The Complex Function of Hsp70 in Metastatic Cancer
Abstract
:1. Introduction
2. Hsp70 Supports Metastatic Cancer Cell Growth through Chaperone and Antiapoptotic Functions
3. Hsp70 Supports Metastasis through Promoting Invasion Steps
4. Impact of Hsp70 Trafficking on Metastasis
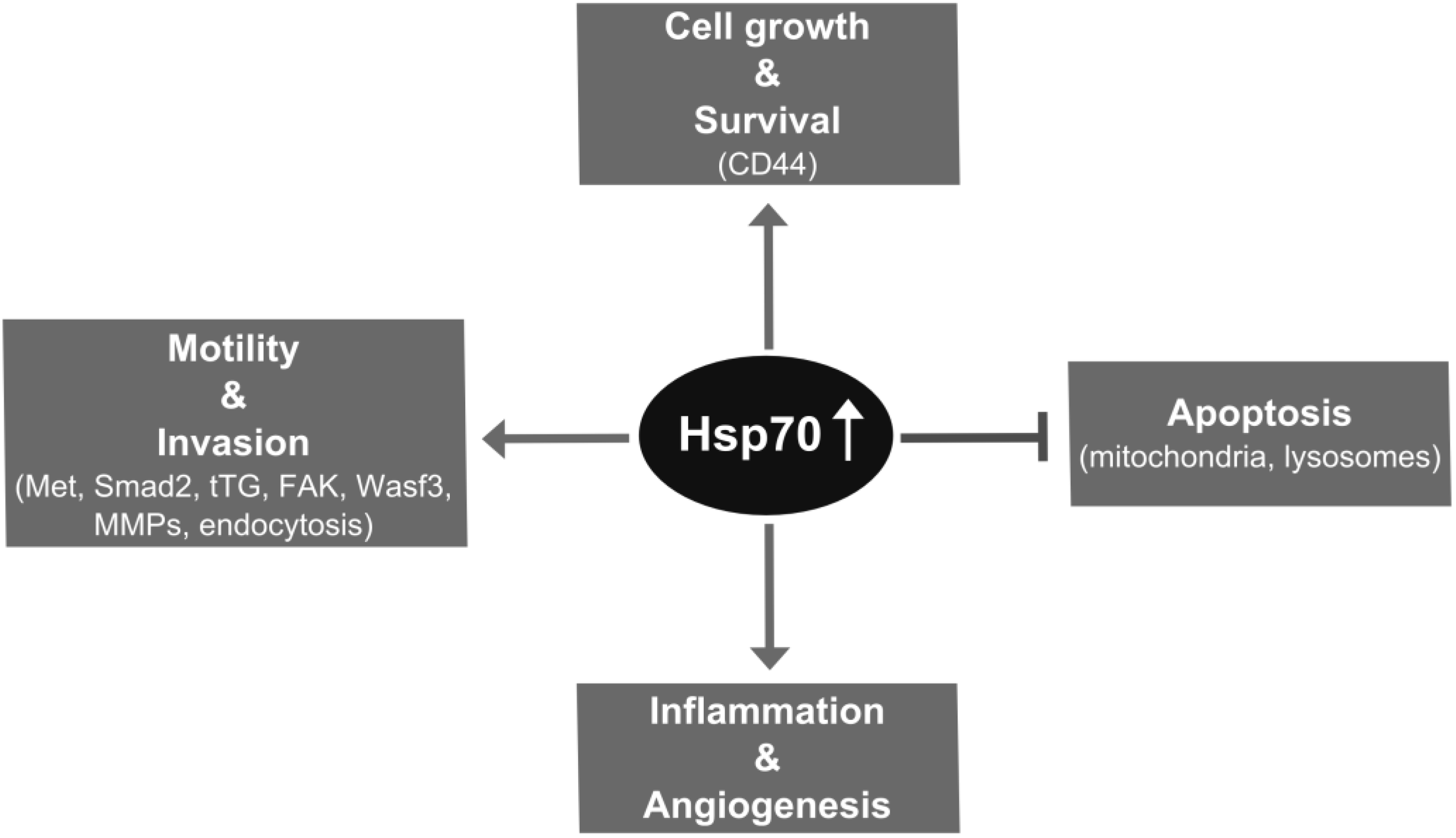
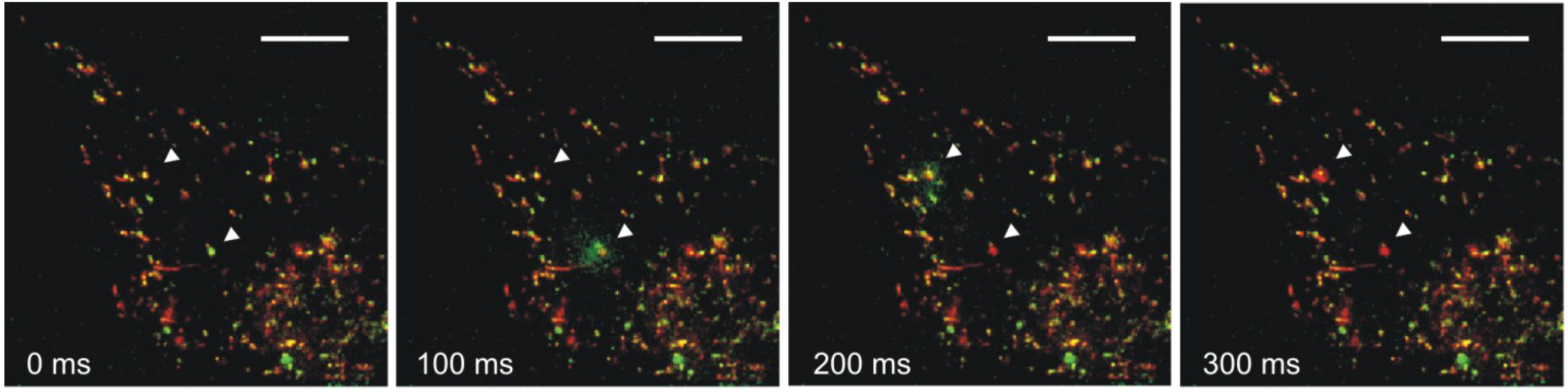
5. Extracellular Functions of Hsp70

6. Targeting Hsp70 in Metastatic Cancer
7. Concluding Remarks
Abbreviations
| AIF | apoptosis-inducing factor |
| Apaf-1 | apoptotic protease activating factor-1 |
| APCs | antigen-presenting cells |
| Ask-1 | apoptosis signal-regulated kinase 1 |
| ASM | acid sphingomyelinase |
| BMDCs | bone marrow-derived cells |
| BMP | bis(monoacylglycero)-phosphate |
| CHIP | carboxyl-terminus of Hsp70 interacting protein |
| DCs | dendritic cells |
| ECM | extracellular matrix |
| EGFR | epidermal growth factor receptor |
| EMT | epithelial-mesenchymal transition |
| ERK | extracellular signal-regulated kinase |
| FAK | focal adhesion kinase |
| HMGB1 | high mobility group protein B1 |
| Hop | Hsp70/Hsp90 organizing protein |
| HSF | heat shock factor |
| Hsp70 | heat shock protein 70 |
| HspBP1 | Hsp70 binding protein 1 |
| JNK | c-Jun N-terminal kinase |
| LAMP-1 | lysosomal-associated membrane protein-1 |
| MAPK | mitogen-activated protein kinase |
| MDSC | myeloid-derived suppressor cells |
| MMPs | matrix metalloproteases |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK | natural killer |
| NO | nitric oxide |
| PMA | phorbol 12-myristate 13-acetate |
| pMHC | peptide-loaded major histocompatibility complex |
| ROS | reactive oxygen species |
| TCR | T cell receptor |
| TGF-beta | transforming growth factor-beta |
| tTG | tissue transglutaminase |
| TLR | Toll-like receptor |
| TNF | tumor necrosis factor |
| Wasf3 | Wiskott-Aldrich syndrome protein family member 3 |
Supplementary Information
Acknowledgements
Conflicts of Interest
References
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef]
- Morimoto, R.I. Cells in stress: Transcriptional activation of heat shock genes. Science 1993, 259, 1409–1410. [Google Scholar]
- Dubois, M.F.; Bensaude, O. MAP kinase activation during heat shock in quiescent and exponentially growing mammalian cells. FEBS Lett. 1993, 324, 191–195. [Google Scholar] [CrossRef]
- Adler, V.; Schaffer, A.; Kim, J.; Dolan, L.; Ronai, Z. UV irradiation and heat shock mediate JNK activation via alternate pathways. J. Biol. Chem. 1995, 270, 26071–26077. [Google Scholar] [CrossRef]
- Soti, C.; Pál, C.; Papp, B.; Csermely, P. Molecular chaperones as regulatory elements of cellular networks. Curr. Opin. Cell Biol. 2005, 17, 210–215. [Google Scholar] [CrossRef]
- Xie, K.; Huang, S. Regulation of cancer metastasis by stress pathways. Clin. Exp. Metastasis 2003, 20, 31–43. [Google Scholar] [CrossRef]
- Santarosa, M.; Favaro, D.; Quaia, M.; Galligioni, E. Expression of heat shock protein 72 in renal cell carcinoma: Possible role and prognostic implications in cancer patients. Eur. J. Cancer 1997, 33, 873–877. [Google Scholar] [CrossRef]
- Nanbu, K.; Konishi, I.; Mandai, M.; Kuroda, H.; Hamid, A.A.; Komatsu, T.; Mori, T. Prognostic significance of heat shock proteins HSP70 and HSP90 in endometrial carcinomas. Cancer Detect. Prev. 1998, 22, 549–555. [Google Scholar] [CrossRef]
- Mosser, D.D.; Morimoto, R.I. Molecular chaperones and the stress of oncogenesis. Oncogene 2004, 23, 2907–2918. [Google Scholar] [CrossRef]
- Whitesell, L.; Lindquist, S. Inhibiting the transcription factor HSF1 as an anticancer strategy. Expert Opin. Ther. Targets 2009, 13, 469–478. [Google Scholar] [CrossRef]
- Powers, M.V.; Workman, P. Inhibitors of the heat shock response: Biology and pharmacology. FEBS Lett. 2007, 581, 3758–3769. [Google Scholar] [CrossRef]
- Wigmore, S.J.; Sangster, K.; McNally, S.J.; Harrison, E.M.; Ross, J.A.; Fearon, K.C.H.; Garden, O.J. De-repression of heat shock transcription factor-1 in interleukin-6-treated hepatocytes is mediated by downregulation of glycogen synthase kinase 3beta and MAPK/ERK-1. Int. J. Mol. Med. 2007, 19, 413–420. [Google Scholar]
- Nylandsted, J.; Rohde, M.; Brand, K.; Bastholm, L.; Elling, F.; Jäättelä, M. Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2. Proc. Natl. Acad. Sci. USA 2000, 97, 7871–7876. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Zhao, X.; Kariya, Y.; Teshigawara, K.; Uchida, A. Inhibition of proliferation and induction of apoptosis by abrogation of heat-shock protein (HSP) 70 expression in tumor cells. Cancer Immunol. Immunother. 1995, 40, 73–78. [Google Scholar] [CrossRef]
- Kaur, J.; Kaur, J.; Ralhan, R. Induction of apoptosis by abrogation of HSP70 expression in human oral cancer cells. Int. J. Cancer J. Int. Cancer 2000, 85, 1–5. [Google Scholar] [CrossRef]
- Jäättelä, M. Over-expression of hsp70 confers tumorigenicity to mouse fibrosarcoma cells. Int. J. Cancer 1995, 60, 689–693. [Google Scholar] [CrossRef]
- Seo, J.S.; Park, Y.M.; Kim, J.I.; Shim, E.H.; Kim, C.W.; Jang, J.J.; Kim, S.H.; Lee, W.H. T cell lymphoma in transgenic mice expressing the human Hsp70 gene. Biochem. Biophys. Res. Commun. 1996, 218, 582–587. [Google Scholar] [CrossRef]
- Volloch, V.Z.; Sherman, M.Y. Oncogenic potential of Hsp72. Oncogene 1999, 18, 3648–3651. [Google Scholar] [CrossRef]
- Gurbuxani, S.; Bruey, J.M.; Fromentin, A.; Larmonier, N.; Parcellier, A.; Jäättelä, M.; Martin, F.; Solary, E.; Garrido, C. Selective depletion of inducible HSP70 enhances immunogenicity of rat colon cancer cells. Oncogene 2001, 20, 7478–7485. [Google Scholar] [CrossRef]
- Ralhan, R.; Kaur, J. Differential expression of Mr 70,000 heat shock protein in normal, premalignant, and malignant human uterine cervix. Clin. Cancer Res. 1995, 1, 1217–1222. [Google Scholar]
- Lazaris, A.C.; Theodoropoulos, G.E.; Aroni, K.; Saetta, A.; Davaris, P.S. Immunohistochemical expression of C-myc oncogene, heat shock protein 70 and HLA-DR molecules in malignant cutaneous melanoma. Virchows Arch. Int. J. Pathol. 1995, 426, 461–467. [Google Scholar]
- Kaur, J.; Srivastava, A.; Ralhan, R. Expression of 70-kDa heat shock protein in oral lesions: Marker of biological stress or pathogenicity. Oral Oncol. 1998, 34, 496–501. [Google Scholar] [CrossRef]
- Syrigos, K.N.; Harrington, K.J.; Karayiannakis, A.J.; Sekara, E.; Chatziyianni, E.; Syrigou, E.I.; Waxman, J. Clinical significance of heat shock protein-70 expression in bladder cancer. Urology 2003, 61, 677–680. [Google Scholar] [CrossRef]
- Abe, M.; Manola, J.B.; Oh, W.K.; Parslow, D.L.; George, D.J.; Austin, C.L.; Kantoff, P.W. Plasma levels of heat shock protein 70 in patients with prostate cancer: A potential biomarker for prostate cancer. Clin. Prostate Cancer 2004, 3, 49–53. [Google Scholar] [CrossRef]
- Chuma, M.; Sakamoto, M.; Yamazaki, K.; Ohta, T.; Ohki, M.; Asaka, M.; Hirohashi, S. Expression profiling in multistage hepatocarcinogenesis: Identification of HSP70 as a molecular marker of early hepatocellular carcinoma. Hepatology 2003, 37, 198–207. [Google Scholar] [CrossRef]
- Ciocca, D.R.; Clark, G.M.; Tandon, A.K.; Fuqua, S.A.; Welch, W.J.; McGuire, W.L. Heat shock protein hsp70 in patients with axillary lymph node-negative breast cancer: Prognostic implications. J. Natl. Cancer Inst. 1993, 85, 570–574. [Google Scholar] [CrossRef]
- Thanner, F.; Sütterlin, M.W.; Kapp, M.; Rieger, L.; Kristen, P.; Dietl, J.; Gassel, A.M.; Müller, T. Heat-shock protein 70 as a prognostic marker in node-negative breast cancer. Anticancer Res. 2003, 23, 1057–1062. [Google Scholar]
- Thomas, X.; Campos, L.; Mounier, C.; Cornillon, J.; Flandrin, P.; Le, Q.-H.; Piselli, S.; Guyotat, D. Expression of heat-shock proteins is associated with major adverse prognostic factors in acute myeloid leukemia. Leuk. Res. 2005, 29, 1049–1058. [Google Scholar] [CrossRef]
- Sun, X.F.; Zhang, H.; Carstensen, J.; Jansson, A.; Nordenskjöld, B. Heat shock protein 72/73 in relation to cytoplasmic p53 expression and prognosis in colorectal adenocarcinomas. Int. J. Cancer 1997, 74, 600–604. [Google Scholar] [CrossRef]
- Kawanishi, K.; Shiozaki, H.; Doki, Y.; Sakita, I.; Inoue, M.; Yano, M.; Tsujinaka, T.; Shamma, A.; Monden, M. Prognostic significance of heat shock proteins 27 and 70 in patients with squamous cell carcinoma of the esophagus. Cancer 1999, 85, 1649–1657. [Google Scholar] [CrossRef]
- Shiozaki, H.; Doki, Y.; Kawanishi, K.; Shamma, A.; Yano, M.; Inoue, M.; Monden, M. Clinical application of malignancy potential grading as a prognostic factor of human esophageal cancers. Surgery 2000, 127, 552–561. [Google Scholar] [CrossRef]
- Maehara, Y.; Oki, E.; Abe, T.; Tokunaga, E.; Shibahara, K.; Kakeji, Y.; Sugimachi, K. Overexpression of the heat shock protein HSP70 family and p53 protein and prognosis for patients with gastric cancer. Oncology 2000, 58, 144–151. [Google Scholar] [CrossRef]
- Nakajima, M.; Kato, H.; Miyazaki, T.; Fukuchi, M.; Masuda, N.; Fukai, Y.; Sohda, M.; Ahmad, F.; Kuwano, H. Tumor immune systems in esophageal cancer with special reference to heat-shock protein 70 and humoral immunity. Anticancer Res. 2009, 29, 1595–606. [Google Scholar]
- Trieb, K.; Lechleitner, T.; Lang, S.; Windhager, R.; Kotz, R.; Dirnhofer, S. Heat shock protein 72 expression in osteosarcomas correlates with good response to neoadjuvant chemotherapy. Hum. Pathol. 1998, 29, 1050–1055. [Google Scholar] [CrossRef]
- Multhoff, G.; Botzler, C.; Wiesnet, M.; Müller, E.; Meier, T.; Wilmanns, W.; Issels, R.D. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int. J. Cancer 1995, 61, 272–279. [Google Scholar] [CrossRef]
- Multhoff, G.; Botzler, C.; Jennen, L.; Schmidt, J.; Ellwart, J.; Issels, R. Heat shock protein 72 on tumor cells: A recognition structure for natural killer cells. J. Immunol. 1997, 158, 4341–4350. [Google Scholar]
- Gastpar, R.; Gehrmann, M.; Bausero, M.A.; Asea, A.; Gross, C.; Schroeder, J.A.; Multhoff, G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005, 65, 5238–5247. [Google Scholar] [CrossRef]
- Korbelik, M.; Sun, J.; Cecic, I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: Relevance for tumor response. Cancer Res. 2005, 65, 1018–1026. [Google Scholar]
- Asea, A.; Kraeft, S.K.; Kurt-Jones, E.A.; Stevenson, M.A.; Chen, L.B.; Finberg, R.W.; Koo, G.C.; Calderwood, S.K. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med. 2000, 6, 435–442. [Google Scholar] [CrossRef]
- Garg, M.; Kanojia, D.; Saini, S.; Suri, S.; Gupta, A.; Surolia, A.; Suri, A. Germ cell-specific heat shock protein 70-2 is expressed in cervical carcinoma and is involved in the growth, migration, and invasion of cervical cells. Cancer 2010, 116, 3785–3796. [Google Scholar] [CrossRef]
- Simard, J.P.; Reynolds, D.N.; Kraguljac, A.P.; Smith, G.S.T.; Mosser, D.D. Overexpression of HSP70 inhibits cofilin phosphorylation and promotes lymphocyte migration in heat-stressed cells. J. Cell Sci. 2011, 124, 2367–2374. [Google Scholar] [CrossRef]
- Chiu, C.-C.; Lin, C.-Y.; Lee, L.-Y.; Chen, Y.-J.; Lu, Y.-C.; Wang, H.-M.; Liao, C.-T.; Chang, J.T.-C.; Cheng, A.-J. Molecular Chaperones as a Common Set of Proteins That Regulate the Invasion Phenotype of Head and Neck Cancer. Clin. Cancer Res. 2011, 17, 4629–4641. [Google Scholar] [CrossRef]
- Kluger, H.M.; Lev, D.C.; Kluger, Y.; McCarthy, M.M.; Kiriakova, G.; Camp, R.L.; Rimm, D.L.; Price, J.E. Using a Xenograft Model of Human Breast Cancer Metastasis to Find Genes Associated with Clinically Aggressive Disease. Cancer Res. 2005, 65, 5578–5587. [Google Scholar] [CrossRef]
- Becker, B.; Multhoff, G.; Farkas, B.; Wild, P.-J.; Landthaler, M.; Stolz, W.; Vogt, T. Induction of Hsp90 protein expression in malignant melanomas and melanoma metastases. Exp. Dermatol. 2004, 13, 27–32. [Google Scholar]
- Gabai, V.L.; Yaglom, J.A.; Waldman, T.; Sherman, M.Y. Heat shock protein Hsp72 controls oncogene-induced senescence pathways in cancer cells. Mol. Cell. Biol. 2009, 29, 559–569. [Google Scholar] [CrossRef]
- Gurbuxani, S.; Schmitt, E.; Cande, C.; Parcellier, A.; Hammann, A.; Daugas, E.; Kouranti, I.; Spahr, C.; Pance, A.; Kroemer, G.; Garrido, C. Heat shock protein 70 binding inhibits the nuclear import of apoptosis-inducing factor. Oncogene 2003, 22, 6669–6678. [Google Scholar] [CrossRef]
- Rohde, M.; Daugaard, M.; Jensen, M.H.; Helin, K.; Nylandsted, J.; Jäättelä, M. Members of the heat-shock protein 70 family promote cancer cell growth by distinct mechanisms. Genes Dev. 2005, 19, 570–582. [Google Scholar]
- Gabai, V.L.; Budagova, K.R.; Sherman, M.Y. Increased expression of the major heat shock protein Hsp72 in human prostate carcinoma cells is dispensable for their viability but confers resistance to a variety of anticancer agents. Oncogene 2005, 24, 3328–3338. [Google Scholar] [CrossRef]
- Wang, X.-P.; Wang, Q.-X.; Ying, X.-P. Correlation between clinicopathology and expression of heat shock protein 72 and glycoprotein 96 in human gastric adenocarcinoma. Tohoku J. Exp. Med. 2007, 212, 35–41. [Google Scholar] [CrossRef]
- Calderwood, S.K. Molecular Cochaperones: Tumor Growth and Cancer Treatment. Scientifica 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Dorsey, W.C.; Tchounwou, P.B. CYP1a1, HSP70, P53, and c-fos expression in human liver carcinoma cells (HepG2) exposed to pentachlorophenol. Biomed. Sci. Instrum. 2003, 39, 389–396. [Google Scholar]
- Hwang, T.S.; Han, H.S.; Choi, H.K.; Lee, Y.J.; Kim, Y.-J.; Han, M.-Y.; Park, Y.-M. Differential, stage-dependent expression of Hsp70, Hsp110 and Bcl-2 in colorectal cancer. J. Gastroenterol. Hepatol. 2003, 18, 690–700. [Google Scholar] [CrossRef]
- Zorzi, E.; Bonvini, P. Inducible Hsp70 in the regulation of cancer cell survival: Analysis of chaperone induction, expression and activity. Cancers 2011, 3, 3921–3956. [Google Scholar] [CrossRef]
- Fidler, I.J.; Nicolson, G.L. Fate of recirculating B16 melanoma metastatic variant cells in parabiotic syngeneic recipients. J. Natl. Cancer Inst. 1977, 58, 1867–1872. [Google Scholar]
- Liotta, L.A.; Vembu, D.; Saini, R.K.; Boone, C. In vivo monitoring of the death rate of artificial murine pulmonary micrometastases. Cancer Res. 1978, 38, 1231–1236. [Google Scholar]
- Wang, X.; Chen, W.; Li, X.; Lin, H.; Wang, Q. Heat shock protein 72 associated with CD44v6 in human colonic adenocarcinoma. Cell Biol. Int. 2008, 32, 860–864. [Google Scholar] [CrossRef]
- Ponta, H.; Sherman, L.; Herrlich, P.A. CD44: From adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003, 4, 33–45. [Google Scholar] [CrossRef]
- Jäättelä, M.; Wissing, D.; Bauer, P.A.; Li, G.C. Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J. 1992, 11, 3507–3512. [Google Scholar]
- Jäättelä, M.; Wissing, D.; Kokholm, K.; Kallunki, T.; Egeblad, M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 1998, 17, 6124–6134. [Google Scholar] [CrossRef]
- Jäättelä, M.; Wissing, D. Heat-shock proteins protect cells from monocyte cytotoxicity: Possible mechanism of self-protection. J. Exp. Med. 1993, 177, 231–236. [Google Scholar] [CrossRef]
- Simon, M.M.; Reikerstorfer, A.; Schwarz, A.; Krone, C.; Luger, T.A.; Jäättelä, M.; Schwarz, T. Heat shock protein 70 overexpression affects the response to ultraviolet light in murine fibroblasts. Evidence for increased cell viability and suppression of cytokine release. J. Clin. Invest. 1995, 95, 926–933. [Google Scholar] [CrossRef]
- Mosser, D.D.; Caron, A.W.; Bourget, L.; Denis-Larose, C.; Massie, B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol. Cell. Biol. 1997, 17, 5317–5327. [Google Scholar]
- Buzzard, K.A.; Giaccia, A.J.; Killender, M.; Anderson, R.L. Heat shock protein 72 modulates pathways of stress-induced apoptosis. J. Biol. Chem. 1998, 273, 17147–17153. [Google Scholar] [CrossRef]
- Vayssier, M.; Banzet, N.; François, D.; Bellmann, K.; Polla, B.S. Tobacco smoke induces both apoptosis and necrosis in mammalian cells: Differential effects of HSP70. Am. J. Physiol. 1998, 275, L771–L779. [Google Scholar]
- Nylandsted, J.; Gyrd-Hansen, M.; Danielewicz, A.; Fehrenbacher, N.; Lademann, U.; Høyer-Hansen, M.; Weber, E.; Multhoff, G.; Rohde, M.; Jäättelä, M. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J. Exp. Med. 2004, 200, 425–435. [Google Scholar] [CrossRef]
- Mosser, D.D.; Caron, A.W.; Bourget, L.; Meriin, A.B.; Sherman, M.Y.; Morimoto, R.I.; Massie, B. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol. Cell. Biol. 2000, 20, 7146–7159. [Google Scholar] [CrossRef]
- Gabai, V.L.; Meriin, A.B.; Mosser, D.D.; Caron, A.W.; Rits, S.; Shifrin, V.I.; Sherman, M.Y. Hsp70 prevents activation of stress Kinases a novel pathway of cellular thermotolerance. J. Biol. Chem. 1997, 272, 18033–18037. [Google Scholar] [CrossRef]
- Park, H.-S.; Cho, S.-G.; Kim, C.K.; Hwang, H.S.; Noh, K.T.; Kim, M.-S.; Huh, S.-H.; Kim, M.J.; Ryoo, K.; Kim, E.K.; et al. Heat shock protein Hsp72 is a negative regulator of apoptosis signal-regulating Kinase 1. Mol. Cell. Biol. 2002, 22, 7721–7730. [Google Scholar] [CrossRef]
- Park, H.-S.; Lee, J.-S.; Huh, S.-H.; Seo, J.-S.; Choi, E.-J. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J. 2001, 20, 446–456. [Google Scholar] [CrossRef]
- Lee, J.-S.; Lee, J.-J.; Seo, J.-S. HSP70 Deficiency Results in Activation of c-Jun N-terminal Kinase, Extracellular Signal-regulated Kinase, and Caspase-3 in Hyperosmolarity-induced Apoptosis. J. Biol. Chem. 2005, 280, 6634–6641. [Google Scholar] [CrossRef]
- Yaglom, J.; O’Callaghan-Sunol, C.; Gabai, V.; Sherman, M.Y. Inactivation of dual-specificity phosphatases is involved in the regulation of extracellular signal-regulated kinases by heat shock and hsp72. Mol. Cell. Biol. 2003, 23, 3813–3824. [Google Scholar] [CrossRef]
- Song, J.; Takeda, M.; Morimoto, R.I. Bag1-Hsp70 mediates a physiological stress signalling pathway that regulates Raf-1/ERK and cell growth. Nat. Cell Biol. 2001, 3, 276–282. [Google Scholar] [CrossRef]
- Yaglom, J.A.; Gabai, V.L.; Sherman, M.Y. High levels of heat shock protein Hsp72 in cancer cells suppress default senescence pathways. Cancer Res. 2007, 67, 2373–2381. [Google Scholar] [CrossRef]
- Beere, H.M.; Wolf, B.B.; Cain, K.; Mosser, D.D.; Mahboubi, A.; Kuwana, T.; Tailor, P.; Morimoto, R.I.; Cohen, G.M.; Green, D.R. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2000, 2, 469–475. [Google Scholar] [CrossRef]
- Ravagnan, L.; Gurbuxani, S.; Susin, S.A.; Maisse, C.; Daugas, E.; Zamzami, N.; Mak, T.; Jäättelä, M.; Penninger, J.M.; Garrido, C.; et al. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat. Cell Biol. 2001, 3, 839–843. [Google Scholar] [CrossRef]
- Doulias, P.-T.; Kotoglou, P.; Tenopoulou, M.; Keramisanou, D.; Tzavaras, T.; Brunk, U.; Galaris, D.; Angelidis, C. Involvement of heat shock protein-70 in the mechanism of hydrogen peroxide-induced DNA damage: The role of lysosomes and iron. Free Radic. Biol. Med. 2007, 42, 567–577. [Google Scholar] [CrossRef]
- Schmitt, E.; Gehrmann, M.; Brunet, M.; Multhoff, G.; Garrido, C. Intracellular and extracellular functions of heat shock proteins: Repercussions in cancer therapy. J. Leukoc. Biol. 2007, 81, 15–27. [Google Scholar]
- Felding-Habermann, B. Integrin adhesion receptors in tumor metastasis. Clin. Exp. Metastasis 2003, 20, 203–213. [Google Scholar] [CrossRef]
- Mehlen, P.; Puisieux, A. Metastasis: A question of life or death. Nat. Rev. Cancer 2006, 6, 449–458. [Google Scholar] [CrossRef]
- Gyrd-Hansen, M.; Nylandsted, J.; Jäättelä, M. Heat shock protein 70 promotes cancer cell viability by safeguarding lysosomal integrity. Cell Cycle 2004, 3, 1484–1485. [Google Scholar] [CrossRef]
- Jang, K.W.; Lee, J.E.; Kim, S.Y.; Kang, M.-W.; Na, M.H.; Lee, C.S.; Song, K.S.; Lim, S.P. The C-terminus of Hsp70-interacting protein promotes Met receptor degradation. J. Thorac. Oncol. 2011, 6, 679–687. [Google Scholar] [CrossRef]
- Mao, H.; Li, F.; Ruchalski, K.; Mosser, D.D.; Schwartz, J.H.; Wang, Y.; Borkan, S.C. Hsp72 inhibits focal adhesion kinase degradation in atp-depleted renal epithelial cells. J. Biol. Chem. 2003, 278, 18214–18220. [Google Scholar] [CrossRef]
- Hofmann, U.B.; Houben, R.; Bröcker, E.-B.; Becker, J.C. Role of matrix metalloproteinases in melanoma cell invasion. Biochimie 2005, 87, 307–314. [Google Scholar] [CrossRef]
- Abraham, R.; Schäfer, J.; Rothe, M.; Bange, J.; Knyazev, P.; Ullrich, A. Identification of MMP-15 as an anti-apoptotic factor in cancer cells. J. Biol. Chem. 2005, 280, 34123–34132. [Google Scholar] [CrossRef]
- Strand, S.; Vollmer, P.; van den Abeelen, L.; Gottfried, D.; Alla, V.; Heid, H.; Kuball, J.; Theobald, M.; Galle, P.R.; Strand, D. Cleavage of CD95 by matrix metalloproteinase-7 induces apoptosis resistance in tumour cells. Oncogene 2004, 23, 3732–3736. [Google Scholar] [CrossRef]
- Sims, J.D.; McCready, J.; Jay, D.G. Extracellular heat shock protein (Hsp)70 and Hsp90α assist in matrix metalloproteinase-2 activation and breast cancer cell migration and invasion. PLoS One 2011, 6, e18848. [Google Scholar] [CrossRef]
- Lee, K.-J.; Kim, Y.M.; Kim, D.Y.; Jeoung, D.; Han, K.; Lee, S.-T.; Lee, Y.-S.; Park, K.H.; Park, J.H.; Kim, D.J.; et al. Release of heat shock protein 70 (Hsp70) and the effects of extracellular Hsp70 on matric metalloproteinase-9 expression in human monocytic U937 cells. Exp. Mol. Med. 2006, 38, 364–374. [Google Scholar] [CrossRef]
- Cannito, S.; Novo, E.; Compagnone, A.; Valfrè di Bonzo, L.; Busletta, C.; Zamara, E.; Paternostro, C.; Povero, D.; Bandino, A.; Bozzo, F.; et al. Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells. Carcinogenesis 2008, 29, 2267–2278. [Google Scholar] [CrossRef]
- Zhong, Q.; Zhou, B.; Ann, D.K.; Minoo, P.; Liu, Y.; Banfalvi, A.; Krishnaveni, M.S.; Dubourd, M.; Demaio, L.; Willis, B.C.; et al. Role of endoplasmic reticulum stress in epithelial-mesenchymal transition of alveolar epithelial cells: Effects of misfolded surfactant protein. Am. J. Respir. Cell Mol. Biol. 2011, 45, 498–509. [Google Scholar] [CrossRef]
- Canöz, O.; Belenli, O.; Patiroglu, T.E. General features of gastric carcinomas and comparison of HSP70 and NK cell immunoreactivity with prognostic factors. Pathol. Oncol. Res. 2002, 8, 262–269. [Google Scholar] [CrossRef]
- Teng, Y.; Ngoka, L.; Mei, Y.; Lesoon, L.; Cowell, J.K. HSP90 and HSP70 proteins are essential for stabilization and activation of WASF3 metastasis-promoting protein. J. Biol. Chem. 2012, 287, 10051–10059. [Google Scholar] [CrossRef]
- Birchmeier, C.; Birchmeier, W.; Gherardi, E.; vande Woude, G.F. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003, 4, 915–925. [Google Scholar] [CrossRef]
- Li, Y.; Kang, X.; Wang, Q. HSP70 decreases receptor-dependent phosphorylation of Smad2 and blocks TGF-β-induced epithelial-mesenchymal transition. J. Genet. Genomics 2011, 38, 111–116. [Google Scholar] [CrossRef]
- Mao, H.; Wang, Y.; Li, Z.; Ruchalski, K.L.; Yu, X.; Schwartz, J.H.; Borkan, S.C. Hsp72 interacts with paxillin and facilitates the reassembly of focal adhesions during recovery from ATP depletion. J. Biol. Chem. 2004, 279, 15472–15480. [Google Scholar] [CrossRef]
- Willmer, T.; Contu, L.; Blatch, G.L.; Edkins, A.L. Knockdown of Hop downregulates RhoC expression, and decreases pseudopodia formation and migration in cancer cell lines. Cancer Lett. 2013, 328, 252–260. [Google Scholar] [CrossRef]
- Boroughs, L.K.; Antonyak, M.A.; Johnson, J.L.; Cerione, R.A. A unique role for heat shock protein 70 and its binding partner tissue transglutaminase in cancer cell migration. J. Biol. Chem. 2011, 286, 37094–37107. [Google Scholar] [CrossRef]
- Sossey-Alaoui, K.; Safina, A.; Li, X.; Vaughan, M.M.; Hicks, D.G.; Bakin, A.V.; Cowell, J.K. Down-regulation of WAVE3, a metastasis promoter gene, inhibits invasion and metastasis of breast cancer cells. Am. J. Pathol. 2007, 170, 2112–2121. [Google Scholar] [CrossRef]
- Sossey-Alaoui, K.; Li, X.; Ranalli, T.A.; Cowell, J.K. WAVE3-mediated cell migration and lamellipodia formation are regulated downstream of phosphatidylinositol 3-Kinase. J. Biol. Chem. 2005, 280, 21748–21755. [Google Scholar] [CrossRef]
- Teng, Y.; Ren, M.Q.; Cheney, R.; Sharma, S.; Cowell, J.K. Inactivation of the WASF3 gene in prostate cancer cells leads to suppression of tumorigenicity and metastases. Br. J. Cancer 2010, 103, 1066–1075. [Google Scholar] [CrossRef]
- Iwasaki, M.; Homma, S.; Hishiya, A.; Dolezal, S.J.; Reed, J.C.; Takayama, S. BAG3 regulates motility and adhesion of epithelial cancer cells. Cancer Res. 2007, 67, 10252–10259. [Google Scholar] [CrossRef]
- Suzuki, M.; Iwasaki, M.; Sugio, A.; Hishiya, A.; Tanaka, R.; Endo, T.; Takayama, S.; Saito, T. BAG3 (BCL2-associated athanogene 3) interacts with MMP-2 to positively regulate invasion by ovarian carcinoma cells. Cancer Lett. 2011, 303, 65–71. [Google Scholar] [CrossRef]
- Song, X.; Wang, X.; Zhuo, W.; Shi, H.; Feng, D.; Sun, Y.; Liang, Y.; Fu, Y.; Zhou, D.; Luo, Y. The Regulatory mechanism of extracellular Hsp90α on matrix metalloproteinase-2 processing and tumor angiogenesis. J. Biol. Chem. 2010, 285, 40039–40049. [Google Scholar] [CrossRef]
- Walsh, N.; Larkin, A.; Swan, N.; Conlon, K.; Dowling, P.; McDermott, R.; Clynes, M. RNAi knockdown of Hop (Hsp70/Hsp90 organising protein) decreases invasion via MMP-2 down regulation. Cancer Lett. 2011, 306, 180–189. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef]
- Leber, M.F.; Efferth, T. Molecular principles of cancer invasion and metastasis. Int. J. Oncol. 2009, 34, 881–895. [Google Scholar]
- Chetty, C.; Vanamala, S.K.; Gondi, C.S.; Dinh, D.H.; Gujrati, M.; Rao, J.S. MMP-9 induces CD44 cleavage and CD44 mediated cell migration in glioblastoma xenograft cells. Cell. Signal. 2012, 24, 549–559. [Google Scholar] [CrossRef]
- Sherman, M.; Multhoff, G. Heat shock proteins in cancer. Ann. NY Acad. Sci. 2007, 1113, 192–201. [Google Scholar] [CrossRef]
- Arispe, N.; Doh, M.; Simakova, O.; Kurganov, B.; de Maio, A. Hsc70 and Hsp70 interact with phosphatidylserine on the surface of PC12 cells resulting in a decrease of viability. FASEB J. 2004, 18, 1636–1645. [Google Scholar] [CrossRef]
- Arispe, N.; Doh, M.; de Maio, A. Lipid interaction differentiates the constitutive and stress-induced heat shock proteins Hsc70 and Hsp70. Cell Stress Chaperones 2002, 7, 330–338. [Google Scholar] [CrossRef]
- Bausero, M.A.; Gastpar, R.; Multhoff, G.; Asea, A. Alternative mechanism by which IFN-gamma enhances tumor recognition: Active release of heat shock protein 72. J. Immunol. 2005, 175, 2900–2912. [Google Scholar]
- Broquet, A.H.; Thomas, G.; Masliah, J.; Trugnan, G.; Bachelet, M. Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J. Biol. Chem. 2003, 278, 21601–21606. [Google Scholar] [CrossRef]
- Hunter-Lavin, C.; Davies, E.L.; Bacelar, M.M.F.V.G.; Marshall, M.J.; Andrew, S.M.; Williams, J.H.H. Hsp70 release from peripheral blood mononuclear cells. Biochem. Biophys. Res. Commun. 2004, 324, 511–517. [Google Scholar] [CrossRef]
- Lancaster, G.I.; Febbraio, M.A. Exosome-dependent trafficking of HSP70: A novel secretory pathway for cellular stress proteins. J. Biol. Chem. 2005, 280, 23349–23355. [Google Scholar] [CrossRef]
- Mambula, S.S.; Calderwood, S.K. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J. Immunol. 2006, 177, 7849–7857. [Google Scholar]
- Lv, L.-H.; Wan, Y.-L.; Lin, Y.; Zhang, W.; Yang, M.; Li, G.-L.; Lin, H.-M.; Shang, C.-Z.; Chen, Y.-J.; Min, J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J. Biol. Chem. 2012, 287, 15874–15885. [Google Scholar] [CrossRef]
- Radons, J.; Multhoff, G. Immunostimulatory functions of membrane-bound and exported heat shock protein 70. Exerc. Immunol. Rev. 2005, 11, 17–33. [Google Scholar]
- Evdonin, A.L.; Guzhova, I.V.; Margulis, B.A.; Medvedeva, N.D. Extracellular heat shock protein 70 mediates heat stress-induced epidermal growth factor receptor transactivation in A431 carcinoma cells. FEBS Lett. 2006, 580, 6674–6678. [Google Scholar] [CrossRef]
- Juhász, K.; Thuenauer, R.; Spachinger, A.; Duda, E.; Horváth, I.; Vígh, L.; Sonnleitner, A.; Balogi, Z. Lysosomal rerouting of Hsp70 trafficking as a potential immune activating tool for targeting melanoma. Curr. Pharm. Des. 2013, 19, 430–440. [Google Scholar] [CrossRef]
- Petersen, N.H.T.; Kirkegaard, T.; Olsen, O.D.; Jäättelä, M. Connecting Hsp70, sphingolipid metabolism and lysosomal stability. Cell Cycle 2010, 9, 2305–2309. [Google Scholar] [CrossRef]
- Kurz, T.; Brunk, U.T. Autophagy of HSP70 and chelation of lysosomal iron in a non-redox-active form. Autophagy 2009, 5, 93–95. [Google Scholar] [CrossRef]
- Yang, Y.; Rao, R.; Shen, J.; Tang, Y.; Fiskus, W.; Nechtman, J.; Atadja, P.; Bhalla, K. Role of acetylation and extracellular location of heat shock protein 90alpha in tumor cell invasion. Cancer Res. 2008, 68, 4833–4842. [Google Scholar] [CrossRef]
- Kirkegaard, T.; Roth, A.G.; Petersen, N.H.T.; Mahalka, A.K.; Olsen, O.D.; Moilanen, I.; Zylicz, A.; Knudsen, J.; Sandhoff, K.; Arenz, C.; et al. Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature 2010, 463, 549–553. [Google Scholar] [CrossRef]
- Fehrenbacher, N.; Jäättelä, M. Lysosomes as targets for cancer therapy. Cancer Res. 2005, 65, 2993–2995. [Google Scholar]
- Vasiljeva, O.; Reinheckel, T.; Peters, C.; Turk, D.; Turk, V.; Turk, B. Emerging roles of cysteine cathepsins in disease and their potential as drug targets. Curr. Pharm. Des. 2007, 13, 387–403. [Google Scholar] [CrossRef]
- Joyce, J.A.; Baruch, A.; Chehade, K.; Meyer-Morse, N.; Giraudo, E.; Tsai, F.-Y.; Greenbaum, D.C.; Hager, J.H.; Bogyo, M.; Hanahan, D. Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell 2004, 5, 443–453. [Google Scholar] [CrossRef]
- Tu, C.; Ortega-Cava, C.F.; Chen, G.; Fernandes, N.D.; Cavallo-Medved, D.; Sloane, B.F.; Band, V.; Band, H. Lysosomal cathepsin B participates in the podosome-mediated extracellular matrix degradation and invasion via secreted lysosomes in v-Src fibroblasts. Cancer Res. 2008, 68, 9147–9156. [Google Scholar] [CrossRef]
- Juhasz, K.; Lehner, M.; Hesse, J.; Haselgruebler, T.; Balogi, Z.; Center for Advanced Bioanalysis GmbH, Linz, Austria. Unpublished work. 2013.
- Yano, Y.; Yano, A.; Oishi, S.; Sugimoto, Y.; Tsujimoto, G.; Fujii, N.; Matsuzaki, K. Coiled-coil tag—Probe system for quick labeling of membrane receptors in living cell. ACS Chem. Biol. 2008, 3, 341–345. [Google Scholar] [CrossRef]
- Vega, V.L.; Charles, W.; de Maio, A. A new feature of the stress response: Increase in endocytosis mediated by Hsp70. Cell Stress Chaperones 2010, 15, 517–527. [Google Scholar] [CrossRef]
- Vega, V.L.; Rodriguez-Silva, M.; Frey, T.; Gehrmann, M.; Diaz, J.C.; Steinem, C.; Multhoff, G.; Arispe, N.; de Maio, A. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J. Immunol. 2008, 180, 4299–4307. [Google Scholar]
- Delneste, Y.; Magistrelli, G.; Gauchat, J.-F.; Haeuw, J.-F.; Aubry, J.-P.; Nakamura, K.; Kawakami-Honda, N.; Goetsch, L.; Sawamura, T.; Bonnefoy, J.-Y. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity 2002, 17, 353–362. [Google Scholar] [CrossRef]
- Thériault, J.R.; Mambula, S.S.; Sawamura, T.; Stevenson, M.A.; Calderwood, S.K. Extracellular HSP70 binding to surface receptors present on antigen presenting cells and endothelial/epithelial cells. FEBS Lett. 2005, 579, 1951–1960. [Google Scholar] [CrossRef]
- Basu, S.; Binder, R.J.; Ramalingam, T.; Srivastava, P.K. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 2001, 14, 303–313. [Google Scholar] [CrossRef]
- Thériault, J.R.; Adachi, H.; Calderwood, S.K. Role of scavenger receptors in the binding and internalization of heat shock protein 70. J. Immunol. 2006, 177, 8604–8611. [Google Scholar]
- Bendz, H.; Ruhland, S.C.; Pandya, M.J.; Hainzl, O.; Riegelsberger, S.; Brauchle, C.; Mayer, M.P.; Buchner, J.; Issels, R.D.; Noessner, E. Human heat shock protein 70 enhances tumor antigen presentation through complex formation and intracellular antigen delivery without innate immune signaling. J. Biol. Chem. 2007, 282, 31688–31702. [Google Scholar] [CrossRef]
- Noessner, E.; Gastpar, R.; Milani, V.; Brandl, A.; Hutzler, P.J.; Kuppner, M.C.; Roos, M.; Kremmer, E.; Asea, A.; Calderwood, S.K.; et al. Tumor-derived heat shock protein 70 peptide complexes are cross-presented by human dendritic cells. J. Immunol. 2002, 169, 5424–5432. [Google Scholar]
- Murshid, A.; Gong, J.; Calderwood, S.K. The role of heat shock proteins in antigen cross presentation. Front. Immunol. 2012, 3, 63. [Google Scholar]
- Arnold-Schild, D.; Hanau, D.; Spehner, D.; Schmid, C.; Rammensee, H.G.; de la Salle, H.; Schild, H. Cutting edge: Receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J. Immunol. 1999, 162, 3757–3760. [Google Scholar]
- Schild, H.; Arnold-Schild, D.; Lammert, E.; Rammensee, H.G. Stress proteins and immunity mediated by cytotoxic T lymphocytes. Curr. Opin. Immunol. 1999, 11, 109–113. [Google Scholar] [CrossRef]
- Enomoto, Y.; Bharti, A.; Khaleque, A.A.; Song, B.; Liu, C.; Apostolopoulos, V.; Xing, P.; Calderwood, S.K.; Gong, J. Enhanced immunogenicity of heat shock protein 70 peptide complexes from dendritic cell-tumor fusion cells. J. Immunol. 2006, 177, 5946–5955. [Google Scholar]
- Stangl, S.; Gehrmann, M.; Riegger, J.; Kuhs, K.; Riederer, I.; Sievert, W.; Hube, K.; Mocikat, R.; Dressel, R.; Kremmer, E.; et al. Targeting membrane heat-shock protein 70 (Hsp70) on tumors by cmHsp70.1 antibody. Proc. Natl. Acad. Sci. USA 2011, 108, 733–738. [Google Scholar] [CrossRef]
- Multhoff, G.; Pfister, K.; Gehrmann, M.; Hantschel, M.; Gross, C.; Hafner, M.; Hiddemann, W. A 14-mer Hsp70 peptide stimulates natural killer (NK) cell activity. Cell Stress Chaperones 2001, 6, 337–344. [Google Scholar] [CrossRef]
- Gross, C.; Schmidt-Wolf, I.G.; Nagaraj, S.; Gastpar, R.; Ellwart, J.; Kunz-Schughart, L.A.; Multhoff, G. Heat shock protein 70-reactivity is associated with increased cell surface density of CD94/CD56 on primary natural killer cells. Cell Stress Chaperones 2003, 8, 348–360. [Google Scholar] [CrossRef]
- Gastpar, R.; Gross, C.; Rossbacher, L.; Ellwart, J.; Riegger, J.; Multhoff, G. The cell surface-localized heat shock protein 70 epitope TKD induces migration and cytolytic activity selectively in human NK cells. J. Immunol. 2004, 172, 972–980. [Google Scholar]
- Figueiredo, C.; Wittmann, M.; Wang, D.; Dressel, R.; Seltsam, A.; Blasczyk, R.; Eiz-Vesper, B. Heat shock protein 70 (HSP70) induces cytotoxicity of T-helper cells. Blood 2009, 113, 3008–3016. [Google Scholar] [CrossRef]
- Clayton, A.; Turkes, A.; Navabi, H.; Mason, M.D.; Tabi, Z. Induction of heat shock proteins in B-cell exosomes. J. Cell Sci. 2005, 118, 3631–3638. [Google Scholar] [CrossRef]
- Asea, A.; Rehli, M.; Kabingu, E.; Boch, J.A.; Bare, O.; Auron, P.E.; Stevenson, M.A.; Calderwood, S.K. Novel signal transduction pathway utilized by extracellular HSP70: Role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002, 277, 15028–15034. [Google Scholar] [CrossRef]
- Vabulas, R.M.; Ahmad-Nejad, P.; Ghose, S.; Kirschning, C.J.; Issels, R.D.; Wagner, H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J. Biol. Chem. 2002, 277, 15107–15112. [Google Scholar] [CrossRef]
- Basu, S.; Binder, R.J.; Suto, R.; Anderson, K.M.; Srivastava, P.K. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int. Immunol. 2000, 12, 1539–1546. [Google Scholar] [CrossRef]
- Panjwani, N.N.; Popova, L.; Srivastava, P.K. Heat shock proteins gp96 and hsp70 activate the release of nitric oxide by APCs. J. Immunol. 2002, 168, 2997–3003. [Google Scholar]
- Sanchez-Perez, L.; Kottke, T.; Daniels, G.A.; Diaz, R.M.; Thompson, J.; Pulido, J.; Melcher, A.; Vile, R.G. Killing of normal melanocytes, combined with heat shock protein 70 and CD40L expression, cures large established melanomas. J. Immunol. 2006, 177, 4168–4177. [Google Scholar]
- Wang, Y.; Whittall, T.; McGowan, E.; Younson, J.; Kelly, C.; Bergmeier, L.A.; Singh, M.; Lehner, T. Identification of stimulating and inhibitory epitopes within the heat shock protein 70 molecule that modulate cytokine production and maturation of dendritic cells. J. Immunol. 2005, 174, 3306–3316. [Google Scholar]
- Kuppner, M.C.; Gastpar, R.; Gelwer, S.; Nössner, E.; Ochmann, O.; Scharner, A.; Issels, R.D. The role of heat shock protein (hsp70) in dendritic cell maturation: Hsp70 induces the maturation of immature dendritic cells but reduces DC differentiation from monocyte precursors. Eur. J. Immunol. 2001, 31, 1602–1609. [Google Scholar] [CrossRef]
- Chen, T.; Guo, J.; Han, C.; Yang, M.; Cao, X. Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J. Immunol. 2009, 182, 1449–1459. [Google Scholar]
- Stocki, P.; Dickinson, A.M. The immunosuppressive activity of heat shock protein 70. Autoimmune Dis. 2012, 2012, 617213. [Google Scholar]
- Van Eden, W.; Spiering, R.; Broere, F.; van der Zee, R. A case of mistaken identity: HSPs are no DAMPs but DAMPERs. Cell Stress Chaperones 2012, 17, 281–292. [Google Scholar] [CrossRef]
- Henderson, B.; Calderwood, S.K.; Coates, A.R.M.; Cohen, I.; van Eden, W.; Lehner, T.; Pockley, A.G. Caught with their PAMPs down? The extracellular signalling actions of molecular chaperones are not due to microbial contaminants. Cell Stress Chaperones 2010, 15, 123–141. [Google Scholar] [CrossRef]
- Tsan, M.-F.; Gao, B. Heat shock proteins and immune system. J. Leukoc. Biol. 2009, 85, 905–910. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Murshid, A.; Gong, J. Heat shock proteins: Conditional mediators of inflammation in tumor immunity. Front. Immunol. 2012, 3, 75. [Google Scholar]
- Multhoff, G.; Molls, M.; Radons, J. Chronic inflammation in cancer development. Front. Immunol. 2011, 2, 98. [Google Scholar]
- Wachstein, J.; Tischer, S.; Figueiredo, C.; Limbourg, A.; Falk, C.; Immenschuh, S.; Blasczyk, R.; Eiz-Vesper, B. HSP70 enhances immunosuppressive function of CD4(+)CD25(+)FoxP3(+) T regulatory cells and cytotoxicity in CD4(+)CD25(−) T cells. PLoS One 2012, 7, e51747. [Google Scholar] [CrossRef]
- Chalmin, F.; Ladoire, S.; Mignot, G.; Vincent, J.; Bruchard, M.; Remy-Martin, J.-P.; Boireau, W.; Rouleau, A.; Simon, B.; Lanneau, D.; et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Invest. 2010, 120, 457–471. [Google Scholar] [Green Version]
- Van Eden, W.; van der Zee, R.; Prakken, B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat. Rev. Immunol. 2005, 5, 318–330. [Google Scholar] [CrossRef]
- Borges, T.J.; Wieten, L.; van Herwijnen, M.J.; Broere, F.; van der Zee, R.; Bonorino, C.; van Eden, W. The anti-inflammatory mechanisms of Hsp70. Front. Immunol. 2012, 3, 95. [Google Scholar]
- Stocki, P.; Wang, X.N.; Dickinson, A.M. Inducible heat shock protein 70 reduces T cell responses and stimulatory capacity of monocyte-derived dendritic cells. J. Biol. Chem. 2012, 287, 12387–12394. [Google Scholar] [CrossRef]
- Evdonin, A.L.; Kropacheva, I.V.; Medvedeva, N.D. Extracellular Hsp70 stimulates multiple signaling pathways in A431 carcinoma cells. Biochem. Mosc. Suppl. Ser. A Membr. Cell Biol. 2009, 3, 291–297. [Google Scholar] [CrossRef]
- Ellerbroek, S.M.; Hudson, L.G.; Stack, M.S. Proteinase requirements of epidermal growth factor-induced ovarian cancer cell invasion. Int. J. Cancer 1998, 78, 331–337. [Google Scholar] [CrossRef]
- Jijon, H.B.; Buret, A.; Hirota, C.L.; Hollenberg, M.D.; Beck, P.L. The EGF receptor and HER2 participate in TNF-α-dependent MAPK activation and IL-8 secretion in intestinal epithelial cells. Mediators Inflamm. 2012, 2012, 207398. [Google Scholar]
- Wells, A. Tumor invasion: Role of growth factor-induced cell motility. Adv. Cancer Res. 2000, 78, 31–101. [Google Scholar] [CrossRef]
- Wu, F.-H.; Yuan, Y.; Li, D.; Liao, S.-J.; Yan, B.; Wei, J.-J.; Zhou, Y.-H.; Zhu, J.-H.; Zhang, G.-M.; Feng, Z.-H. Extracellular HSPA1A promotes the growth of hepatocarcinoma by augmenting tumor cell proliferation and apoptosis-resistance. Cancer Lett. 2012, 317, 157–164. [Google Scholar] [CrossRef]
- Gong, W.; Wang, Z.-Y.; Chen, G.-X.; Liu, Y.-Q.; Gu, X.-Y.; Liu, W.-W. Invasion potential of H22 hepatocarcinoma cells is increased by HMGB1-induced tumor NF-κB signaling via initiation of HSP70. Oncol. Rep. 2013. [Google Scholar] [CrossRef]
- Ellerman, J.E.; Brown, C.K.; de Vera, M.; Zeh, H.J.; Billiar, T.; Rubartelli, A.; Lotze, M.T. Masquerader: High mobility group box-1 and cancer. Clin. Cancer Res. 2007, 13, 2836–2848. [Google Scholar] [CrossRef]
- Klink, M.; Nowak, M.; Kielbik, M.; Bednarska, K.; Blus, E.; Szpakowski, M.; Szyllo, K.; Sulowska, Z. The interaction of HspA1A with TLR2 and TLR4 in the response of neutrophils induced by ovarian cancer cells in vitro. Cell Stress Chaperones 2012, 17, 661–674. [Google Scholar] [CrossRef]
- Ushio-Fukai, M.; Alexander, R.W. Reactive oxygen species as mediators of angiogenesis signaling: Role of NAD(P)H oxidase. Mol. Cell. Biochem. 2004, 264, 85–97. [Google Scholar] [CrossRef]
- De Larco, J.E.; Wuertz, B.R.K.; Furcht, L.T. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin. Cancer Res. 2004, 10, 4895–4900. [Google Scholar] [CrossRef]
- Wheeler, D.S.; Chase, M.A.; Senft, A.P.; Poynter, S.E.; Wong, H.R.; Page, K. Extracellular Hsp72, an endogenous DAMP, is released by virally infected airway epithelial cells and activates neutrophils via Toll-like receptor (TLR)-4. Respir. Res. 2009, 10, 31. [Google Scholar] [CrossRef]
- Becker, T.; Hartl, F.-U.; Wieland, F. CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes. J. Cell Biol. 2002, 158, 1277–1285. [Google Scholar] [CrossRef]
- Wang, Y.; Kelly, C.G.; Karttunen, J.T.; Whittall, T.; Lehner, P.J.; Duncan, L.; MacAry, P.; Younson, J.S.; Singh, M.; Oehlmann, W.; et al. CD40 is a cellular receptor mediating mycobacterial heat shock protein 70 stimulation of CC-chemokines. Immunity 2001, 15, 971–983. [Google Scholar] [CrossRef]
- Van Kooten, C.; Banchereau, J. CD40-CD40 ligand. J. Leukoc. Biol. 2000, 67, 2–17. [Google Scholar]
- Futagami, S.; Hiratsuka, T.; Shindo, T.; Hamamoto, T.; Horie, A.; Ueki, N.; Kusunoki, M.; Gudis, K.; Miyake, K.; Tsukui, T.; et al. Extracellular HSP70 blocks CD40L-induced apoptosis and tubular formation in endothelial cells. J. Gastroenterol. Hepatol. 2008, 23, S222–S228. [Google Scholar] [CrossRef]
- Zhan, R.; Leng, X.; Liu, X.; Wang, X.; Gong, J.; Yan, L.; Wang, L.; Wang, Y.; Wang, X.; Qian, L.-J. Heat shock protein 70 is secreted from endothelial cells by a non-classical pathway involving exosomes. Biochem. Biophys. Res. Commun. 2009, 387, 229–233. [Google Scholar] [CrossRef]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [Green Version]
- Goloudina, A.R.; Demidov, O.N.; Garrido, C. Inhibition of HSP70: A challenging anti-cancer strategy. Cancer Lett. 2012, 325, 117–124. [Google Scholar] [CrossRef]
- Murshid, A.; Gong, J.; Stevenson, M.A.; Calderwood, S.K. Heat shock proteins and cancer vaccines: Developments in the past decade and chaperoning in the decade to come. Expert Rev. Vaccines 2011, 10, 1553–1568. [Google Scholar] [CrossRef]
- Ciocca, D.R.; Cayado-Gutierrez, N.; Maccioni, M.; Cuello-Carrion, F.D. Heat shock proteins (HSPs) based anti-cancer vaccines. Curr. Mol. Med. 2012, 12, 1183–1197. [Google Scholar] [CrossRef]
- Garg, M.; Kanojia, D.; Seth, A.; Kumar, R.; Gupta, A.; Surolia, A.; Suri, A. Heat-shock protein 70-2 (HSP70-2) expression in bladder urothelial carcinoma is associated with tumour progression and promotes migration and invasion. Eur. J. Cancer 2010, 46, 207–215. [Google Scholar] [CrossRef]
- Du, X.L.; Jiang, T.; Wen, Z.Q.; Gao, R.; Cui, M.; Wang, F. Silencing of heat shock protein 70 expression enhances radiotherapy efficacy and inhibits cell invasion in endometrial cancer cell line. Croat. Med. J. 2009, 50, 143–150. [Google Scholar] [CrossRef]
- Weber, G.F. Why does cancer therapy lack effective anti-metastasis drugs? Cancer Lett. 2013, 328, 207–211. [Google Scholar] [CrossRef]
- Weng, D.; Penzner, J.H.; Song, B.; Koido, S.; Calderwood, S.K.; Gong, J. Metastasis is an early event in mouse mammary carcinomas and is associated with cells bearing stem cell markers. Breast Cancer Res. 2012, 14, R18. [Google Scholar] [CrossRef]
- Botzler, C.; Issels, R.; Multhoff, G. Heat-shock protein 72 cell-surface expression on human lung carcinoma cells in associated with an increased sensitivity to lysis mediated by adherent natural killer cells. Cancer Immunol. Immunother. 1996, 43, 226–230. [Google Scholar] [CrossRef]
- Stangl, S.; Wortmann, A.; Guertler, U.; Multhoff, G. Control of metastasized pancreatic carcinomas in SCID/beige mice with human IL-2/TKD-activated NK cells. J. Immunol. 2006, 176, 6270–6276. [Google Scholar]
- Botzler, C.; Schmidt, J.; Luz, A.; Jennen, L.; Issels, R.; Multhoff, G. Differential Hsp70 plasma-membrane expression on primary human tumors and metastases in mice with severe combined immunodeficiency. Int. J. Cancer 1998, 77, 942–948. [Google Scholar] [CrossRef]
- Multhoff, G.; Pfister, K.; Botzler, C.; Jordan, A.; Scholz, R.; Schmetzer, H.; Burgstahler, R.; Hiddemann, W. Adoptive transfer of human natural killer cells in mice with severe combined immunodeficiency inhibits growth of Hsp70-expressing tumors. Int. J. Cancer 2000, 88, 791–797. [Google Scholar] [CrossRef]
- Moser, C.; Schmidbauer, C.; Gürtler, U.; Gross, C.; Gehrmann, M.; Thonigs, G.; Pfister, K.; Multhoff, G. Inhibition of tumor growth in mice with severe combined immunodeficiency is mediated by heat shock protein 70 (Hsp70)-peptide-activated, CD94 positive natural killer cells. Cell Stress Chaperones 2002, 7, 365–373. [Google Scholar] [CrossRef]
- Krause, S.W.; Gastpar, R.; Andreesen, R.; Gross, C.; Ullrich, H.; Thonigs, G.; Pfister, K.; Multhoff, G. Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: A clinical phase i trial. Clin. Cancer Res. 2004, 10, 3699–3707. [Google Scholar] [CrossRef]
- Milani, V.; Stangl, S.; Issels, R.; Gehrmann, M.; Wagner, B.; Hube, K.; Mayr, D.; Hiddemann, W.; Molls, M.; Multhoff, G. Anti-tumor activity of patient-derived NK cells after cell-based immunotherapy—A case report. J. Transl. Med. 2009, 7, 50. [Google Scholar] [CrossRef]
- Gehrmann, M.; Stangl, S.; Kirschner, A.; Foulds, G.A.; Sievert, W.; Doss, B.T.; Walch, A.; Pockley, A.G.; Multhoff, G. Immunotherapeutic targeting of membrane Hsp70-expressing tumors using recombinant human granzyme B. PLoS One 2012, 7, e41341. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Juhasz, K.; Lipp, A.-M.; Nimmervoll, B.; Sonnleitner, A.; Hesse, J.; Haselgruebler, T.; Balogi, Z. The Complex Function of Hsp70 in Metastatic Cancer. Cancers 2014, 6, 42-66. https://doi.org/10.3390/cancers6010042
Juhasz K, Lipp A-M, Nimmervoll B, Sonnleitner A, Hesse J, Haselgruebler T, Balogi Z. The Complex Function of Hsp70 in Metastatic Cancer. Cancers. 2014; 6(1):42-66. https://doi.org/10.3390/cancers6010042
Chicago/Turabian StyleJuhasz, Kata, Anna-Maria Lipp, Benedikt Nimmervoll, Alois Sonnleitner, Jan Hesse, Thomas Haselgruebler, and Zsolt Balogi. 2014. "The Complex Function of Hsp70 in Metastatic Cancer" Cancers 6, no. 1: 42-66. https://doi.org/10.3390/cancers6010042



