Androgen Receptor Signaling in Salivary Gland Cancer
Abstract
:1. Introduction
2. AR Expression in SGC
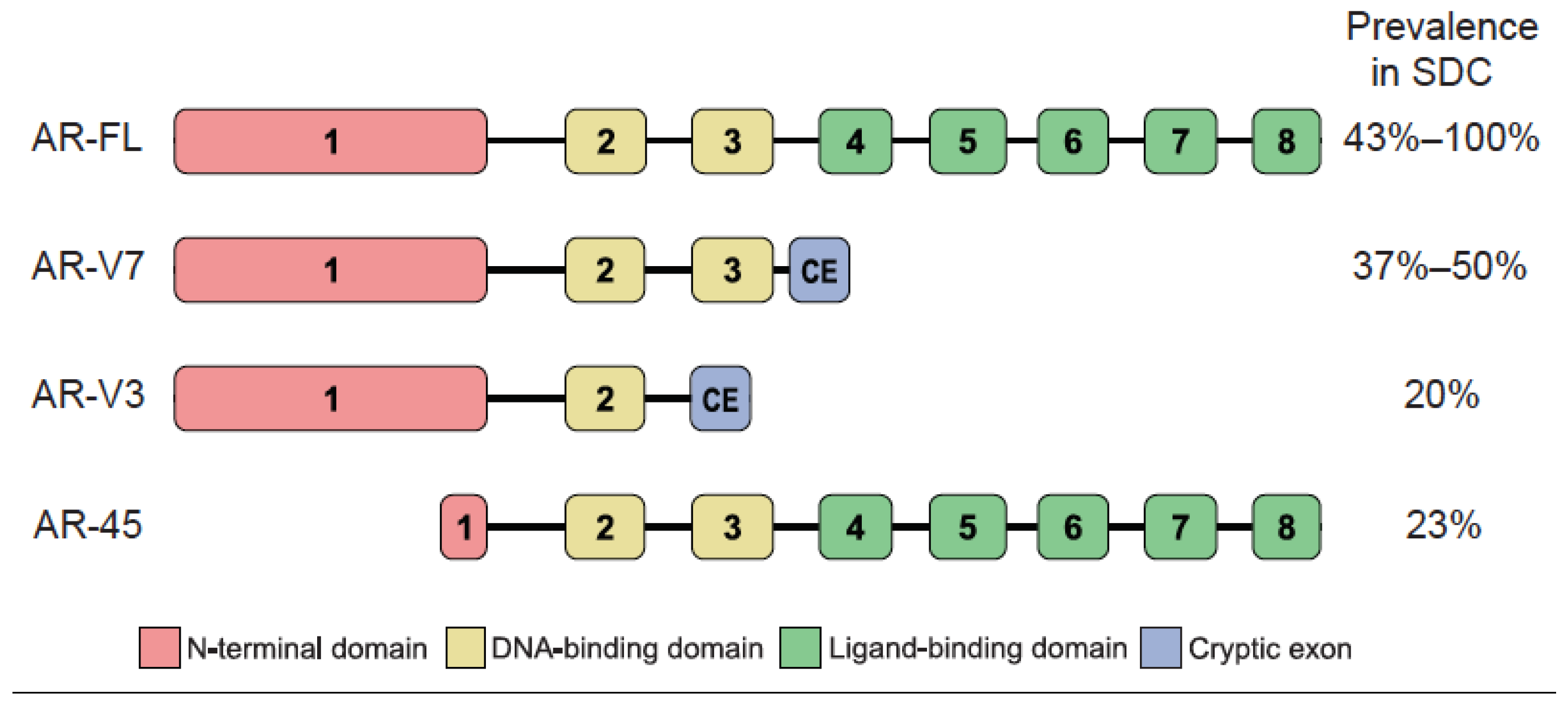
3. Expression of AR Splice Variants
4. Genetic Alterations Affecting AR Signaling
5. Anti-Androgen Therapy in Patients with SGC
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eveson, J.W.; Auclair, P.; Gnepp, D.R.; El-Naggar, A.K. Tumors of the salivary glands. In Pathology and Genetics of Head and Neck Tumours: International Agency for Research on Cancer; World Health Organization: Geneva, Switzerland, 2005; pp. 210–281. [Google Scholar]
- Terhaard, C.H.; Lubsen, H.; Van der Tweel, I.; Hilgers, F.J.; Eijkenboom, W.M.; Marres, H.A.; Tjho-Heslinga, R.E.; de Jong, J.M.; Roodenburg, J.L. Salivary gland carcinoma: Independent prognostic factors for locoregional control, distant metastases, and overall survival: Results of the dutch head and neck oncology cooperative group. Head Neck 2004, 26, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.; Gleave, E.N.; Hancock, B.D.; Smith, P.; McGurk, M. Long-term follow-up of over 1000 patients with salivary gland tumours treated in a single centre. Br. J. Surg. 1996, 83, 1750–1754. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, P.J.; Theriault, C. Malignant salivary gland tumors. Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 1743–1747. [Google Scholar] [CrossRef]
- Panwar, A.; Kozel, J.A.; Lydiatt, W.M. Cancers of major salivary glands. Surg. Oncol. Clin. N. Am. 2015, 24, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Laurie, S.A.; Licitra, L. Systemic therapy in the palliative management of advanced salivary gland cancers. J. Clin. Oncol. 2006, 24, 2673–2678. [Google Scholar] [CrossRef] [PubMed]
- Licitra, L.; Grandi, C.; Prott, F.J.; Schornagel, J.H.; Bruzzi, P.; Molinari, R. Major and minor salivary glands tumours. Crit. Rev. Oncol. Hematol. 2003, 45, 215–225. [Google Scholar] [CrossRef]
- Dalin, M.G.; Desrichard, A.; Katabi, N.; Makarov, V.; Walsh, L.A.; Lee, K.W.; Wang, Q.; Armenia, J.; West, L.; Dogan, S.; et al. Comprehensive molecular characterization of salivary duct carcinoma reveals actionable targets and similarity to apocrine breast cancer. Clin. Cancer Res. 2016, 22, 4623–4633. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.S.; Kannan, K.; Roy, D.M.; Morris, L.G.; Ganly, I.; Katabi, N.; Ramaswami, D.; Walsh, L.A.; Eng, S.; Huse, J.T.; et al. The mutational landscape of adenoid cystic carcinoma. Nat. Genet. 2013, 45, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Ku, B.M.; Jung, H.A.; Sun, J.M.; Ko, Y.H.; Jeong, H.S.; Son, Y.I.; Baek, C.H.; Park, K.; Ahn, M.J. High-throughput profiling identifies clinically actionable mutations in salivary duct carcinoma. J. Trans. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, I.; Piscuoglio, S.; Martelotto, L.G.; Waggott, D.; Ng, C.K.; Perez-Ordonez, B.; Harding, N.J.; Alfaro, J.; Chu, K.C.; Viale, A.; et al. Hotspot activating PRKD1 somatic mutations in polymorphous low-grade adenocarcinomas of the salivary glands. Nat. Genet. 2014, 46, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Tan, M.; Bishop, J.A.; Jones, S.; Sausen, M.; Ha, P.K.; Agrawal, N. Whole-exome sequencing of salivary gland mucoepidermoid carcinoma. Clin. Cancer Res. 2017, 23, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Skalova, A.; Vanecek, T.; Simpson, R.H.; Laco, J.; Majewska, H.; Baneckova, M.; Steiner, P.; Michal, M. Mammary analogue secretory carcinoma of salivary glands: Molecular analysis of 25 ETV6 gene rearranged tumors with lack of detection of classical ETV6-NTRK3 fusion transcript by standard RT-PCR: Report of 4 cases harboring ETV6-X gene fusion. Am. J. Surg. Pathol. 2016, 40, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Ruizeveld de Winter, J.A.; Trapman, J.; Vermey, M.; Mulder, E.; Zegers, N.D.; van der Kwast, T.H. Androgen receptor expression in human tissues: An immunohistochemical study. J. Histochem. Cytochem. 1991, 39, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.Z.; Wardell, S.E.; Burnstein, K.L.; Defranco, D.; Fuller, P.J.; Giguere, V.; Hochberg, R.B.; McKay, L.; Renoir, J.M.; Weigel, N.L.; et al. International union of pharmacology. Lxv. The pharmacology and classification of the nuclear receptor superfamily: Glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol. Rev. 2006, 58, 782–797. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Van Allen, E.M.; Wu, Y.M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.M.; Montgomery, B.; Taplin, M.E.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Dalton, J.T. Androgen receptor: A complex therapeutic target for breast cancer. Cancers 2016. [Google Scholar] [CrossRef] [PubMed]
- Perlmutter, M.A.; Lepor, H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev. Urol. 2007, 9, S3–S8. [Google Scholar] [PubMed]
- Godoy, G.; Gakis, G.; Smith, C.L.; Fahmy, O. Effects of androgen and estrogen receptor signaling pathways on bladder cancer initiation and progression. Bladder Cancer 2016, 2, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Di Lauro, L.; Barba, M.; Pizzuti, L.; Vici, P.; Sergi, D.; Di Benedetto, A.; Mottolese, M.; Speirs, V.; Santini, D.; De Maria, R.; et al. Androgen receptor and antiandrogen therapy in male breast cancer. Cancer lett. 2015, 368, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Jiang, X.; Yokosuka, O. Androgen receptor signaling in hepatocellular carcinoma and pancreatic cancers. World J. Gastroenterol. 2014, 20, 9229–9236. [Google Scholar] [PubMed]
- Gomella, L.G. Effective testosterone suppression for prostate cancer: Is there a best castration therapy? Rev. Urol. 2009, 11, 52–60. [Google Scholar] [PubMed]
- Grivas, P.D.; Robins, D.M.; Hussain, M. Predicting response to hormonal therapy and survival in men with hormone sensitive metastatic prostate cancer. Crit. Rev. Oncol. Hematol. 2013, 85, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Luk, P.P.; Weston, J.D.; Yu, B.; Selinger, C.I.; Ekmejian, R.; Eviston, T.J.; Lum, T.; Gao, K.; Boyer, M.; O’Toole, S.A.; et al. Salivary duct carcinoma: Clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck 2016, 38, E1838–E1847. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hao, J.; Chen, S.; Deng, R. Salivary duct carcinoma: A clinopathological report of 11 cases. Oncol. Lett. 2015, 10, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Mitani, Y.; Rao, P.H.; Maity, S.N.; Lee, Y.C.; Ferrarotto, R.; Post, J.C.; Licitra, L.; Lippman, S.M.; Kies, M.S.; Weber, R.S.; et al. Alterations associated with androgen receptor gene activation in salivary duct carcinoma of both sexes: Potential therapeutic ramifications. Clin. Cancer Res. 2014, 20, 6570–6581. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.T.; Spector, M.E.; Thomas, D.; McDaniel, A.S.; McHugh, J.B. An immunohistochemical panel for reliable differentiation of salivary duct carcinoma and mucoepidermoid carcinoma. Head Neck Pathol. 2014, 8, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Cros, J.; Sbidian, E.; Hans, S.; Roussel, H.; Scotte, F.; Tartour, E.; Brasnu, D.; Laurent-Puig, P.; Bruneval, P.; Blons, H.; et al. Expression and mutational status of treatment-relevant targets and key oncogenes in 123 malignant salivary gland tumours. Ann. Oncol. 2013, 24, 2624–2629. [Google Scholar] [CrossRef] [PubMed]
- Masubuchi, T.; Tada, Y.; Maruya, S.; Osamura, Y.; Kamata, S.E.; Miura, K.; Fushimi, C.; Takahashi, H.; Kawakita, D.; Kishimoto, S.; et al. Clinicopathological significance of androgen receptor, HER2, KI-67 and EGFR expressions in salivary duct carcinoma. Int. J. Clin. Oncol. 2015, 20, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.D.; Roberts, D.; Blumenschein, G.R., Jr.; Temam, S.; Kies, M.S.; Rosenthal, D.I.; Weber, R.S.; El-Naggar, A.K. Differential expression of hormonal and growth factor receptors in salivary duct carcinomas: Biologic significance and potential role in therapeutic stratification of patients. Am. J. Surg. Pathol. 2007, 31, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Thompson, L.D.; Seethala, R.R.; Weinreb, I.; Assaad, A.M.; Tuluc, M.; Ud Din, N.; Purgina, B.; Lai, C.; Griffith, C.C.; et al. Salivary duct carcinoma: The predominance of apocrine morphology, prevalence of histologic variants, and androgen receptor expression. Am. J. Surg. Pathol. 2015, 39, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Nasser, S.M.; Faquin, W.C.; Dayal, Y. Expression of androgen, estrogen, and progesterone receptors in salivary gland tumors. Frequent expression of androgen receptor in a subset of malignant salivary gland tumors. Am. J. Clin. Pathol. 2003, 119, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Locati, L.D.; Perrone, F.; Losa, M.; Mela, M.; Casieri, P.; Orsenigo, M.; Cortelazzi, B.; Negri, T.; Tamborini, E.; Quattrone, P.; et al. Treatment relevant target immunophenotyping of 139 salivary gland carcinomas (SGCS). Oral Oncol. 2009, 45, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Sygut, D.; Bien, S.; Ziolkowska, M.; Sporny, S. Immunohistochemical expression of androgen receptor in salivary gland cancers. Pol. J. Pathol. 2008, 59, 205–210. [Google Scholar] [PubMed]
- Thompson, L.D.; Aslam, M.N.; Stall, J.N.; Udager, A.M.; Chiosea, S.; McHugh, J.B. Clinicopathologic and immunophenotypic characterization of 25 cases of acinic cell carcinoma with high-grade transformation. Head Neck Pathol. 2016, 10, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Ito, F.A.; Ito, K.; Coletta, R.D.; Vargas, P.A.; Lopes, M.A. Immunohistochemical study of androgen, estrogen and progesterone receptors in salivary gland tumors. Braz. Oral Res. 2009, 23, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Ito, Y.; Fujii, K.; Masaki, A.; Beppu, S.; Kawakita, D.; Ijichi, K.; Shimozato, K.; Inagaki, H. Androgen receptor-positive mucoepidermoid carcinoma: Case report and literature review. Int. J. Surg. Pathol. 2015, 23, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Seethala, R.R.; Richmond, J.A.; Hoschar, A.P.; Barnes, E.L. New variants of epithelial-myoepithelial carcinoma: Oncocytic-sebaceous and apocrine. Arch. Pathol. Lab. Med. 2009, 133, 950–959. [Google Scholar] [PubMed]
- Di Palma, S. Carcinoma ex pleomorphic adenoma, with particular emphasis on early lesions. Head Neck Pathol. 2013, 7, S68–S76. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Kishimoto, T.; Nagai, Y.; Yamada, M.; Iida, Y.; Okamoto, Y.; Ishida, Y.; Nakatani, Y.; Ichinose, M. Expressions of androgen receptor and its co-regulators in carcinoma ex pleomorphic adenoma of salivary gland. Pathology 2009, 41, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.Y.; Melhem, M.F.; Hosal, A.S.; Grandis, J.R.; Barnes, E.L. Expression of androgen receptor, epidermal growth factor receptor, and transforming growth factor alpha in salivary duct carcinoma. Arch. Otolaryngol. Head Neck Surg. 2001, 127, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Di Palma, S.; Simpson, R.H.; Marchio, C.; Skalova, A.; Ungari, M.; Sandison, A.; Whitaker, S.; Parry, S.; Reis-Filho, J.S. Salivary duct carcinomas can be classified into luminal androgen receptor-positive, HER2 and basal-like phenotypes. Histopathology 2012, 61, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Minami, S.; Fujii, M. Clinicopathologic study of salivary duct carcinoma and the efficacy of androgen deprivation therapy. Am. J. Otolaryngol. 2014, 35, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Chiosea, S.I.; Williams, L.; Griffith, C.C.; Thompson, L.D.; Weinreb, I.; Bauman, J.E.; Luvison, A.; Roy, S.; Seethala, R.R.; Nikiforova, M.N. Molecular characterization of apocrine salivary duct carcinoma. Am. J. Surg. Pathol. 2015, 39, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Boon, E.; Bel, M.; van der Graaf, W.T.A.; van Es, R.J.J.; Eerenstein, S.; de Jong, R.B.; van den Brekel, M.; van der Velden, L.-A.; Witjes, M.; Hoeben, A.; et al. Salivary duct carcinoma: Clinical outcomes and prognostic factors in 157 patients and results of androgen deprivation therapy in recurrent disease (n = 31)—Study of the dutch head and neck society (DHNS). J. Clin. Oncol. 2016, 34, Suppl. abstract 6016. [Google Scholar]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Dehm, S.M.; Schmidt, L.J.; Heemers, H.V.; Vessella, R.L.; Tindall, D.J. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008, 68, 5469–5477. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yang, X.; Sun, F.; Jiang, R.; Linn, D.E.; Chen, H.; Chen, H.; Kong, X.; Melamed, J.; Tepper, C.G.; et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009, 69, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Sprenger, C.C.; Vessella, R.L.; Haugk, K.; Soriano, K.; Mostaghel, E.A.; Page, S.T.; Coleman, I.M.; Nguyen, H.M.; Sun, H.; et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J. Clin. Investig. 2010, 120, 2715–2730. [Google Scholar] [CrossRef]
- Watson, P.A.; Chen, Y.F.; Balbas, M.D.; Wongvipat, J.; Socci, N.D.; Viale, A.; Kim, K.; Sawyers, C.L. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc. Natl. Acad. Sci. USA 2010, 107, 16759–16765. [Google Scholar] [CrossRef] [PubMed]
- Metzger, E.; Muller, J.M.; Ferrari, S.; Buettner, R.; Schule, R. A novel inducible transactivation domain in the androgen receptor: Implications for prk in prostate cancer. EMBO J. 2003, 22, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Augello, M.A.; Hickey, T.E.; Knudsen, K.E. Foxa1: Master of steroid receptor function in cancer. EMBO J. 2011, 30, 3885–3894. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.; Holmes, K.A.; Carroll, J.S. FOXA1 mutations in hormone-dependent cancers. Front. Oncol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Niu, Y.; Lee, S.O.; Yeh, S.; Shang, Z.; Gao, H.; Li, Y.; Chou, F.; Chang, C. Targeting fatty acid synthase with ASC-J9 suppresses proliferation and invasion of prostate cancer cells. Mol. Carcinog. 2016, 55, 2278–2290. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.M.; Gao, A.C. Drug resistance in castration resistant prostate cancer: Resistance mechanisms and emerging treatment strategies. Am. J. Clin. Exp. Urol. 2015, 3, 64–76. [Google Scholar] [PubMed]
- Merseburger, A.S.; Alcaraz, A.; von Klot, C.A. Androgen deprivation therapy as backbone therapy in the management of prostate cancer. OncoTargets Ther. 2016, 9, 7263–7274. [Google Scholar] [CrossRef] [PubMed]
- Kamata, Y.U.; Sumida, T.; Murase, R.; Nakano, H.; Yamada, T.; Mori, Y. Blockade of androgen-induced malignant phenotypes by flutamide administration in human salivary duct carcinoma cells. Anticancer Res. 2016, 36, 6071–6075. [Google Scholar] [CrossRef] [PubMed]
- Locati, L.D.; Perrone, F.; Cortelazzi, B.; Lo Vullo, S.; Bossi, P.; Dagrada, G.; Quattrone, P.; Bergamini, C.; Potepan, P.; Civelli, E.; et al. Clinical activity of androgen deprivation therapy in patients with metastatic/relapsed androgen receptor-positive salivary gland cancers. Head Neck 2016, 38, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, H.C.; Verbist, B.M.; Schoffelen, R.; Mattijssen, V.; Slootweg, P.J.; van der Graaf, W.T.; van Herpen, C.M. Androgen receptor-positive salivary duct carcinoma: A disease entity with promising new treatment options. J. Clin. Oncol. 2011, 29, e473–e476. [Google Scholar] [CrossRef] [PubMed]
- Locati, L.D.; Quattrone, P.; Bossi, P.; Marchiano, A.V.; Cantu, G.; Licitra, L. A complete remission with androgen-deprivation therapy in a recurrent androgen receptor-expressing adenocarcinoma of the parotid gland. Ann. Oncol. 2003, 14, 1327–1328. [Google Scholar] [CrossRef] [PubMed]
- Van der Hulst, R.W.; van Krieken, J.H.; van der Kwast, T.H.; Gerritsen, J.J.; Baatenburg de Jong, R.J.; Lycklama a Nijeholt, A.A.; Meinders, A.E. Partial remission of parotid gland carcinoma after goserelin. Lancet 1994. [Google Scholar] [CrossRef]
- Urban, D.; Rischin, D.; Angel, C.; D’Costa, I.; Solomon, B. Abiraterone in metastatic salivary duct carcinoma. J. Natl. Compr. Cancer Netw. 2015, 13, 288–290. [Google Scholar]
- Locati, L.D.; Perrone, F.; Cortelazzi, B.; Imbimbo, M.; Bossi, P.; Potepan, P.; Civelli, E.; Rinaldi, G.; Quattrone, P.; Licitra, L.; et al. Activity of abiraterone in rechallenging two ar-expressing salivary gland adenocarcinomas, resistant to androgen-deprivation therapy. Cancer Biol. Ther. 2014, 15, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Soper, M.S.; Iganej, S.; Thompson, L.D. Definitive treatment of androgen receptor-positive salivary duct carcinoma with androgen deprivation therapy and external beam radiotherapy. Head Neck 2014, 36, E4–E7. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Sakurai, T.; Yamada, M.; Uemura, N.; Ono, M.; Abe, T.; Fujii, S.; Maeda, M.; Kohda, K.; Obata, M.; et al. Effective treatment by both anti-androgen therapy and chemotherapy for a patient with advanced salivary duct carcinoma. Jpn. J. Cancer Chemother. 2011, 38, 627–630. [Google Scholar]
| Histology | AR Positivity 1 | Reported Range 2 | References |
|---|---|---|---|
| SDC | 615/713 (86%) | 43%–100% | [8,24,25,26,27,28,29,30,31,33,34,41,42,43,44,45] |
| AC NOS | 11/43 (26%) | 21%–33% | [28,33,34] |
| AcCC | 6/40 (15%) | 0%–31% | [28,32,34,35] |
| MEC | 7/135 (5%) | 0%–20% | [27,28,32,33,34,36] |
| ACC | 7/145 (5%) | 0%–20% | [28,32,33,34,36] |
| EMC | 0/6 (0%) | N/A | [28] |
| MECA | 0/7 (0%) | N/A | [28,33] |
| BCAC | 2/2 (100%) | N/A | [32] |
| PLGA | 1/2 (50%) | N/A | [28] |
| Patient ID 1 | Histology | Sex | Age 2 | ADT Agents | Response | PFS (Months) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | AC NOS | m | 73 | Bicalutamide + triptorelin | CR | N.K. | [60] |
| 2 | AC NOS | m | 72 | Bicalutamide + triptorelin | CR | 2 | [58] |
| 3 | AC NOS | m | N.K. | Goserelin | PR | N.K. | [61] |
| 4 | AC NOS | m | 59 | Bicalutamide + triptorelin | PR | 12 | [63] |
| 5 | AC NOS | m | 44 | Bicalutamide + triptorelin | PR | 25 | [63] |
| 6 | AC NOS | m | 67 | Bicalutamide + triptorelin | PR | 22 | [58] |
| 7 | AC NOS | m | 67 | Bicalutamide + triptorelin | PR | 22 | [58] |
| 8 | AC NOS | m | 46 | Bicalutamide + triptorelin | PR | 58 | [58] |
| 9 | AC NOS | m | 49 | Bicalutamide + triptorelin | PR | 7 | [58] |
| 10 | AC NOS | m | 62 | Bicalutamide + triptorelin | PR | 9 | [58] |
| 11 | AC NOS | m | 69 | Bicalutamide + triptorelin | SD | 20 | [58] |
| 12 | Cyst AC | m | 79 | Bicalutamide + triptorelin | PR | 14 | [58] |
| 13 | Cyst AC | f | 68 | Triptorelin + cyproterone | PD | 0 | [58] |
| 14 | Poor diff. | m | 54 | Bicalutamide + triptorelin | PD | 0 | [58] |
| 15 | SDC | f | 87 | Bicalutamide + leuprolide 3 | CR | 24 | [64] |
| 16 | SDC | m | 44 | Bicalutamide + triptorelin | CR | 39 | [58] |
| 17 | SDC | m | 67 | Bicalutamide + triptorelin | CR | 11 | [58] |
| 18 | SDC | m | 66 | Bicalutamide | PR | 14 | [43] |
| 19 | SDC | m | 50 | Bicalutamide | PR | 8 | [59] |
| 20 | SDC | f | 83 | Bicalutamide | PR | 26 | [59] |
| 21 | SDC | m | 45 | Goserelin | PR | 4 | [62] |
| 22 | SDC | m | 45 | Bicalutamide + goserelin | PR | 10 | [62] |
| 23 | SDC | m | 45 | Abiraterone + goserelin | PR | 10 | [62] |
| 24 | SDC | m | 51 | Bicalutamide + triptorelin | PR | 6 | [58] |
| 25 | SDC | m | 67 | Bicalutamide + triptorelin | PR | 7 | [58] |
| 26 | SDC | f | 68 | Bicalutamide + leuprolide | SD | 17 | [8] |
| 27 | SDC | m | 57 | Bicalutamide | SD | 14 | [59] |
| 28 | SDC | m | 56 | Bicalutamide + goserelin | SD | 12 | [59] |
| 29 | SDC | m | 67 | Bicalutamide + goserelin | SD | 8 | [59] |
| 30 | SDC | m | 75 | Bicalutamide + triptorelin | SD | 8 | [58] |
| 31 | SDC | m | 54 | Bicalutamide + triptorelin | SD | 10 | [58] |
| 32 | SDC | m | 68 | Bicalutamide + triptorelin | SD | 23 | [58] |
| 33 | SDC | f | 48 | Bicalutamide + leuprolide | PD | 0 | [8] |
| 34 | SDC | f | 69 | Bicalutamide + leuprolide | PD | 0 | [8] |
| 35 | SDC | m | 77 | Bicalutamide + leuprolide | PD | 0 | [8] |
| 36 | SDC | m | 73 | Bicalutamide + goserelin | PD | 0 | [59] |
| 37 | SDC | m | 68 | Bicalutamide + goserelin | PD | 0 | [59] |
| 38 | SDC | f | 64 | Bicalutamide | PD | 0 | [59] |
| 39 | SDC | m | 39 | Bicalutamide | PD | 0 | [59] |
| 40 | SDC | m | 73 | Bicalutamide | PD | 0 | [59] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalin, M.G.; Watson, P.A.; Ho, A.L.; Morris, L.G.T. Androgen Receptor Signaling in Salivary Gland Cancer. Cancers 2017, 9, 17. https://doi.org/10.3390/cancers9020017
Dalin MG, Watson PA, Ho AL, Morris LGT. Androgen Receptor Signaling in Salivary Gland Cancer. Cancers. 2017; 9(2):17. https://doi.org/10.3390/cancers9020017
Chicago/Turabian StyleDalin, Martin G., Philip A. Watson, Alan L. Ho, and Luc G. T. Morris. 2017. "Androgen Receptor Signaling in Salivary Gland Cancer" Cancers 9, no. 2: 17. https://doi.org/10.3390/cancers9020017





