HGF/Met Signaling in Cancer Invasion: The Impact on Cytoskeleton Remodeling
Abstract
:1. Introduction
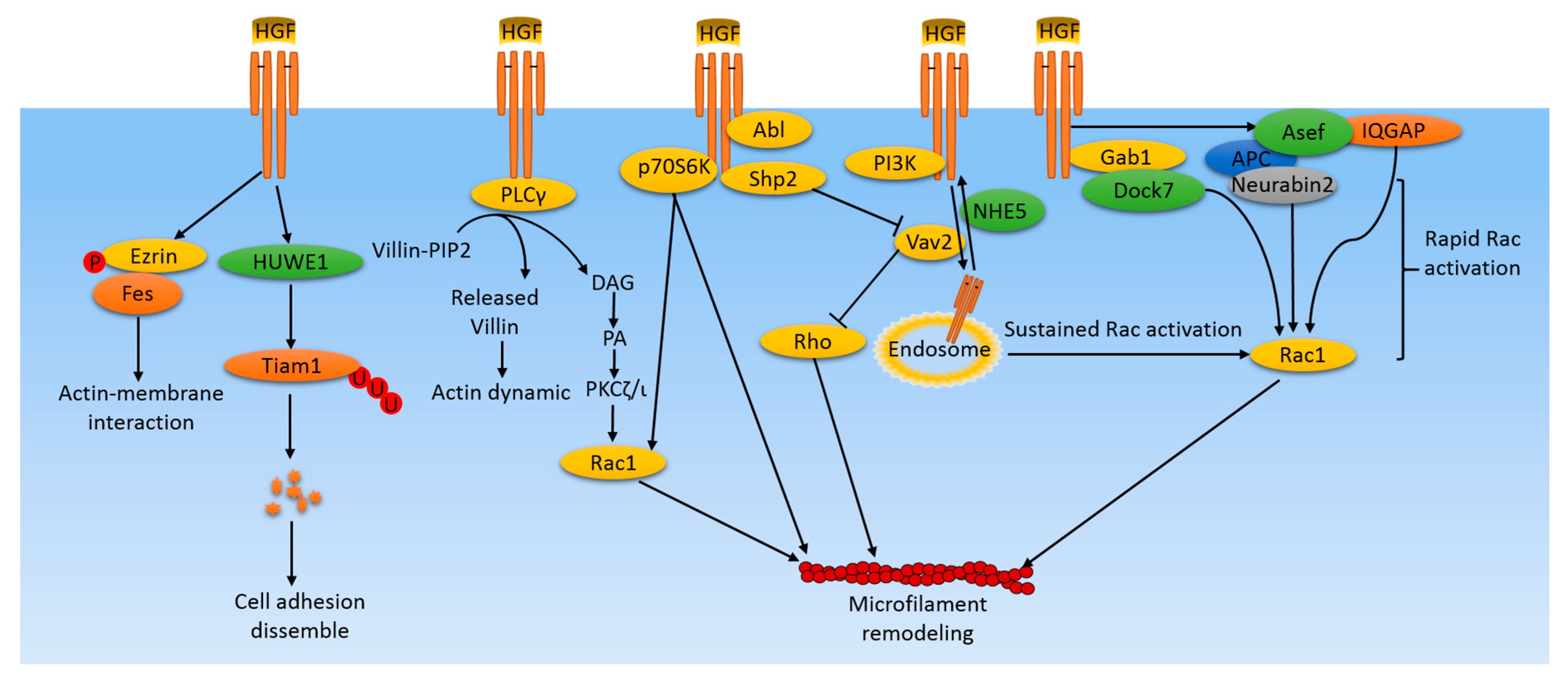
2. HGF and Microfilaments in Cancer
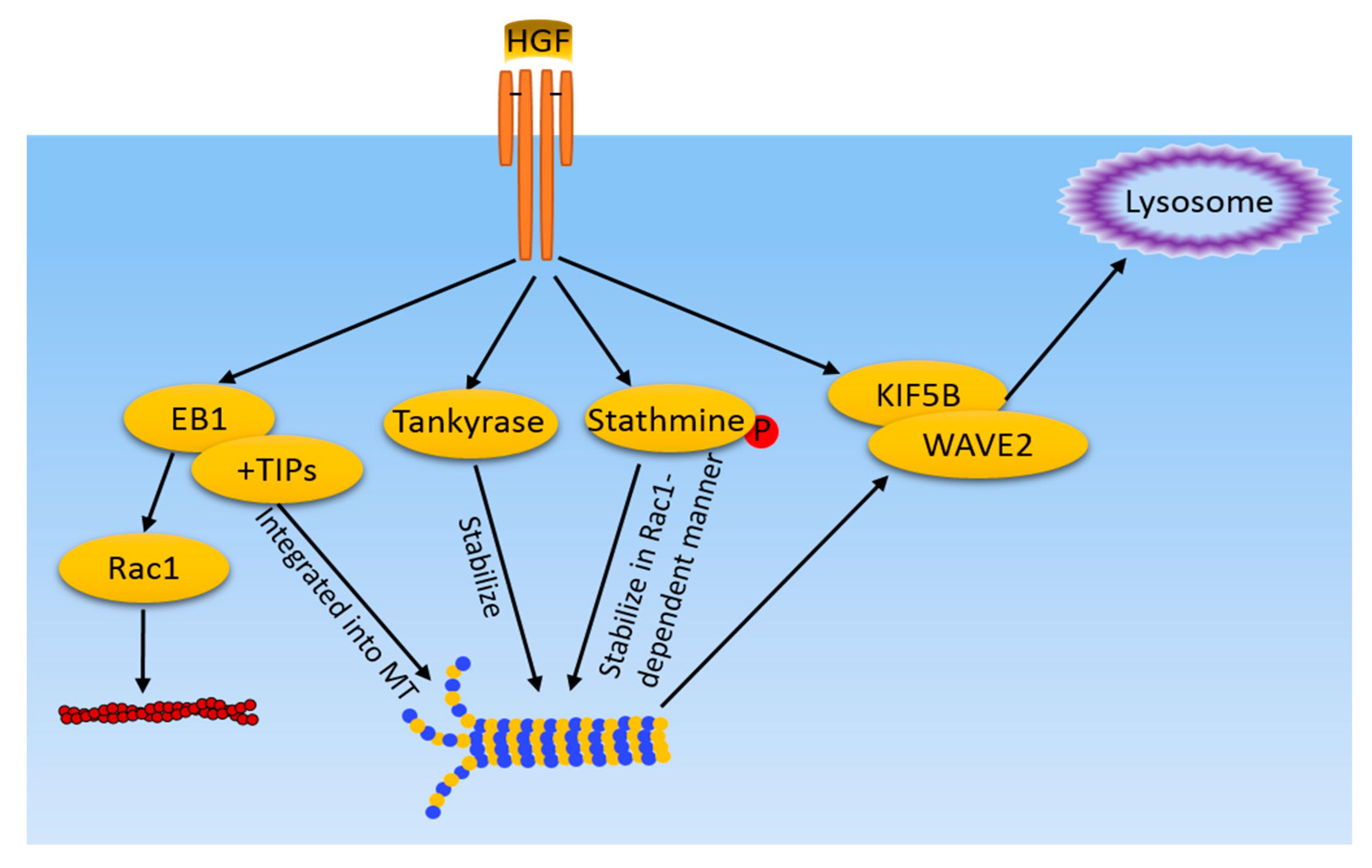
3. HGF and Microtubule in Cancer
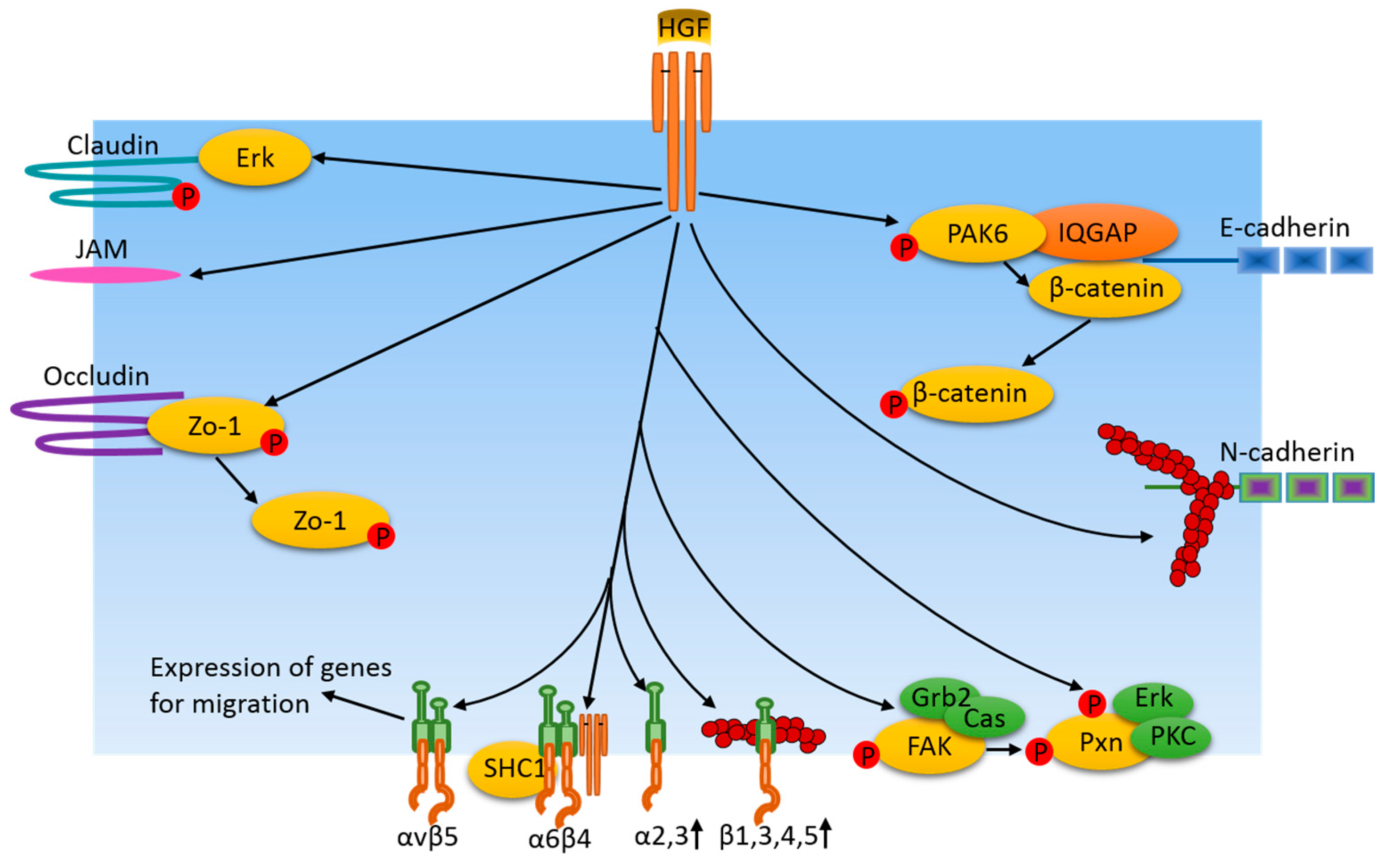
4. HGF and Cell-Cell Junctions in Cancer
5. HGF and Focal Adhesions in Cancer
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jeffers, M.; Schmidt, L.; Nakaigawa, N.; Webb, C.P.; Weirich, G.; Kishida, T.; Zbar, B.; Vande Woude, G.F. Activating mutations for the met tyrosine kinase receptor in human cancer. Proc. Natl. Acad. Sci. USA 1997, 94, 11445–11450. [Google Scholar] [CrossRef] [PubMed]
- Baraniak, P.R.; McDevitt, T.C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Catizone, A.; Galdieri, M. Pleiotropic activity of hepatocyte growth factor during embryonic mouse testis development. Mech. Dev. 2002, 118, 19–28. [Google Scholar] [CrossRef]
- Bevan, D.; Gherardi, E.; Fan, T.P.; Edwards, D.; Warn, R. Diverse and potent activities of HGF/SF in skin wound repair. J. Pathol. 2004, 203, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Chmielowiec, J.; Borowiak, M.; Morkel, M.; Stradal, T.; Munz, B.; Werner, S.; Wehland, J.; Birchmeier, C.; Birchmeier, W. c-Met is essential for wound healing in the skin. J. Cell Biol. 2007, 177, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Tekkesin, N.; Taga, Y.; Sav, A.; Almaata, I.; Ibrisim, D. Induction of HGF and VEGF in hepatic regeneration after hepatotoxin-induced cirrhosis in mice. Hepatogastroenterology 2011, 58, 971–979. [Google Scholar] [PubMed]
- Rosen, E.M.; Lamszus, K.; Laterra, J.; Polverini, P.J.; Rubin, J.S.; Goldberg, I.D. HGF/SF in angiogenesis. Ciba Found. Symp. 1997, 212, 215–226. [Google Scholar] [PubMed]
- Ding, S.; Merkulova-Rainon, T.; Han, Z. C.; Tobelem, G. HGF receptor up-regulation contributes to the angiogenic phenotype of human endothelial cells and promotes angiogenesis in vitro. Blood 2003, 101, 4816–4822. [Google Scholar] [CrossRef] [PubMed]
- Bae-Jump, V.; Segreti, E.M.; Vandermolen, D.; Kauma, S. Hepatocyte growth factor (HGF) induces invasion of endometrial carcinoma cell lines in vitro. Gynecol. Oncol. 1999, 73, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Jeffers, M.; Rong, S.; Vande Woude, G.F. Enhanced tumorigenicity and invasion-metastasis by hepatocyte growth factor/scatter factor-met signalling in human cells concomitant with induction of the urokinase proteolysis network. Mol. Cell. Biol. 1996, 16, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.W.; Didier, S.; Chan, A.; Paulino, V.; Van Aelst, L.; Ruggieri, R.; Tran, N.L.; Byrne, A.T.; Symons, M. Guanine nucleotide exchange factor Dock7 mediates HGF-induced glioblastoma cell invasion via Rac activation. Br. J. Cancer 2014, 110, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Spitzer, E.; Meyer, D.; Sachs, M.; Niemann, C.; Hartmann, G.; Weidner, K.M.; Birchmeier, C.; Birchmeier, W. Sequential requirement of hepatocyte growth factor and neuregulin in the morphogenesis and differentiation of the mammary gland. J. Cell Biol. 1995, 131, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Etienne-Manneville, S. Microtubules in cell migration. Annu. Rev. Cell Dev. Biol. 2013, 29, 471–499. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.; Srivastava, J.; Madson, N.; Wittmann, T.; Barber, D.L. Dynamic actin remodeling during epithelial-mesenchymal transition depends on increased moesin expression. Mol. Biol. Cell 2011, 22, 4750–4764. [Google Scholar] [CrossRef] [PubMed]
- Shankar, J.; Nabi, I.R. Actin cytoskeleton regulation of epithelial mesenchymal transition in metastatic cancer cells. PLoS ONE 2015, 10, e0119954. [Google Scholar]
- Wilson, K.; Lewalle, A.; Fritzsche, M.; Thorogate, R.; Duke, T.; Charras, G. Mechanisms of leading edge protrusion in interstitial migration. Nat. Commun. 2013, 4, 2896. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowska-Wodnicka, M.; Burridge, K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 1996, 133, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, J.S.; Hansen, M.D.; Nelson, W.J. Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion. Dev. Cell 2002, 3, 259–270. [Google Scholar] [CrossRef]
- Mattila, P.K.; Lappalainen, P. Filopodia: Molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.L.; Rehmann, H.; Wittinghofer, A. GEFs and GAPs: Critical elements in the control of small G proteins. Cell 2007, 129, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Sagara, M.; Kawasaki, Y.; Iemura, S.I.; Natsume, T.; Takai, Y.; Akiyama, T. Asef2 and Neurabin2 cooperatively regulate actin cytoskeletal organization and are involved in HGF-induced cell migration. Oncogene 2009, 28, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Gawlak, G.; Shah, A.S.; Higginbotham, K.; Tian, X.; Kawasaki, Y.; Akiyama, T.; Sacks, D.B.; Birukova, A.A. Hepatocyte growth factor-induced Asef-IQGAP1 complex controls cytoskeletal remodeling and endothelial barrier. J. Biol. Chem. 2015, 290, 4097–4109. [Google Scholar] [CrossRef] [PubMed]
- Chianale, F.; Rainero, E.; Cianflone, C.; Bettio, V.; Pighini, A.; Porporato, P.E.; Filigheddu, N.; Serini, G.; Sinigaglia, F.; Baldanzi, G.; et al. Diacylglycerol kinase alpha mediates HGF-induced Rac activation and membrane ruffling by regulating atypical PKC and RhoGDI. Proc. Natl. Acad. Sci. USA 2010, 107, 4182–4187. [Google Scholar] [CrossRef] [PubMed]
- Menard, L.; Parker, P.J.; Kermorgant, S. Receptor tyrosine kinase c-Met controls the cytoskeleton from different endosomes via different pathways. Nat. Commun. 2014, 5, 3907. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.H.; Numata, Y.; Numata, M. Endosomal Na+/H+ exchanger NHE5 influences MET recycling and cell migration. Mol. Biol. Cell 2016, 27, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Ip, C.K.; Cheung, A.N.; Ngan, H.Y.; Wong, A.S. p70 S6 kinase in the control of actin cytoskeleton dynamics and directed migration of ovarian cancer cells. Oncogene 2011, 30, 2420–2432. [Google Scholar] [CrossRef] [PubMed]
- Okano, Y.; Mizuno, K.; Osada, S.; Nakamura, T.; Nozawa, Y. Tyrosine phosphorylation of phospholipase C gamma in c-met/HGF receptor-stimulated hepatocytes: Comparison with HepG2 hepatocarcinoma cells. Biochem. Biophys. Res. Commun. 1993, 190, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Logan, M.R.; Mandato, C.A. Regulation of the actin cytoskeleton by PIP2 in cytokinesis. Biol. Cell 2006, 98, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Royal, I.; Fournier, T.M.; Park, M. Differential requirement of Grb2 and PI3-kinase in HGF/SF-induced cell motility and tubulogenesis. J. Cell. Physiol. 1997, 173, 196–201. [Google Scholar] [CrossRef]
- Kumar, N.; Zhao, P.; Tomar, A.; Galea, C.A.; Khurana, S. Association of villin with phosphatidylinositol 4,5-bisphosphate regulates the actin cytoskeleton. J. Biol. Chem. 2004, 279, 3096–3110. [Google Scholar] [CrossRef] [PubMed]
- Athman, R.; Louvard, D.; Robine, S. Villin enhances hepatocyte growth factor-induced actin cytoskeleton remodeling in epithelial cells. Mol. Biol. Cell 2003, 14, 4641–4653. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Knight, J.F.; Park, M.; Pendergast, A.M. Abl Kinases Regulate HGF/Met Signaling Required for Epithelial Cell Scattering, Tubulogenesis and Motility. PLoS ONE 2015, 10, e0124960. [Google Scholar] [CrossRef] [PubMed]
- Santhana Kumar, K.; Tripolitsioti, D.; Ma, M.; Grahlert, J.; Egli, K.B.; Fiaschetti, G.; Shalaby, T.; Grotzer, M.A.; Baumgartner, M. The Ser/Thr kinase MAP4K4 drives c-Met-induced motility and invasiveness in a cell-based model of SHH medulloblastoma. Springerplus 2015, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Kodama, A.; Matozaki, T.; Fukuhara, A.; Kikyo, M.; Ichihashi, M.; Takai, Y. Involvement of an SHP-2-Rho small G protein pathway in hepatocyte growth factor/scatter factor-induced cell scattering. Mol. Biol. Cell 2000, 11, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, L.; Tan, C.T.; Chapman, A.; Nonaka, D.; Mack, N.A.; Smith, D.; Booton, R.; Hurlstone, A.F.; Malliri, A. HUWE1 ubiquitylates and degrades the RAC activator TIAM1 promoting cell-cell adhesion disassembly, migration, and invasion. Cell Rep. 2015, 10, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Crepaldi, T.; Gautreau, A.; Comoglio, P.M.; Louvard, D.; Arpin, M. Ezrin is an effector of hepatocyte growth factor-mediated migration and morphogenesis in epithelial cells. J. Cell Biol. 1997, 138, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Reverdy, C.; Louvard, D.; Arpin, M. Spatial recruitment and activation of the Fes kinase by ezrin promotes HGF-induced cell scattering. EMBO J. 2008, 27, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Kaverina, I.; Straube, A. Regulation of cell migration by dynamic microtubules. Semin. Cell Dev. Biol. 2011, 22, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.B.; Howard, L.; Compton, D.A. Chromosome movement in mitosis requires microtubule anchorage at spindle poles. J. Cell Biol. 2001, 52, 425–434. [Google Scholar] [CrossRef]
- Sun, B.O.; Fang, Y.; Li, Z.; Chen, Z.; Xiang, J. Role of cellular cytoskeleton in epithelial-mesenchymal transition process during cancer progression. Biomed. Rep. 2015, 3, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Akhmanova, A.; Steinmetz, M.O. Tracking the ends: A dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol. 2008, 9, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Etienne-Manneville, S. APC in cell migration. Adv. Exp. Med. Biol. 2009, 656, 30–40. [Google Scholar] [PubMed]
- Kumar, P.; Lyle, K.S.; Gierke, S.; Matov, A.; Danuser, G.; Wittmann, T. GSK3beta phosphorylation modulates CLASP-microtubule association and lamella microtubule attachment. J. Cell Biol. 2009, 184, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shen, Q.T.; Oristian, D.S.; Lu, C.P.; Zheng, Q.; Wang, H.W.; Fuchs, E. Skin stem cells orchestrate directional migration by regulating microtubule-ACF7 connections through GSK3beta. Cell 2010, 144, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Honnappa, S.; Gouveia, S.M.; Weisbrich, A.; Damberger, F.F.; Bhavesh, N.S.; Jawhari, H.; Grigoriev, I.; van Rijssel, F.J.; Buey, R.M.; Lawera, A.; et al. An EB1-binding motif acts as a microtubule tip localization signal. Cell 2009, 138, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Gierke, S.; Wittmann, T. EB1-recruited microtubule +TIP complexes coordinate protrusion dynamics during 3D epithelial remodeling. Curr. Biol. 2012, 2, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Ohmura, T.; Shah, A.S.; Son, S.; Tian, Y.; Birukova, A.A. Role of End Binding Protein-1 in endothelial permeability response to barrier-disruptive and barrier-enhancing agonists. Cell. Signal. 2017, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lupo, B.; Vialard, J.; Sassi, F.; Angibaud, P.; Puliafito, A.; Pupo, E.; Lanzetti, L.; Comoglio, P.M.; Bertotti, A.; Trusolino, L. Tankyrase inhibition impairs directional migration and invasion of lung cancer cells by affecting microtubule dynamics and polarity signals. BMC Biol. 2016, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.E.; Lytle, N.K.; Zuniga, A.; Goldstein, L.S. The Microtubule Regulatory Protein Stathmin Is Required to Maintain the Integrity of Axonal Microtubules in Drosophila. PLoS ONE 2013, 8, e68324. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Tian, Y.; Moldobaeva, N.; Sarich, N.; Birukova, A.A. Microtubule dynamics control HGF-induced lung endothelial barrier enhancement. PLoS ONE 2014, 9, e105912. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Suzuki, K. Requirement of kinesin-mediated membrane transport of WAVE2 along microtubules for lamellipodia formation promoted by hepatocyte growth factor. Exp. Cell Res. 2008, 314, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Steffan, J.J.; Williams, B.C.; Welbourne, T.; Cardelli, J.A. HGF-induced invasion by prostate tumor cells requires anterograde lysosome trafficking and activity of Na+-H+ exchangers. J. Cell Sci. 2010, 123, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A.; Watkins, G.; Mansel, R.E.; Jiang, W.G. Hepatocyte growth factor disrupts tight junctions in human breast cancer cells. Cell Biol. Int. 2004, 28, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Lipschutz, J.H.; Li, S.; Arisco, A.; Balkovetz, D.F. Extracellular signal-regulated kinases 1/2 control claudin-2 expression in Madin-Darby canine kidney strain I and II cells. J. Biol. Chem. 2005, 280, 3780–3788. [Google Scholar] [CrossRef] [PubMed]
- Twiss, F.; Oldenkamp, M.; Hiemstra, A.; Zhou, H.; Matheron, L.; Mohammed, S.; de Rooij, J. HGF signaling regulates Claudin-3 dynamics through its C-terminal tyrosine residues. Tissue Barriers 2013, 1, e27425. [Google Scholar] [CrossRef] [PubMed]
- Fram, S.; King, H.; Sacks, D.B.; Wells, C.M. A PAK6-IQGAP1 complex promotes disassembly of cell-cell adhesions. Cell. Mol. Life Sci. 2014, 71, 2759–2773. [Google Scholar] [CrossRef] [PubMed]
- Hollande, F.; Blanc, E.M.; Bali, J.P.; Whitehead, R.H.; Pelegrin, A.; Baldwin, G.S.; Choquet, A. HGF regulates tight junctions in new nontumorigenic gastric epithelial cell line. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G910–G921. [Google Scholar] [PubMed]
- Mangold, S.; Wu, S.K.; Norwood, S.J.; Collins, B.M.; Hamilton, N.A.; Thorn, P.; Yap, A.S. Hepatocyte growth factor acutely perturbs actin filament anchorage at the epithelial zonula adherens. Curr. Biol. 2011, 21, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Shih, W.; Yamada, S. N-cadherin-mediated cell-cell adhesion promotes cell migration in a three-dimensional matrix. J. Cell Sci. 2012, 125, 3661–3670. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.A.; Brugge, J.S. Integrins and signal transduction pathways: The road taken. Science 1995, 268, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- van der Flier, A.; Sonnenberg, A. Function and interactions of integrins. Cell Tissue Res. 2001, 305, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Trusolino, L.; Cavassa, S.; Angelini, P.; Ando, M.; Bertotti, A.; Comoglio, P.M.; Boccaccio, C. HGF/scatter factor selectively promotes cell invasion by increasing integrin avidity. FASEB J. 2000, 14, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Trusolino, L.; Serini, G.; Cecchini, G.; Besati, C.; Ambesi-Impiombato, F.S.; Marchisio, P.C.; De Filippi, R. Growth factor-dependent activation of alphavbeta3 integrin in normal epithelial cells: Implications for tumor invasion. J. Cell Biol. 1998, 142, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.F.; Kao, S.C.; Jiang, S.T.; Tang, M.J.; Chan, P.C.; Chen, H.C. Involvement of focal adhesion kinase in hepatocyte growth factor-induced scatter of Madin-Darby canine kidney cells. J. Biol. Chem. 2000, 275, 7474–7480. [Google Scholar] [CrossRef] [PubMed]
- Tuck, A.B.; Elliott, B.E.; Hota, C.; Tremblay, E.; Chambers, A.F. Osteopontin-induced, integrin-dependent migration of human mammary epithelial cells involves activation of the hepatocyte growth factor receptor (Met). J. Cell. Biochem. 2000, 78, 465–475. [Google Scholar] [CrossRef]
- Crouch, S.; Spidel, C.S.; Lindsey, J.S. HGF and ligation of alphavbeta5 integrin induce a novel, cancer cell-specific gene expression required for cell scattering. Exp. Cell Res. 2004, 292, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Sulzmaier, F.J.; Jean, C.; Schlaepfer, D.D. FAK in cancer: Mechanistic findings and clinical applications. Nat. Rev. Cancer 2014, 14, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Frame, M.C.; Patel, H.; Serrels, B.; Lietha, D.; Eck, M.J. The FERM domain: Organizing the structure and function of FAK. Nat. Rev. Mol. Cell Biol. 2010, 11, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.D. Cellular functions of FAK kinases: Insight into molecular mechanisms and novel functions. J. Cell Sci. 2010, 123, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.C.; Liang, C.C.; Yu, K.C.; Chang, M.C.; Ho, W.L.; Chen, B.H.; Chen, H.C. Synergistic effect of focal adhesion kinase overexpression and hepatocyte growth factor stimulation on cell transformation. J. Biol. Chem. 2002, 277, 50373–50379. [Google Scholar] [CrossRef] [PubMed]
- Parr, C.; Davies, G.; Nakamura, T.; Matsumoto, K.; Mason, M.D.; Jiang, W.G. The HGF/SF-induced phosphorylation of paxillin, matrix adhesion, and invasion of prostate cancer cells were suppressed by NK4, an HGF/SF variant. Biochem. Biophys. Res. Commun. 2001, 285, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.T.; Cheng, C.C.; Pan, S.M.; Wu, J.R.; Wu, W.S. PKC mediates fluctuant ERK-paxillin signaling for hepatocyte growth factor-induced migration of hepatoma cell HepG2. Cell. Signal. 2013, 25, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, C.; Chen, J.; Fu, P. HGF/Met Signaling in Cancer Invasion: The Impact on Cytoskeleton Remodeling. Cancers 2017, 9, 44. https://doi.org/10.3390/cancers9050044
Xiang C, Chen J, Fu P. HGF/Met Signaling in Cancer Invasion: The Impact on Cytoskeleton Remodeling. Cancers. 2017; 9(5):44. https://doi.org/10.3390/cancers9050044
Chicago/Turabian StyleXiang, Chuan, Junxia Chen, and Panfeng Fu. 2017. "HGF/Met Signaling in Cancer Invasion: The Impact on Cytoskeleton Remodeling" Cancers 9, no. 5: 44. https://doi.org/10.3390/cancers9050044




