Coevolution of Cooperation, Response to Adverse Social Ties and Network Structure
Abstract
:1. Introduction
2. Active Linking
- Coordination or Bistability: and leads to what is called coordination or SH games [65], in which it is always beneficial to follow the strategy of the opponent, turning both C’s and D’s advantageous when rare.
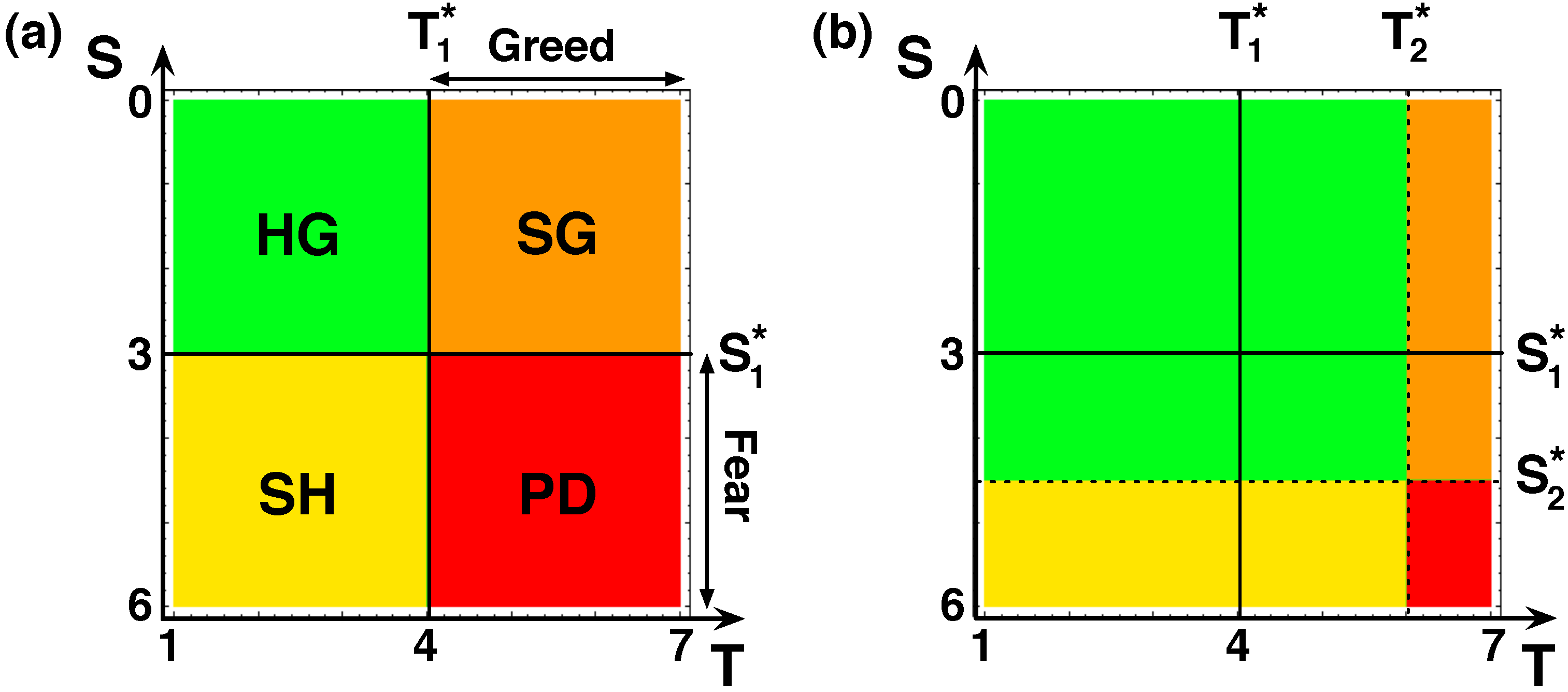
2.1. Network Evolution
2.2. Strategy Evolution
2.3. Separation of Time Scales
2.4. Comparable Time Scales

3. Diversity and Cooperation in the PD
3.1. A Minimal Model
3.2. Two Linking Strategies
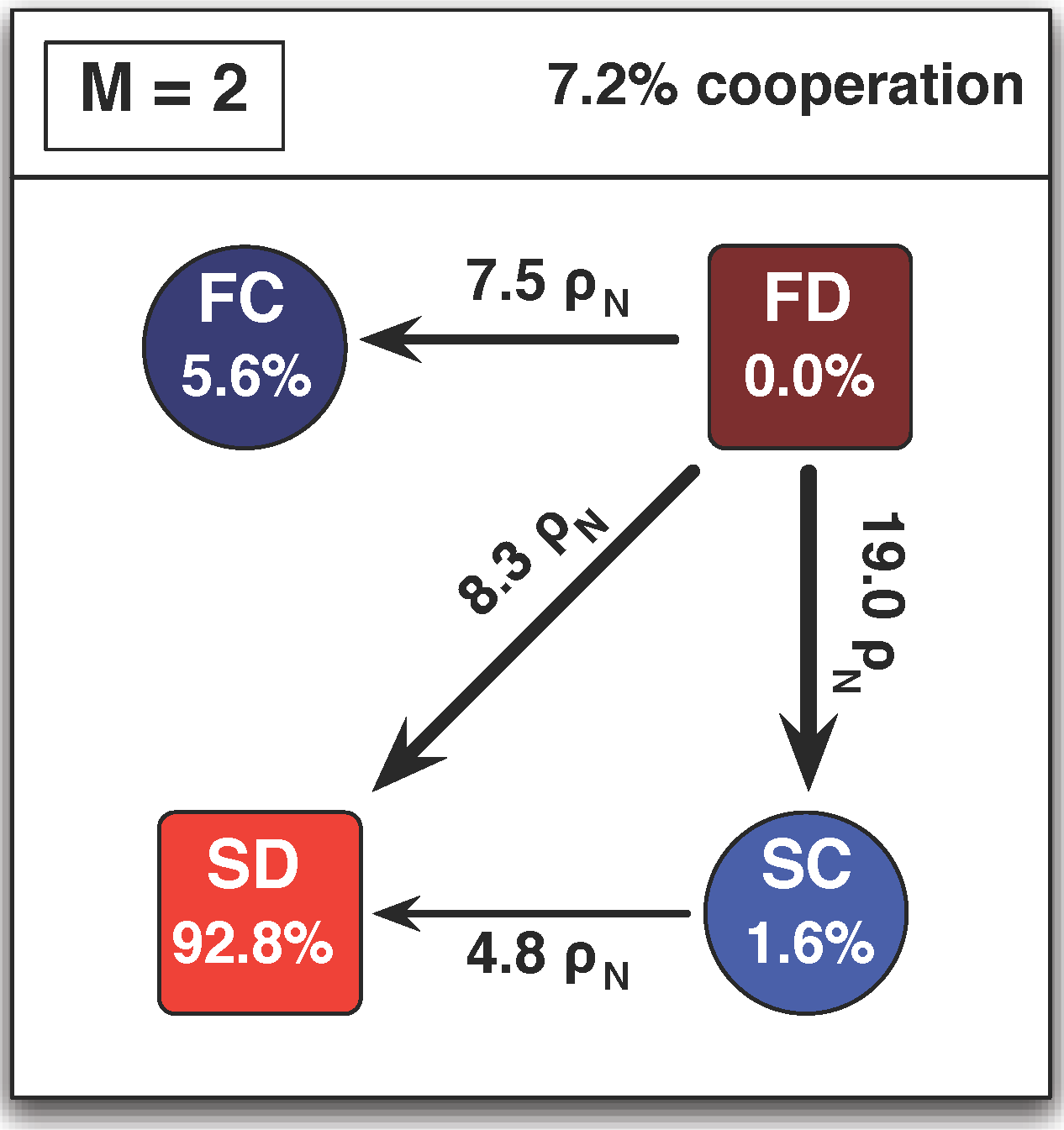
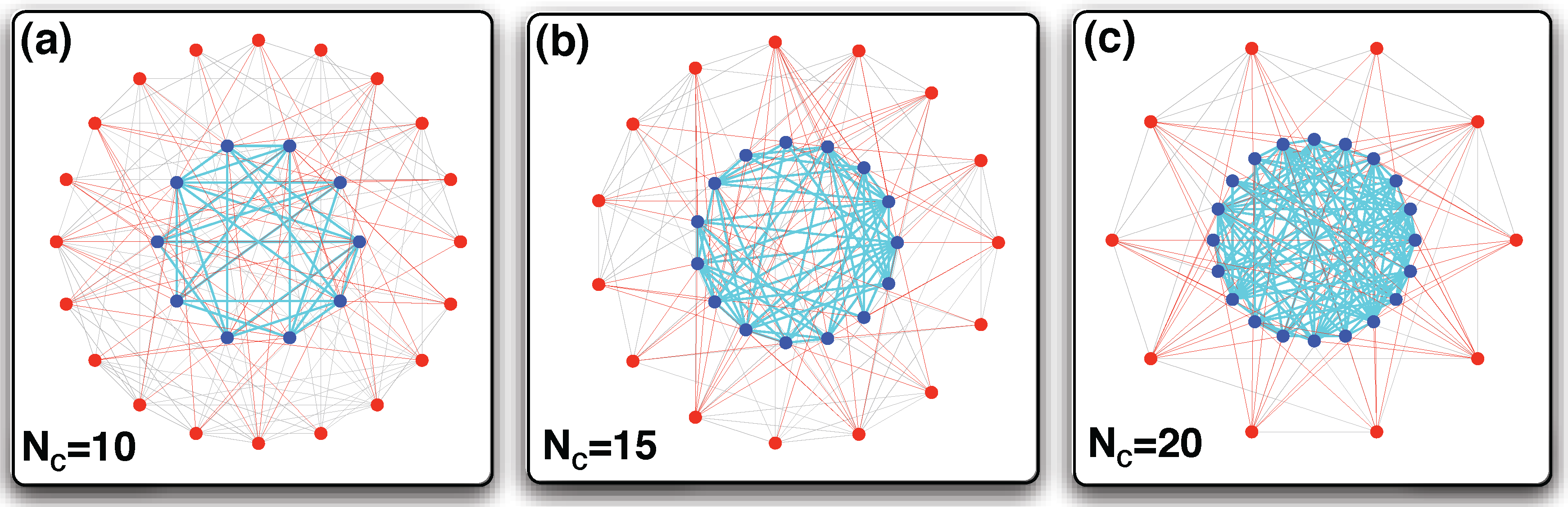
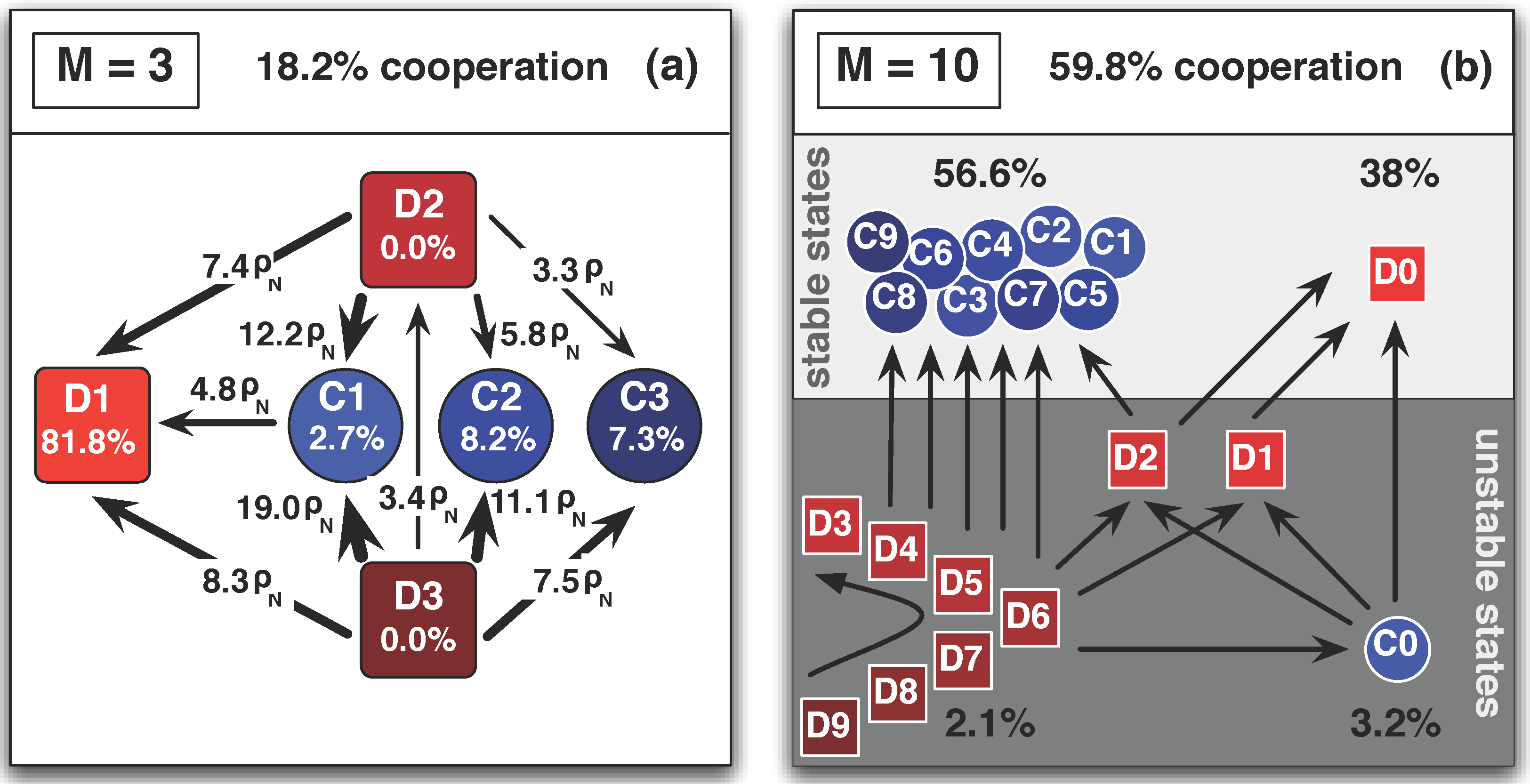
3.3. Arbitrary Number of Linking Strategies

4. Selection Pressure and Behavioral Diversity

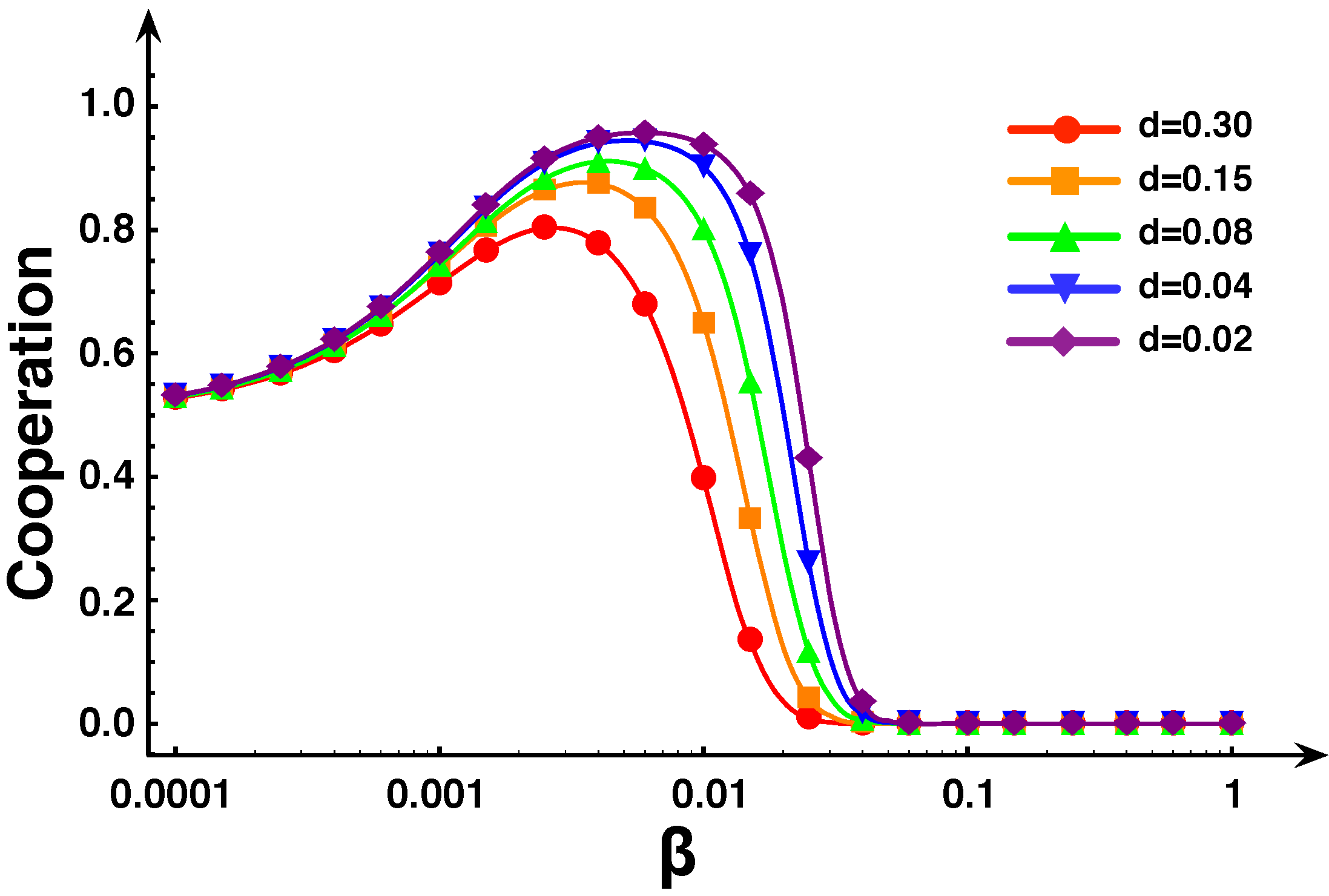
5. Diversity and Cooperation in the Snowdrift and Stag-hunt games
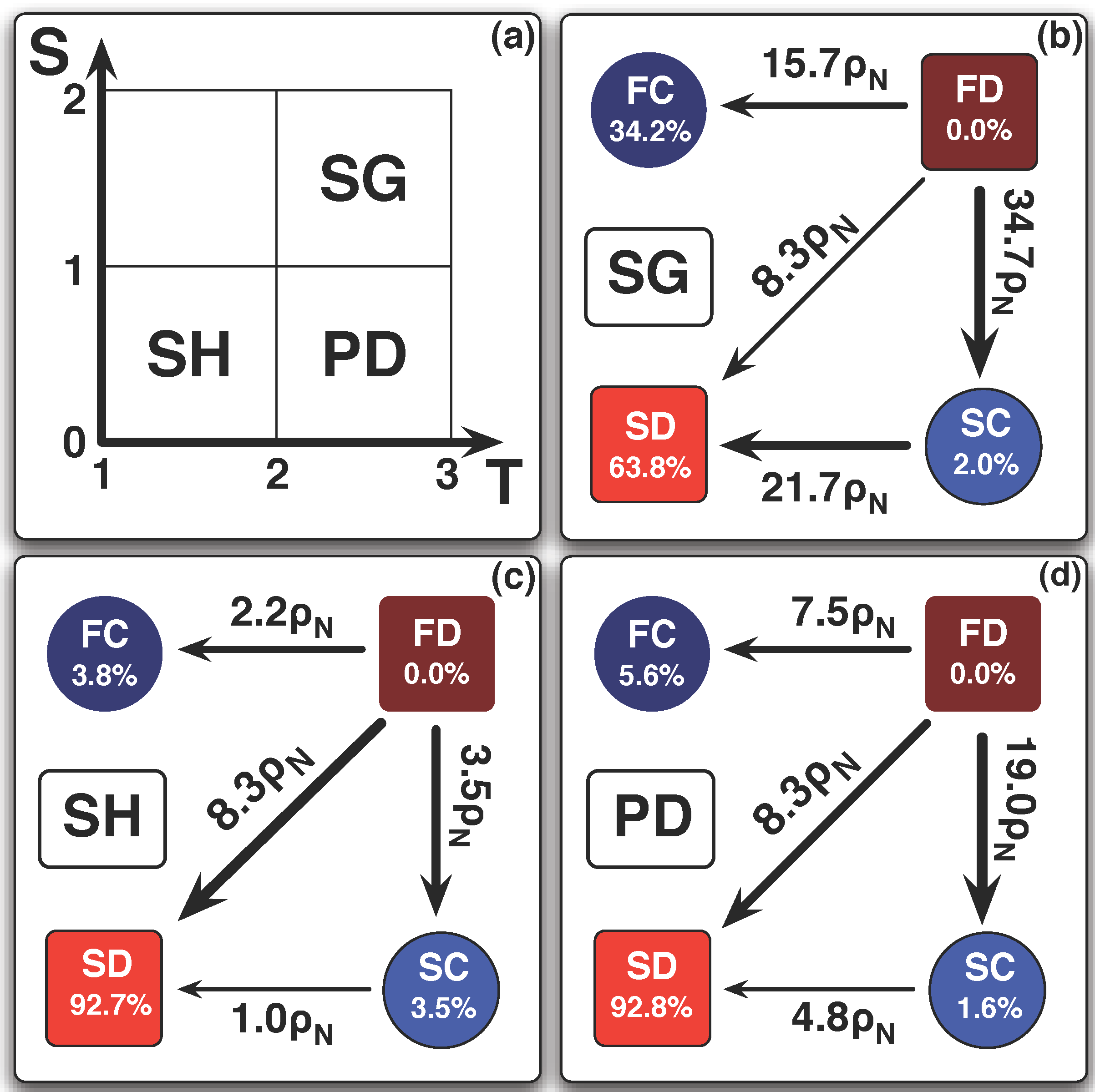

6. Conclusions
Acknowledgements
References
- Singh, J. Collaborative networks as determinants of knowledge diffusion patterns. Manage. Sci. 2005, 51, 756–770. [Google Scholar] [CrossRef]
- Kearns, M.; Suri, S.; Montfort, N. An experimental study on the coloring problem on human subject networks. Science 2006, 313, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Christakis, N.A.; Fowler, J.H. The spread of obesity in a large social network over 32 years. N. Eng. J. Med. 2007, 357, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Kearns, M.; Judds, S.; Tan, J.; Wortman, J. Behavioral experiments on biased voting in networks. Proc. Natl. Acad. Sci. USA 2006, 106, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.W.; Christakis, N.A. Cooperative behavior cascades in human social networks. Proc. Natl. Acad. Sci. USA 2010, 107, 5334–5338. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, E. How did cooperative behavior evolve? Science 2005, 309, 93. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, A.; Chammah, A.M. Prisoner’s Dilemma; University of Michigan Press: Ann Arbor, MI, USA, 1965. [Google Scholar]
- Maynard Smith, J. Evolution and the Theory of Games; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- Hofbauer, J.; Sigmund, K. Evolutionary Games and Population Dynamics; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Gintis, H. Game Theory Evolving; Princeton University Press: Princeton, NJ, USA, 2000. [Google Scholar]
- Nowak, M.A. Evolutionary Dynamics: Exploring the Equations of Life; Belknap Press of Harvard University Press: Cambridge, UK, 2006. [Google Scholar]
- Taylor, P.D.; Jonker, L. Evolutionary stable strategies and game dynamics. Math. Biosci. 1978, 40, 145–156. [Google Scholar] [CrossRef]
- Zeeman, E.C. Population dynamics from game theory. Lect. Note. Math. 1980, 819/1980, 471–497. [Google Scholar]
- Nowak, M.A. Five rules for the evolution of cooperation. Science 2006, 314, 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Nowak, M.A. Transforming the dilemma. Evolution 2007, 61, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Szabó, G.; Fáth, G. Evolutionary games on graphs. Phys. Rep. 2007, 446, 97–216. [Google Scholar] [CrossRef]
- Nowak, M.A.; May, R.M. Evolutionary games and spatial chaos. Nature 1992, 359, 826–829. [Google Scholar] [CrossRef]
- Huberman, B.A.; Glance, N.S. Evolutionary games and computer simulations. Proc. Natl. Acad. Sci. USA 1993, 90, 7716–7718. [Google Scholar] [CrossRef] [PubMed]
- Herz, A.V.M. Collective phenomena in spatially extended evolutionary games. J. Theor. Biol. 1994, 169, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, K.; Nordahl, M.G. Evolutionary dynamics of spatial games. Physica D 1994, 75, 292–309. [Google Scholar] [CrossRef]
- Nowak, M.A.; Bonhoeffer, S.; May, R.M. Spatial games and the maintenance of cooperation. Proc. Natl. Acad. Sci. USA 1994, 91, 4877–4881. [Google Scholar] [CrossRef] [PubMed]
- Nakamaru, M.; Matsuda, H.; Iwasa, Y. The evolution of cooperation in a lattice-structured population. J. Theor. Biol. 1997, 184, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Szabó, G.; Tőke, C. Evolutionary Prisoner’s Dilemma game on a square lattice. Phys. Rev. E 1998, 58, 69. [Google Scholar] [CrossRef]
- Hauert, C. Effects of space in 2 x 2 Games. Int. J. Bifurcat. Chaos 2002, 12, 1531–1548. [Google Scholar] [CrossRef]
- Szabó, G.; Vukov, J.; Szolnoki, A. Phase diagrams for an evolutionary prisoner’s dilemma game on two-dimensional lattices. Phys. Rev. E 2005, 72, 047107. [Google Scholar] [CrossRef] [PubMed]
- Vainstein, M.H.; Arenzon, J.J. Disordered environments in spatial games. Phys. Rev. E 2001, 64, 051905. [Google Scholar] [CrossRef] [PubMed]
- Abramson, G.; Kuperman, M. Social games in a social network. Phys. Rev. E 2001, 63, 030901(R). [Google Scholar] [CrossRef] [PubMed]
- Ebel, H.; Bornholdt, S. Coevolutionary games on networks. Phys. Rev. E 2002, 66, 056118. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Trusina, A.; Holme, P.; Minnhagen, P.; Chung, J.S.; Choi, M.Y. Dynamic instabilities by asymmetric influence: Prisoners’ Dilemma game in small-world networks. Phys. Rev. E 2002, 66, 021907. [Google Scholar] [CrossRef] [PubMed]
- Holme, P.; Trusina, A.; Kim, B.J.; Minnhagen, P. Prisoner’s Dilemma in real-world acquaintance networks: Spikes and quasiequilibria induced by the interplay between structure and dynamics. Phys. Rev. E 2003, 68, 030901(R). [Google Scholar] [CrossRef] [PubMed]
- Szabó, G.; Vukov, J. Cooperation for volunteering and partially random partnerships. Phys. Rev. E 2004, 69, 036107. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, E.; Hauert, C.; Nowak, M.A. Evolutionary dynamics on graphs. Nature 2005, 433, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.C.; Pacheco, J.M. Scale-free networks provide a unifying framework for the emergence of cooperation. Phys. Rev. Lett. 2005, 95, 098104. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.C.; Pacheco, J.M. A new route to the evolution of cooperation. J. Evol. Biol. 2006, 19, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.X.; Xu, X.J.; Huang, Z.G.; Wang, S.J.; Wang, Y.H. Evolutionary prisoner’s dilemma game with dynamic preferential selection. Phys. Rev. E 2006, 74, 021107. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.C.; Pacheco, J.M.; Lenaerts, T. Evolutionary dynamics of social dilemmas in structured heterogeneous populations. Proc. Natl. Acad. Sci. USA 2006, 103, 3490–3494. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, H.; Hauert, C.; Lieberman, E.; Nowak, M.A. A simple rule for the evolution of cooperation on graphs. Nature 2006, 441, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gardeñes, J.; Campillo, M.; Floría, L.M.; Moreno, Y. Dynamical organization of cooperation in complex topologies. Phys. Rev. Lett. 2007, 98, 108103. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Liu, L.H.; Wang, L. Evolutionary Prisoner’s Dilemma on heterogeneous Newman-Watts small-world network. Eur. Phys. J. B 2007, 56, 367–372. [Google Scholar] [CrossRef]
- Tomassini, M.; Luthi, L.; Giacobini, M. Hawks and doves on small-world networks. Phys. Rev. E 2006, 73, 016132. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N. Participation costs dismiss the advantage of heterogeneous networks in the evolution of cooperation. Proc. Biol. Sci. 2007, 274, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Poncela, J.; Gómez-Gardeñes, J.; Floría, L.M.; Moreno, Y. Robustness of cooperation in the evolutionary prisoner’s dilemma on complex networks. New J. Phys. 2007, 9, 184. [Google Scholar] [CrossRef]
- Lozano, S.; Arenas, A.; Sánchez, A. Mesoscopic structure conditions the emergence of cooperation on social networks. PLoS One 2008, 3, e1892. [Google Scholar] [CrossRef] [PubMed]
- Szolnoki, A.; Perc, M.; Danku, Z. Towards effective payoffs in the prisoner’s dilemma game on scale-free networks. Phyica A 2008, 387, 2075–2082. [Google Scholar] [CrossRef]
- Santos, F.C.; Santos, M.D.; Pacheco, J.M. Social diversity promotes the emergence of cooperation in public goods games. Nature 2008, 454, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Vukov, J.; Szabó, G.; Szolnoki, A. Evolutionary prisoner’s dilemma game on Newman-Watts networks. Phys. Rev. E 2008, 77, 026109. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.M.; Pinheiro, F.; Santos, F.C. Population structure induces a symmetry breaking favoring the emergence of cooperation. PLoS Comput. Biol. 2009, 5, e1000596. [Google Scholar] [CrossRef] [PubMed]
- Kossinets, G.; Watts, D.J. Empirical analysis of an evolving social network. Science 2006, 311, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.G.; Eguíluz, V.M.; Cela-Conde, C.; San Miguel, M. Coevolution of dynamical states and interactions in dynamic networks. Phys. Rev. E 2004, 69, 065102. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.G.; Eguíluz, V.M.; San Miguel, M. Cooperation and emergence of role differentiation in the dynamics of social networks. Am. J. Soc. 2005, 110, 977. [Google Scholar]
- Santos, F.C.; Pacheco, J.M.; Lenaerts, T. Cooperation prevails when individuals adjust their social ties. PLoS Comput. Biol. 2006, 2, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.M.; Traulsen, A.; Nowak, M.A. Co-evolution of strategy and structure in complex networks with dynamical linking. Phys. Rev. Lett. 2006, 97, 258103. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.M.; Traulsen, A.; Nowak, M.A. Active linking in evolutionary games. J. Theor. Biol. 2006, 243, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Hanaki, N.; Peterhansl, A.; Dodds, P.S.; Watts, D.J. Cooperation in evolving social networks. Manage. Sci. 2007, 53, 1036–1050. [Google Scholar] [CrossRef]
- Tanimoto, J. Dilemma solving by the coevolution of networks and strategy in a 2 x 2 game. Phys. Rev. E 2007, 76, 021126. [Google Scholar] [CrossRef] [PubMed]
- Gross, T.; Blasius, B. Adaptive coevolutionary networks—A review. J. Roy. Soc. Interface 2008, 5, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Van Segbroeck, S.; Santos, F.C.; Nowé, A.; Pacheco, J.M.; Lenaerts, T. The evolution of prompt reaction to adverse ties. BMC Evol. Biol. 2008, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, J.M.; Traulsen, A.; Ohtsuki, H.; Nowak, M.A. Repeated games and direct reciprocity under active linking. J. Theor. Biol. 2008, 250, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Hauert, C.; Nowak, M.; Wang, L. Reputation-based partner choice promotes cooperation in social networks. Phys. Rev. E 2008, 78, 026117. [Google Scholar] [CrossRef] [PubMed]
- Pestelacci, E.; Tomassini, M.; Luthi, L. Evolution of cooperation and coordination in a dynamically networked society. J. Biol. Theor. 2008, 3, 139–153. [Google Scholar] [CrossRef]
- Szolnoki, A.; Perc, M. Resolving social dilemmas on evolving random networks. Europhys. Lett. 2009, 86, 30007. [Google Scholar] [CrossRef]
- Van Segbroeck, S.; Santos, F.C.; Lenaerts, T.; Pacheco, J.M. Reacting differently to adverse ties promotes cooperation in social networks. Phys. Rev. Lett. 2009, 102, 058105. [Google Scholar] [CrossRef] [PubMed]
- Perc, M.; Szolnoki, A. Coevolutionary games—A mini review. Biosystems 2009, 99, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhou, D.; Fu, F.; Luo, Q.; Wang, L.; Traulsen, A. Evolution of cooperation on stochastical dynamical networks. PLoS One 2010, 5, e11187. [Google Scholar] [CrossRef] [PubMed]
- Skyrms, B. The Stag-Hunt Game and the Evolution of Social Structure; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Sugden, R. The Economics of Rights, Co-operation and Welfare; Blackwell: Oxford, UK, 1986. [Google Scholar]
- Hauert, C.; Doebeli, M. Spatial structure often inhibits the evolution of cooperation in the snowdrift game. Nature 2004, 428, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Doebeli, M.; Hauert, C. Models of cooperation based on the Prisoner’s Dilemma and the Snowdrift game. Ecol. Lett. 2005, 8, 748–766. [Google Scholar] [CrossRef]
- Posch, M.; Pichler, A.; Sigmund, K. The efficiency of adapting aspiration levels. Proc. R. Soc. Lond. B 1999, 266, 1427–1435. [Google Scholar] [CrossRef]
- Macy, M.; Flache, A. Learning dynamics in social dilemmas. Proc. Natl. Acad. Sci. USA 2002, 99, 7229–7236. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.A.N.; Scala, A.; Barthélémy, M.; Stanley, H.E. Classes of small-world networks. Proc. Natl. Acad. Sci. USA 2000, 97, 11149–11152. [Google Scholar] [CrossRef] [PubMed]
- Traulsen, A.; Nowak, M.A.; Pacheco, J.M. Stochastic dynamics of invasion and fixation. Phys. Rev. E 2006, 74, 11909. [Google Scholar] [CrossRef] [PubMed]
- Traulsen, A.; Pacheco, J.M.; Nowak, M.A. Pairwise comparison and selection temperature in evolutionary game dynamics. J. Theor. Biol. 2007, 246, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Liggett, T.M. Interacting Particle Systems; Springer-Verlag: New York, NY, USA, 1985. [Google Scholar]
- Ohtsuki, H.; Nowak, M.A.; Pacheco, J.M. Breaking the symmetry between interaction and replacement in evolutionary dynamics on graphs. Phys. Rev. Lett. 2007, 98, 108106. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, H.; Pacheco, J.M.; Nowak, M.A. Evolutionary graph theory: breaking the symmetry between interaction and replacement. J. Theor. Biol. 2007, 246, 681–94. [Google Scholar] [CrossRef] [PubMed]
- Van Kampen, N.G. Stochastic Processes in Physics and Chemistry; North Holland: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Taylor, P.D.; Day, T.; Wild, G. Evolution of cooperation in a finite homogeneous graph. Nature 2007, 447, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Eckel, C.; Wilson, R. Whom to Trust? Choice of Partner in a Trust Game; Technical Report; Virginia Polytechnic Institute: Blacksburg, VA, USA, 2004; (Working paper). [Google Scholar]
- Imhof, L.A.; Fudenberg, D.; Nowak, M.A. Evolutionary cycles of cooperation and defection. Proc. Natl. Acad. Sci. USA 2005, 102, 10797–10800. [Google Scholar] [CrossRef] [PubMed]
- Hauert, C.; Traulsen, A.; Brandt, H.; Nowak, M.A.; Sigmund, K. Via freedom to coercion: The emergence of costly punishment. Science 2007, 316, 1905–1907. [Google Scholar] [CrossRef] [PubMed]
- Szolnoki, A.; Szabó, G. Cooperation enhanced by inhomogeneous activity of teaching for evolutionary Prisoner’s Dilemma games. Europhys. Lett. 2007, 77, 30004. [Google Scholar] [CrossRef]
- Szolnoki, A.; Perc, M.; Szabó, G. Diversity of reproduction rate supports cooperation in the prisoner’s dilemma game on complex networks. Eur. Phys. J. B 2008, 61, 505–509. [Google Scholar] [CrossRef]
- Van Segbroeck, S.; Santos, F.C.; Pacheco, J.M. Adaptive contact networks change effective disease infectiousness and dynamics. PLoS Comput. Biol. 2010, 6, e1000895. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license http://creativecommons.org/licenses/by/3.0/.
Share and Cite
Van Segbroeck, S.; Santos, F.C.; Pacheco, J.M.; Lenaerts, T. Coevolution of Cooperation, Response to Adverse Social Ties and Network Structure. Games 2010, 1, 317-337. https://doi.org/10.3390/g1030317
Van Segbroeck S, Santos FC, Pacheco JM, Lenaerts T. Coevolution of Cooperation, Response to Adverse Social Ties and Network Structure. Games. 2010; 1(3):317-337. https://doi.org/10.3390/g1030317
Chicago/Turabian StyleVan Segbroeck, Sven, Francisco C. Santos, Jorge M. Pacheco, and Tom Lenaerts. 2010. "Coevolution of Cooperation, Response to Adverse Social Ties and Network Structure" Games 1, no. 3: 317-337. https://doi.org/10.3390/g1030317
APA StyleVan Segbroeck, S., Santos, F. C., Pacheco, J. M., & Lenaerts, T. (2010). Coevolution of Cooperation, Response to Adverse Social Ties and Network Structure. Games, 1(3), 317-337. https://doi.org/10.3390/g1030317



