3.3.1. Power Plants
In power plants, an increase of DeNO
x catalyst layer is directly proportional to the efficiency of nitrogen oxide removal. The power plants of South Korea are removing nitrogen oxides in the exhaust gas using SCR method. Most SCR plant units are often designed to be equipped with four-layer catalysts, though only two layers are now filled with catalyst to satisfy the emission criteria. A four-layer SCR catalytic system also displayed a good efficiency for removal of NO
x. However, they suffered a pressure drop, due to additional catalytic layers, which increased not only the overall cost of the DeNO
x process but also the ammonia distribution in the nozzle of ammonia injection facility [
44].
According to the Korea Electric Power Exchange’s statistics in 2018, the capacity of thermal power plants using gas and coal as raw materials accounts for about 69% of the total capacity (
Table 5). While the number of thermal power generators using gas and coal as raw materials, accounting for 73% of the total number of main power generators. With this power industry environment, the thermal power has a higher number of operating days as compared to other sources of power generation, especially 81.53% for coal-fired power generation (
Table 6) [
45].
The exhaust gas treatment facility of coal-fired power generation requires stable performance for a long period of time. In order to preserve long-term stability, the presence of proper catalysts in coal-fired power-generating SCR facilities is highly recommended. Therefore, long-term stability of operation is required for catalyst injection into power-generating SCR facilities. In addition, for boilers used in thermal power generation, the temperature of the boiler exhaust is higher than 500 °C which is enough to operate any catalyst. However, due to its higher temperature, the SCR catalyst may deactivate during long-term operation. Therefore, since 2000s, when SCR facilities were supplied to South Korea, various studies have been conducted for stable operation of the SCR catalyst.
The catalysts used in the SCR process are V
2O
5-WO
3 (or MoO
3)/TiO
2 have honeycomb and plate type structures (
Figure 18). In particular, the V
2O
5-WO
3 (or MoO
3)/TiO
2 monolith catalyst of the honeycomb type has been consistently used as it has advantages such as high heat and mass transfer rates besides high contact area [
46].
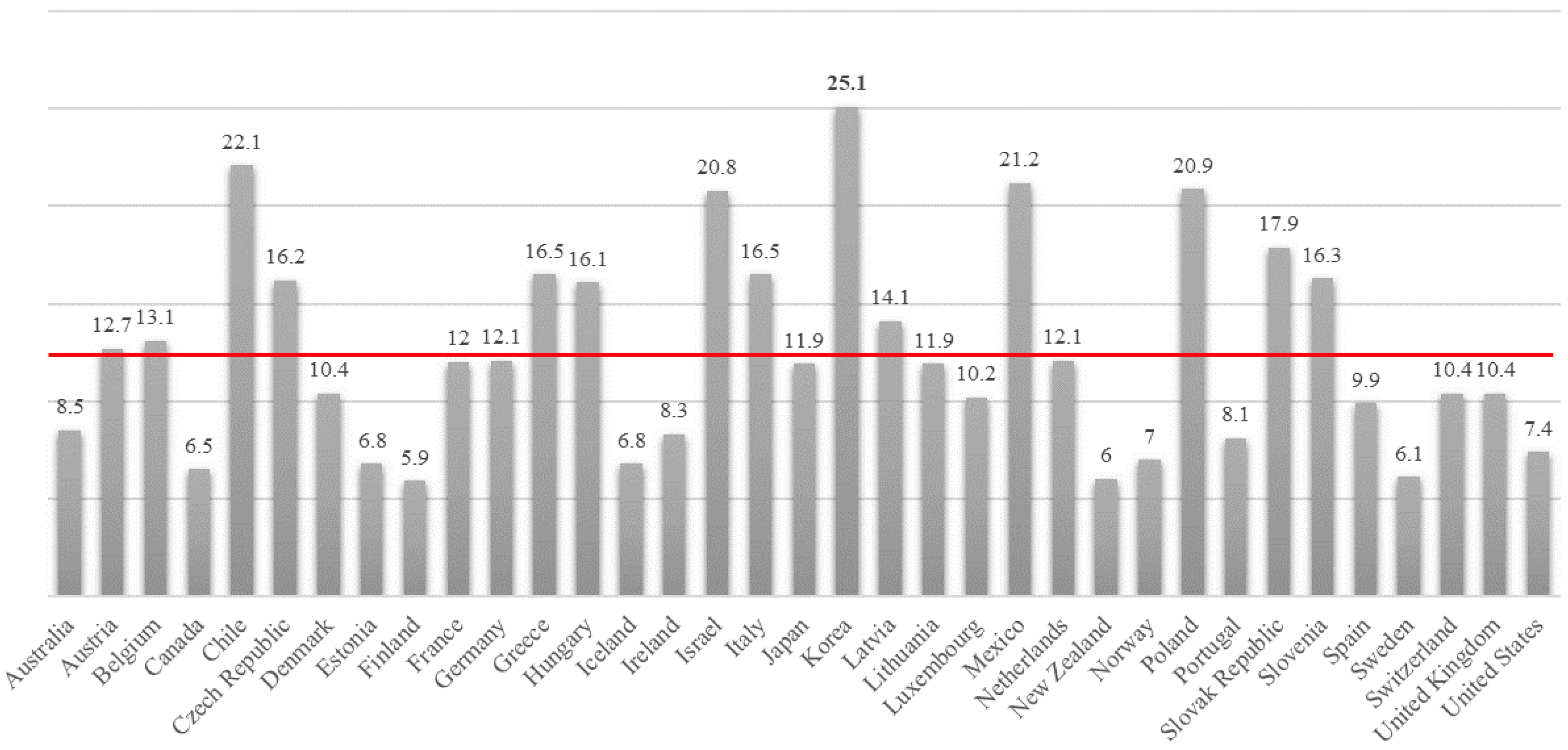
Looking at the amount of installation of SCR catalyst in South Korea in 2015 (
Table 7), the subsidiary power plant of the Korea Electric Power Corporation (KEPCO) is approximately 21,758 m
3, and third-party power plant usage is estimated to be around 4786 m
3. The total use of SCR catalyst for domestic power plants and incinerators is roughly around 26,660 m
3 [
47].
Huang et al., according to V
2O
5 content, investigated the thermal behavior of V
2O
5/TiO
2 catalyst. They changed the pretreatment temperature of the catalyst from 250 to 500 °C. Through this experiment, they reported that at temperatures above 400 °C the phase transition of V
2O
5 occurred and condensation caused the decrease of the specific surface area (
Figure 19) and hence of the catalytic activity [
48].
Cha et al. investigated the thermal deactivation characteristics of V
2O
5-WO
3/TiO
2 catalyst of plate-type. They reported that when the catalyst calcined at temperatures above 600 °C, the NO
x conversion rate was dramatically reduced (
Figure 20). The XRD analysis results showed that the crystalline structure of TiO
2 changed from anatase to rutile, and the surface area and the contribution of the catalyst was significantly reduced at those temperatures due to the formation of CaWO
4 and TiO
2 crystals, which were confirmed by scanning electron microscope (SEM) images (
Figure 21) [
49].
Nam et al. conducted a study on the high-temperature SCR reaction using W/TiO
2 catalyst. They found that TiO
2 alone showed a conversion rate of NO
x of less than 50% at temperatures above 500 °C. However, addition of W with 13 wt% into TiO
2 showed an increased SCR efficiency, but more than 13 wt% content of W on TiO
2 led to lower efficiency. W(13)/TiO
2(B) (where W content is 13 wt% and B = pure anatase) catalyst was pretreated at 600 °C and then loaded SCR reaction operated at 500 °C and 550 °C. The catalytic activity was stable even after more than 900 h (
Figure 22) [
50].
Except for liquefied natural gas (LNG), most fossil fuels contain significant amount of sulfur dioxide within the exhaust gas after combustion of these fuels. SO
2 may be oxidized into SO
3 in the presence of catalysts, which converted again into SO
4− species on the catalyst surface. The new species formed inhibits the catalytic active sites, or SO
42− species may be combined with NH
4+ to create salt, NH
4HSO
4. This salt also made it possible to cover the catalyst active species, and thus greater catalytic deactivation was observed in the presence of SO
2 [
51].
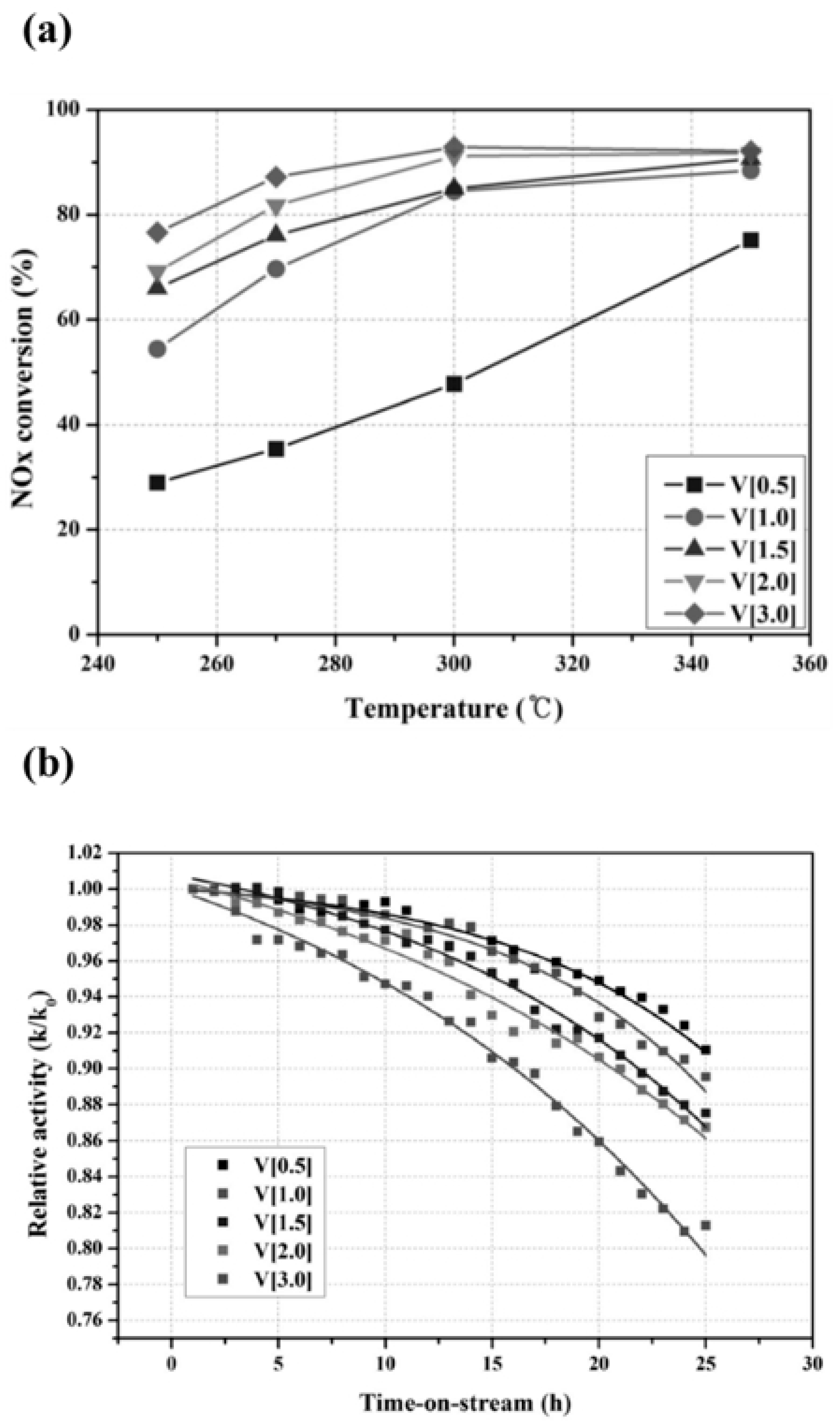
Lee et al. carried out studies to enhance SCR reactivity in the presence of SO
2 on the V/TiO
2 catalyst. They showed different SCR conversion rates and SO
2 durability results depending on the vanadium content. The NO
x conversion was increased by increasing the vanadium amount (from 0.5 to 3.0 wt%) on TiO
2, whereas the relative activity for SCR of NO
x in the presence of SO
2 followed an opposite trend (
Figure 23). They have also tested the relative activity for SCR after the addition of 5 wt% W into the optimized V[2]/TiO
2 (i.e., V[2]W[5]/TiO
2) catalyst which displayed an improved performance of NO
x in the presence of SO
2 500 ppm (
Figure 24a) [
52]. It was observed from SO
2-Temperature Programed Desorption (TPD) (
Figure 24b) that the desorption peak was shifted to lower temperature on V[2]W[5]/TiO
2 catalyst as compared with that of W-free catalyst (V[2]/TiO
2).
Park et al. found that over 90% of catalytic activity was recovered when spent catalyst was washed by acidic solution in the field regeneration of SCR waste catalyst, and the concentration of acidic solution was 3~5 M (
Figure 25). When the acidity of the solution exceeds the optimal point, the dissolution effects of the impurity component deposited on the catalyst surface that caused the catalyst deactivation. Moreover, the leaching rate of vanadium and tungsten, which are the main active components, increases and eventually lead to catalyst deactivation [
53].
Despite many efforts being made to increase the life and stability of the SCR catalysts, they have a limited life depending on the operation conditions applied. The life of the catalyst used in the NH
3-SCR process is usually 3–5 years depending on the operating conditions of catalyst at each process site. In addition, only a few years ago, the activity of the SCR catalyst, which markedly deteriorated and reached the end of its life, was mostly treated by reclaiming the waste. Although research is being conducted worldwide to regenerate and use it, the currently studied regeneration method is to desorb the catalyst from the reactor and transport it to a regeneration plant to regenerate the degraded catalyst. In order to retain the original activity of the degraded catalyst, acid is used to remove the adsorbed coke and other particulate matters on the surface of the catalyst which was generally reported to be a time-consuming process [
53].
3.3.2. Car Engines
Hydrocarbons, carbon monoxide and nitrogen oxides emitted by gasoline engines can be reduced by using three-way catalysts. Diesel engines also emit those reducing gases, although they need to be operated in excess oxygen environments. Due to the dominant concentration of reducing agents such as hydrocarbons and carbon monoxide in the exhaust gas, which actually need to be reduced to N
2 from NO
x, they reacted with excess oxygen. The diesel engine produces higher emissions of particle matter (PM) and NO
x than gasoline engines [
54]. In South Korea, diesel oxidation catalyst (DOC) and diesel particulate filter (DPF) were used to reduce the NO
x as per the Euro-5 regulation. However, Euro-6 regulations, which applied from 2015, require a separate technology called SCR to reduce NO
x as the emission volume of NO
x is significantly strengthened, compared to Euro-5 (
Figure 15). The reduction technique of NO
x can be divided into two methods such as pre-processing technology (engine application technology, where both fuel and oxygen ratio adjust within engine) and post-processing technology (actual SCR catalytic technology) [
54].
Among the post-processing technologies, the lean NO
x trap (LNT) and SCR are the representative technologies that reduce nitrogen oxides. Urea-SCR is the most excellent device for removing NO
x by spraying urea solution into the exhaust pipe to create ammonia by thermal hydrolysis (
Figure 7). However, it should be considered that the system is complex and has problems such as low efficiency at low temperatures, the generation of solid byproducts and the use of low-grade elements, etc. These problems can be avoided by uniform mixing of the urea solution [
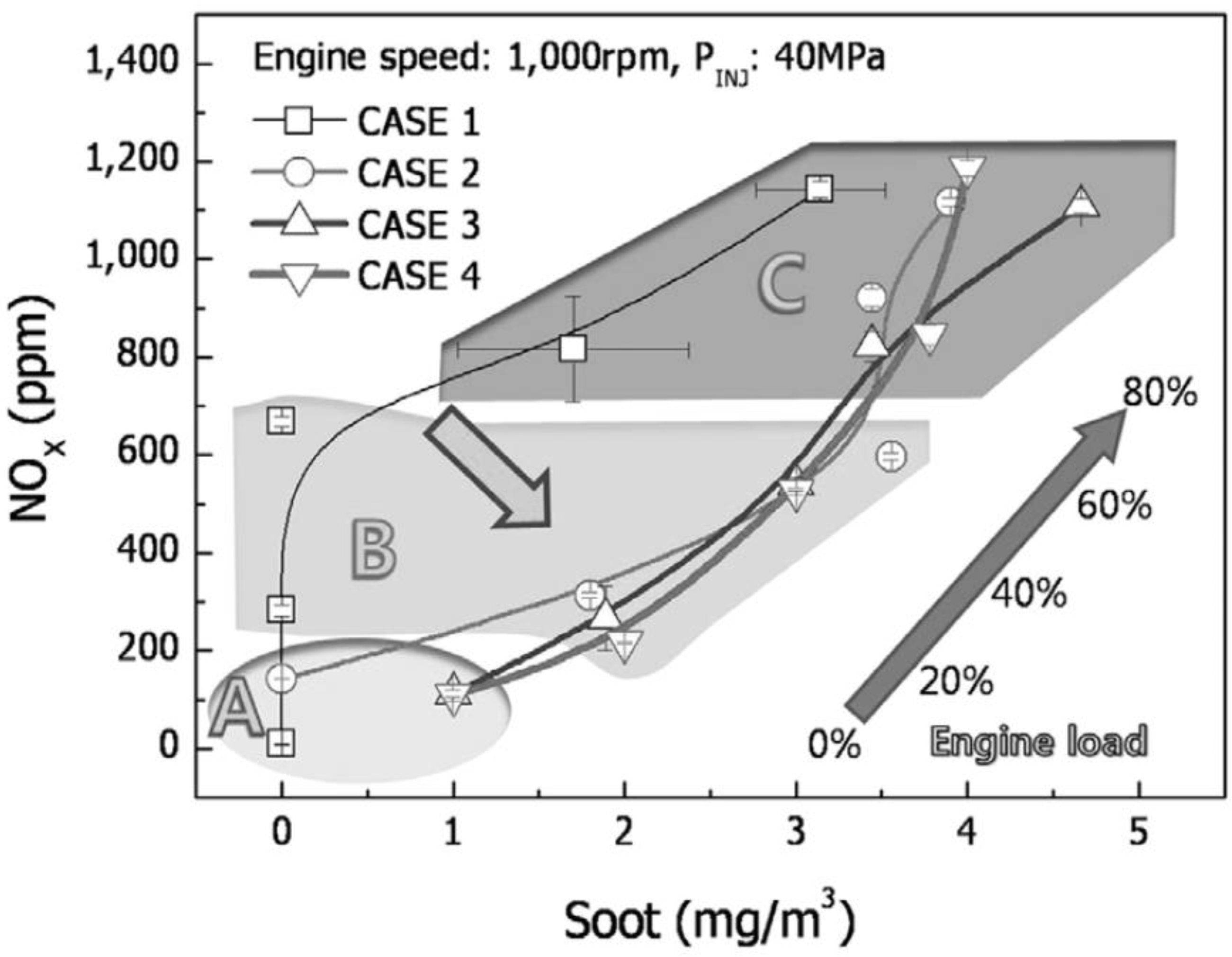
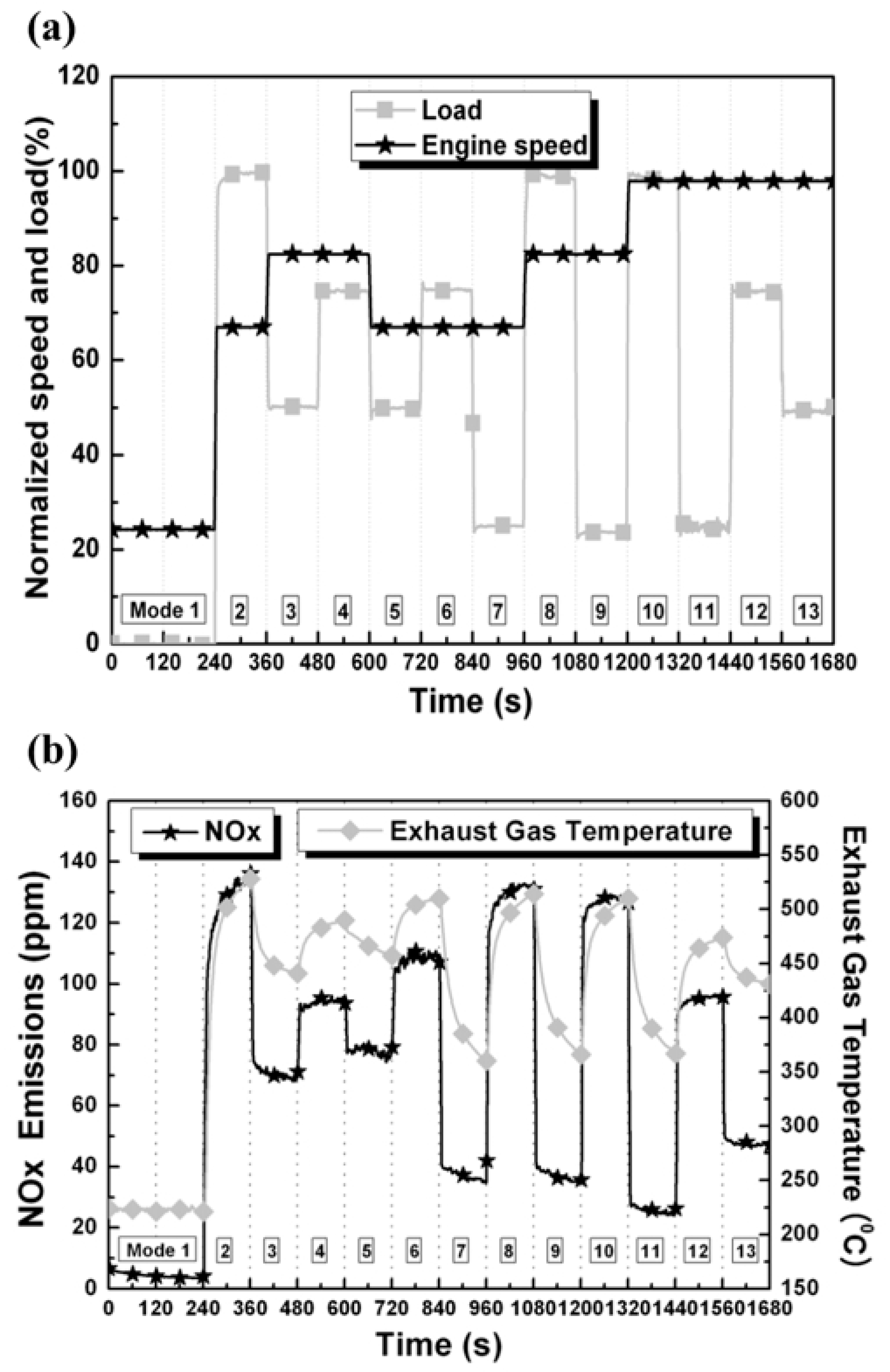
55]. The driving characteristics of the vehicle cause a sudden change in engine load, resulting in different exhaust gas temperatures and concentrations of NO
x (
Figure 26 and
Figure 27) [
56].
The most likely and effective deployment of the current vehicle SCR system is in the order of DOC and DPF followed by SCR. The SCR catalyst will also be exposed to high temperature exhausts during the forced regeneration of DPF PM combustion [
57]. V
2O
5/WO/TiO
2 catalysts, widely used in plant industries, show excellent efficiency in the range of 300–400 °C, but the reaction activity and selectivity are significantly reduced at low temperature (below 200 °C) and high temperature (above 400 °C) [
58]. The Cu-ZSM-5, a typical example of non-V
2O
5 based catalytic system, has also received an attention due to its fast rate of NO decomposition [
59].
The vanadium catalyst generally used in the SCR process has a low deactivation at moderate reaction temperature even in the presence of sulfur, although the catalyst deactivated at high temperature. By contrast, the adsorption amount of ammonia is excellent on the zeolite catalysts in the wide reaction temperature range, but sulfur resistance is low. Han et al. investigated the performance of the catalyst to overcome the shortcomings of vanadium catalyst and zeolite catalyst and maximize the catalyst life time as well as catalytic activity. They reported high temperature stability from the Fe-zeolite catalyst and sulfur resistance from the V
2O
5 catalytic system (
Figure 28). Both the catalyst stability and sulfur resistance were enhanced when V
2O
5 was loaded into the Fe-zeolite catalyst (
Figure 29) [
60].
Huang et al. investigated the NH
3-SCR catalytic reaction on the zeolite catalyst with different structures. In more detail, using commercial zeolite catalyst (Clariant Co., Ltd. Daegu, Korea), they exchanged cations such as Cu and Fe to BEA (zeolite β) and MFI (ZSM-5) respectively. The BEA exchanged with Cu ions displayed a greater low temperature activity, and thus higher NO conversion rate than Fe exchanged on MFI. Furthermore, as shown
Figure 30, NH
3 desorption peaks of BEA zeolite (B) was greatly influenced by the type of ion exchange metal ions rather than the structure of the zeolite’s structure [
61].
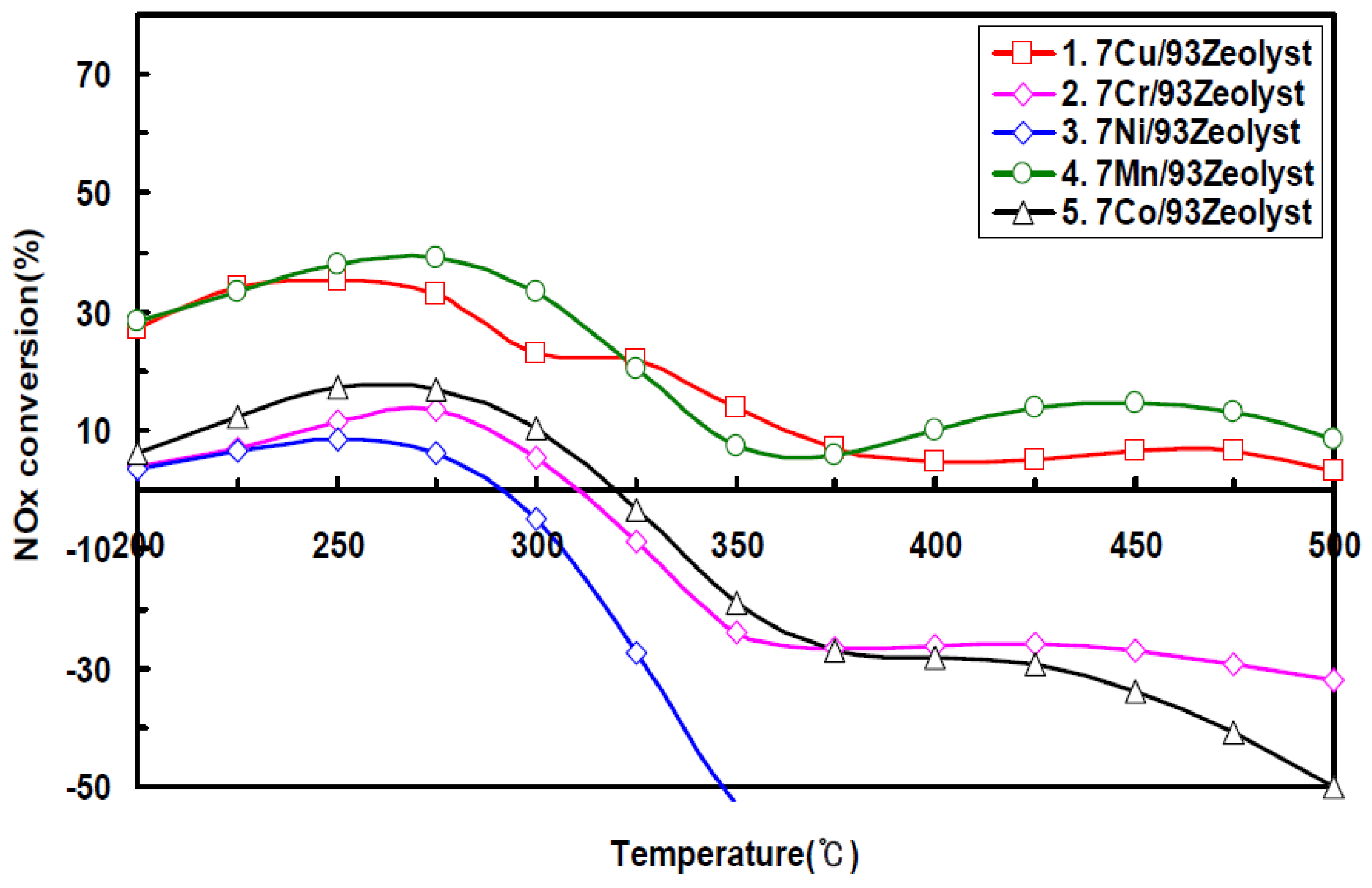
In another research, Seo studied the iron exchanged with Cu, Co, Ni, Mn, and Co in the zeolite, and investigated catalytic performance of DeNO
x. Also, the effects of the catalyst were investigated by adding some cations like Ba, K and Ca to the Cu-zeolite catalyst. The researcher reported that Cu and Mn showed a high NO
x conversion at low reaction temperature of 200 °C (
Figure 31). The Ba-modified zeolite showed a high activity at 500 °C, as compared with that of K- and Ca-modified catalysts, by inhibiting the agglomeration of Cu particles on the zeolite catalyst (
Figure 32) [
62].
In the case of the direct ammonia-injection SCR system, the solid phase ammonia storage material is heated to generate ammonia gas, a reducing agent, and sprayed directly into the exhaust pipe in a gaseous phase. There is no discharge of solid byproducts, and the shape of the exhaust system required for uniform mixing of reducing agent is relatively free. In addition, since a series of processes such as thermal decomposition and hydrolysis are not required in low-temperature areas, which is advantageous in terms of reactivity and efficiency, this technique could be applied in the future [
55].
The characteristics of NO
x reduction rate, reaction rate, and ammonia slip of ammonia gas injection system were evaluated by Jung et al. via preparing ammonia gas injection system, which can replace the existing urea-SCR, using low temperature Cu-ZSM-5 catalyst. They reported that, unlike the urea solution, the response rate of ammonia gas was significantly improved due to the injection of reducing agents, with less stable adsorbed-phase occurring in the SCR catalyst (
Figure 33) [
63].
3.3.3. Marine Engines
The generation of nitrogen oxides and power supply for ships results from the internal combustion engines of ships. The exhaust emission characteristics of a heavy-duty diesel engine with real time analysis are shown in
Figure 34 [
64]. Since engine optimization alone does not satisfy Tier III’s regulatory parameters on the reduction of nitrogen oxide emissions, the development of ship’s exhaust gas post-processing device has become required to satisfy the enhanced emissions regulations of IMO [
65].
Most diesel engines for propulsion use a 2-stroke engine that is simple in structure and has good momentum. Diesel engines for power generation are mainly used with 4-stroke engines [
66]. However, unlike automobile engines which have a temperature of 300 to 400 °C, the 2-stroke diesel engine for ships only has a temperature of 200 to 300 °C, making it difficult for the SCR system to be applied to the system [
65].
In addition, the catalyst used in the ship SCR process built within the ship, so the vibration resistance to withstand the internal engine, operation, and vibration caused by waves must be considered. Great importance should be given to the prevention of catalyst poisoning and the formation of ammonium bisulfate in the presence of SO
2 [
67]. In order to solve this problem, various catalysts having nitrogen oxide reduction performance suitable for ship SCR process environment have been studied.
Kwon et al. studied the low temperature SCR reaction on Mn/TiO
2 catalyst using various commercial TiO
2 as supports (
Figure 35). They reported that low MnO
x surface density caused by high dispersion manganese oxides increased low-temperature SCR activity besides lowering the reduction temperature (from MnO
2 to Mn
2O
3) of surface MnOx on TiO
2 [
68].
As shown in
Figure 36, Kang et al. investigated the NO
x reduction rate using the single Cu and Mn oxide, and Cu-Mn mixed oxides catalyst. The presence of small amounts of copper oxide showed 100% NO
x conversion compared with single metal oxide catalysts such as CuO and MnO
x at wide temperature range (323–473 K) [
69].
Ship diesel fuel contains sulfur (
Table 8), which will go through the combustion process and form SO
2, which is a known catalytic poisoning gas for the active species of the SCR catalysts. In more detail, SO
2 is the biggest problem for cold NH
3-SCR catalysts [
70]. Many research efforts have been made to reduce the poisoning of SCR catalyst in the presence of sulfur.
Ha et al. investigated the effects of moisture and SO
2 in gases (5% H
2O and 100 ppm SO
2) on the selective catalytic recuperative activity of NO at low temperatures on Fe/zeolite catalyst. Additives such as Mn, Zr, and Ce were added into Fe/zeolite catalysts to check their catalytic efficacy. The NO conversion rate of Fe/BEA catalyst was reduced to 47% at 200 °C, which slightly increased to 53% on the MnFe/BEA in the presence of moisture and SO
2. The modified catalyst also displayed a greater stable activity than the Fe/BEA catalyst. Mn showed the effect of increasing the dispersion on Fe as compared to that of Zr and Ce, and also preventing the formation of a valuable aggregate of Fe [
72].
Using the Cu-chabazite SCR catalyst, Nam investigated the effect of reducing nitrogen oxides by SO
2 poisoning at low temperatures between 150 and 300 °C. The researcher was able to measure NO
x reduction for two hours at 150 °C, and then again at the same temperature for another two hours. Although the reduction rate of NO
x was reduced by 50% compared to the new one, it was reported that the higher temperature near to 300 °C, the effect of sulfur poisoning was almost eliminated [
73].
Lee et al. [
74] investigated the effect of ceria loading over Sb-V
2O
5/TiO
2 catalyst for the selective catalytic reduction of NO
x by NH
3. The V
2O
5-Ce/TiO
2 and Sb-V
2O
5-Ce/TiO
2 catalysts were prepared by incipient wetness co-impregnation of vanadia and antimony on the pre-prepared CeO
2/TiO
2 support, which was synthesized by deposition precipitation method by hydrolysis with ammonium hydroxide. The addition of 10% ceria to Sb-V
2O
5/TiO
2 significantly enhanced the total acidity and redox properties, and thus the NO
x conversion as well as N
2 selectivity (>95%) at wide temperature range of 220–500 °C (
Figure 37). The X-ray diffraction (XRD) results indicated the active components of Sb and V were homogeneously dispersed over CeO
2/TiO
2. The results of NO and SO
2 TPD of 10% ceria-loaded Sb-V
2O
5/TiO
2 catalyst showed enhancement of NO adsorption and SO
2 inhibition properties, which is thought to play a significant role in the long-term stability of the catalyst during an SO
2 on–off study for 38 h at 240 °C.
To investigate the effect of pore structure of TiO
2 on sulfur poisoning, Youn et al. studied 5 wt% V
2O
5 supported on the two types of TiO
2 (VT) having distinctive pore, mesopore (DT-51) and micropore (microporous TiO
2) structures for selective catalytic reduction of NO
x with NH
3 (NH
3 SCR). The catalyst prepared by the wet impregnation method of vanadium solution on TiO
2. During the SCR reaction in the presence of SO
2 for 12 h, 5 wt% VT (DT-51) showed more drastic decrease in activity than 5 wt% VT (micro). They reported that a larger amount of SO
2 was desorbed over the DT-51 catalyst used during the temperature-programmed decomposition with the elemental analysis. The 5 wt% VT (micro) having V=O bonds that showed stronger sulfur resistance, but 5 wt% VT (DT-51) having V–O–V bonds that lost their activity more severely than the former. They also summarized that the different vanadium species on the pore structure of TiO
2 have a significant effect on the sulfur poisoning during SCR reaction [
75].