Heterogeneous Catalytic Ozonation of Phenol by a Novel Binary Catalyst of Fe-Ni/MAC
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Fe-Ni/MAC Fabrication
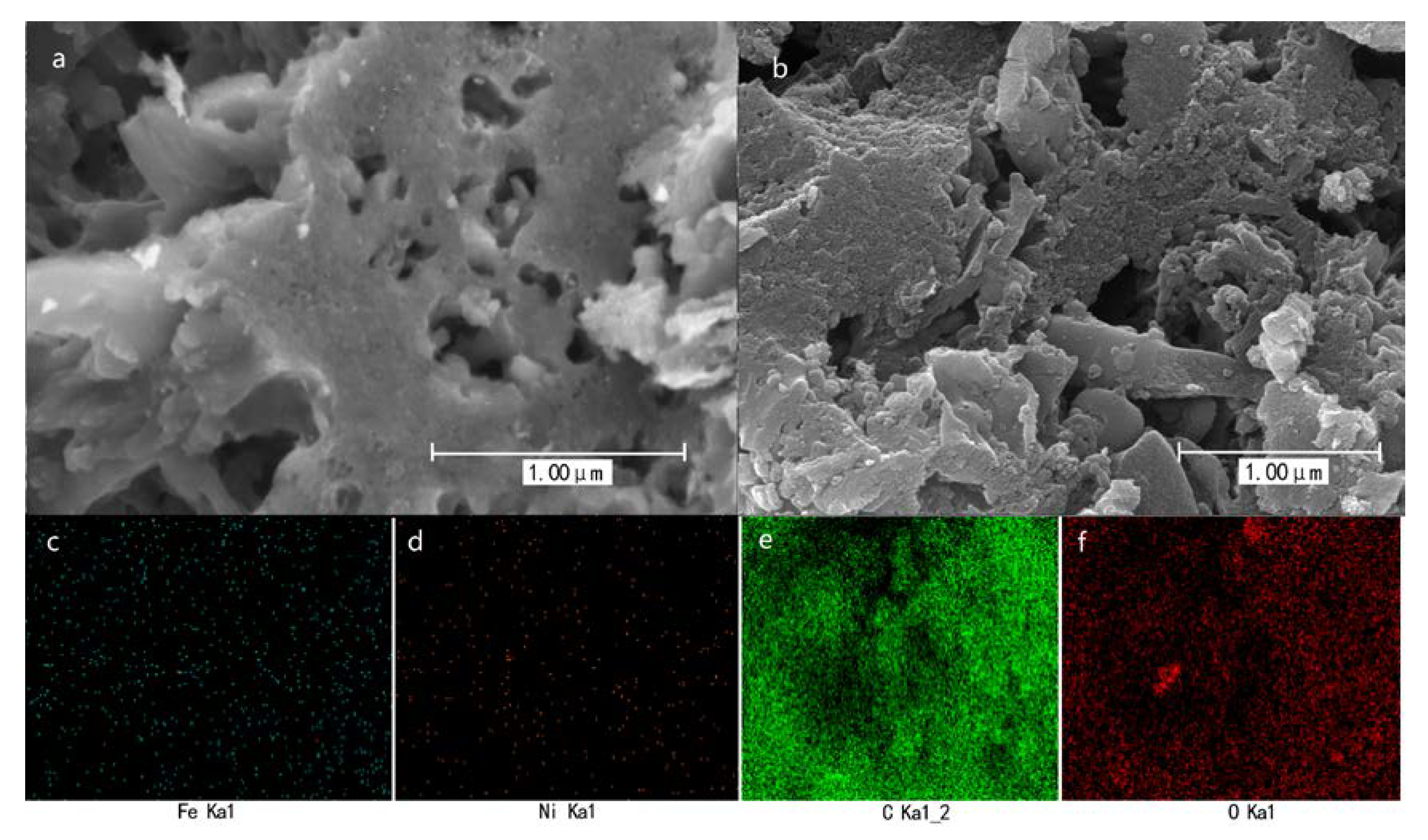
2.2. Characterization of Fe-Ni/MAC
2.3. Catalytic Performance of Fe-Ni/MAC
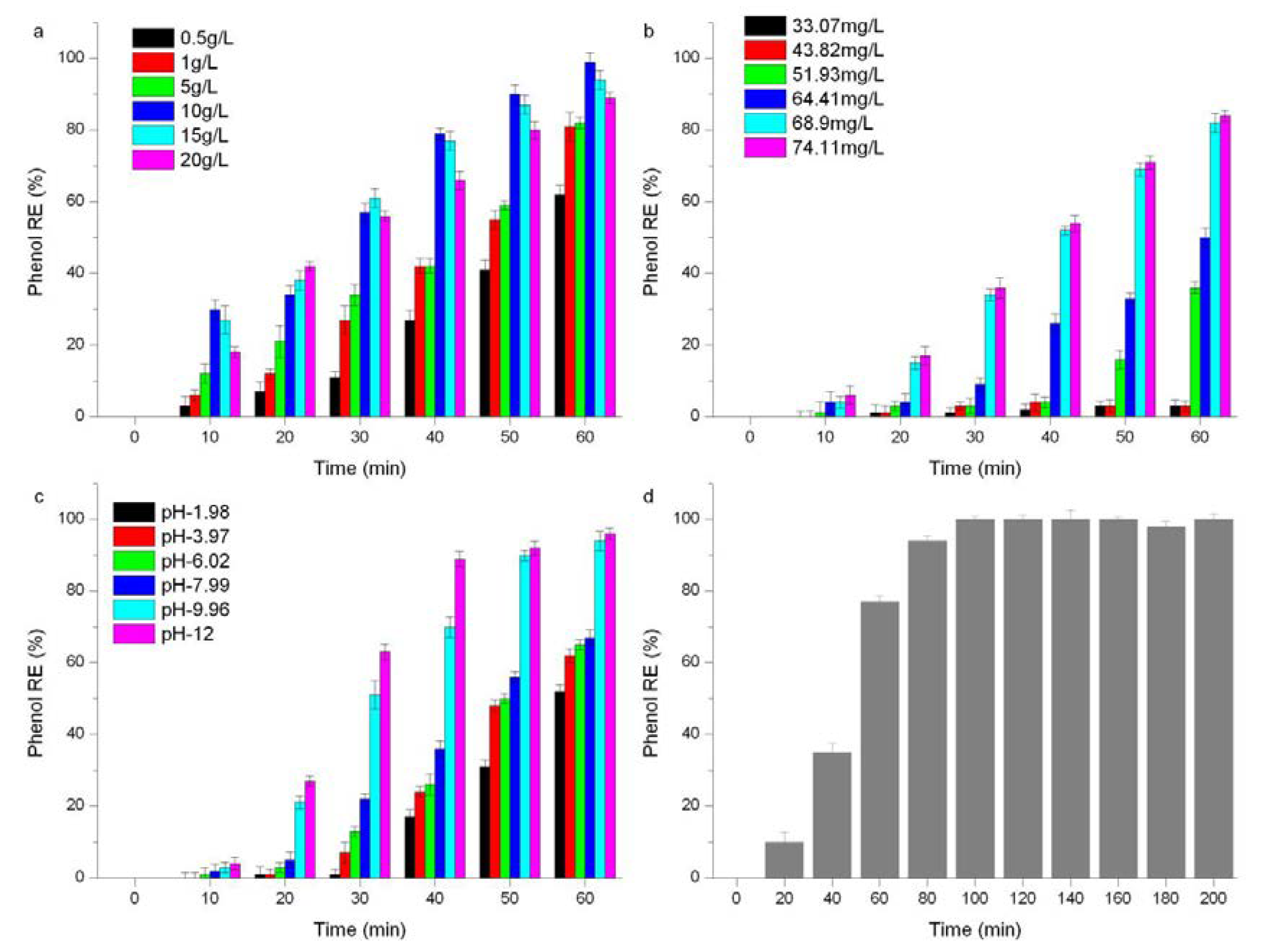
2.3.1. Key Parameters Affecting the Catalytic Performance
2.3.2. Reusability and Stability of Fe-Ni/MAC Catalyst
2.3.3. Real Phenol-Containing Wastewater Treatment by Fe-Ni/MAC
2.4. Investigation of Active Oxidative Species
2.5. Possible Mechanisms Discussion
3. Materials and Methods
3.1. Preparation of Fe-Ni/MAC
3.2. Characterization of Fe-Ni/MAC
3.3. Experimental Procedures
3.3.1. Optimization for Catalyst Preparation
3.3.2. Catalytic Activity Tests
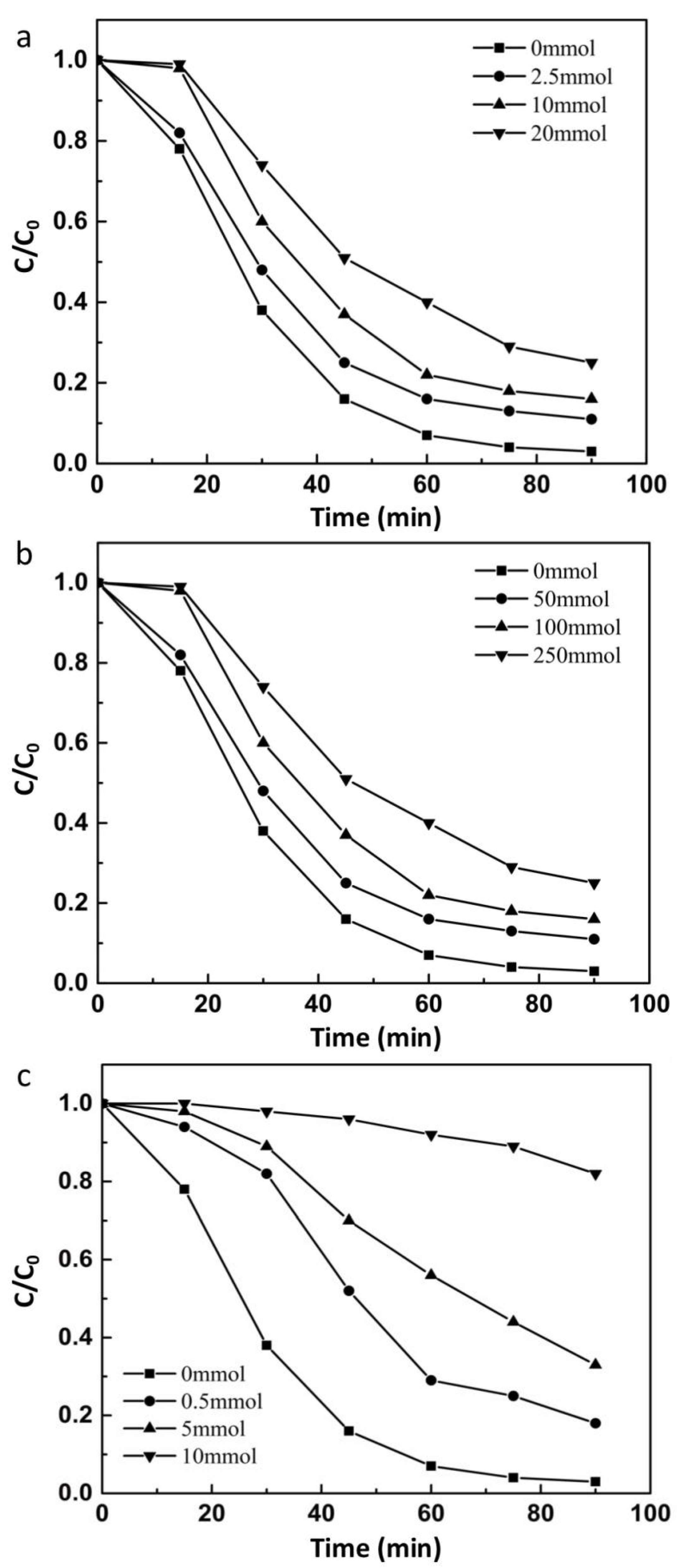
3.3.3. Radical Scavenging Experiments
3.4. Analytical Methods
4. Conclusions
- (1)
- The optimal conditions for catalytic ozonation obtained by response surface methodology (RSM) were catalyst 10 g/L, ozone 68 mg/L, pH 9 and reaction time 90 min.
- (2)
- Despite being reused six times, Fe-Ni/MAC catalysts were stable in the catalytic process with excellent performance and no secondary pollution.
- (3)
- The primary radical responsible for the degradation of phenol was proved to be (1O2), while other radicals played a minor role, based on radical scavenging experiments.
- (4)
- The fluorescence excitation emission matrices (EEMs) indicated that as much as 1243 mg/L phenol in the real wastewater was completely degraded.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Muhammad, S.; Saputra, E.; Sun, H.; Ang, H.-M.; Tadé, M.O.; Wang, S. Heterogeneous Catalytic Oxidation of Aqueous Phenol on Red Mud-Supported Cobalt Catalysts. Ind. Eng. Chem. Res. 2012, 51, 15351–15359. [Google Scholar] [CrossRef]
- Dohnal, V.; Fenclova, D. Air-Water Partitioning and Aqueous Solubility of Phenols. J. Chem. Eng. Data 1995, 40, 478–483. [Google Scholar] [CrossRef]
- Keith, L.; Telliard, W. ES&T Special Report: Priority pollutants: I-a perspective view. Environ. Sci. Technol. 1979, 13, 416–423. [Google Scholar]
- Huang, C.-P.; Huang, Y.-H. Comparison of catalytic decomposition of hydrogen peroxide and catalytic degradation of phenol by immobilized iron oxides. Appl. Catal. A Gen. 2008, 346, 140–148. [Google Scholar] [CrossRef]
- Barrault, J.; Abdellaoui, M.; Bouchoule, C.; Majesté, A.; Tatibouët, J.M.; Louloudi, A.; Papayannakos, N.; Gangas, N.H. Catalytic wet peroxide oxidation over mixed (Al–Fe) pillared clays. Appl. Catal. B Environ. 2000, 27, L225–L230. [Google Scholar] [CrossRef]
- Shukla, P.R.; Wang, S.; Sun, H.; Ang, H.M.; Tadé, M. Activated carbon supported cobalt catalysts for advanced oxidation of organic contaminants in aqueous solution. Appl. Catal. B Environ. 2010, 100, 529–534. [Google Scholar] [CrossRef]
- Esplugas, S.; Giménez, J.; Contreras, S.; Pascual, E.; Rodríguez, M. Comparison of different advanced oxidation processes for phenol degradation. Water Res. 2002, 36, 1034–1042. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.; Jiang, P.; Wang, G.; Zhang, J.; Zhang, C. ZnAl2O4 as a novel high-surface-area ozonation catalyst: One-step green synthesis, catalytic performance and mechanism. Chem. Eng. J. 2015, 260, 623–630. [Google Scholar] [CrossRef]
- Ma, Z.; Zhu, L.; Lu, X.; Xing, S.; Wu, Y.; Gao, Y. Catalytic ozonation of p-nitrophenol over mesoporous Mn–Co–Fe oxide. Sep. Purif. Technol. 2014, 133, 357–364. [Google Scholar] [CrossRef]
- Dadban Shahamat, Y.; Sadeghi, M.; Shahryari, A.; Okhovat, N.; Bahrami Asl, F.; Baneshi, M.M. Heterogeneous catalytic ozonation of 2,4-dinitrophenol in aqueous solution by magnetic carbonaceous nanocomposite: Catalytic activity and mechanism. Desalin. Water Treat. 2016, 57, 20447–20456. [Google Scholar] [CrossRef]
- Fajardo, A.S.; Martins, R.C.; Quinta-Ferreira, R.M. Treatment of a simulated phenolic effluent by heterogeneous catalytic ozonation using Pt/Al2O3. Environ. Technol. 2013, 34, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yang, H.; He, K.; Song, S.; Zhang, A. β-MnO2 nanowires: A novel ozonation catalyst for water treatment. Appl. Catal. B Environ. 2009, 85, 155–161. [Google Scholar] [CrossRef]
- Wei, X.; Shao, S.; Ding, X.; Jiao, W.; Liu, Y. Degradation of phenol with heterogeneous catalytic ozonation enhanced by high gravity technology. J. Clean. Prod. 2020, 248, 119179. [Google Scholar] [CrossRef]
- Shahamat, Y.D.; Farzadkia, M.; Nasseri, S.; Mahvi, A.H.; Gholami, M.; Esrafili, A. Magnetic heterogeneous catalytic ozonation: A new removal method for phenol in industrial wastewater. J. Environ. Health Sci. Eng. 2014, 12, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farzadkia, M.; Dadban Shahamat, Y.; Nasseri, S.; Mahvi, A.H.; Gholami, M.; Shahryari, A. Catalytic Ozonation of Phenolic Wastewater: Identification and Toxicity of Intermediates. J. Eng. 2014, 2014, 520929. [Google Scholar] [CrossRef] [Green Version]
- Aliyu, A.; Abdulkareem, A.S.; Kovo, A.S.; Abubakre, O.K.; Tijani, J.O.; Kariim, I. Synthesize multi-walled carbon nanotubes via catalytic chemical vapour deposition method on Fe-Ni bimetallic catalyst supported on kaolin. Carbon Lett. 2017, 21, 33–50. [Google Scholar] [CrossRef] [Green Version]
- Hong, E.; Bang, S.; Cho, J.H.; Jung, K.D.; Shin, C.-H. Reductive amination of isopropanol to monoisopropylamine over Ni-Fe/γ-Al2O3 catalysts: Synergetic effect of Ni-Fe alloy formation. Appl. Catal. A Gen. 2017, 542, 146–153. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Williams, P.T. Fe–Ni–MCM-41 Catalysts for Hydrogen-Rich Syngas Production from Waste Plastics by Pyrolysis–Catalytic Steam Reforming. Energy Fuels 2017, 31, 8497–8504. [Google Scholar] [CrossRef]
- Ni, S.-Q.; Yang, N. Cation exchange resin immobilized bimetallic nickel–iron nanoparticles to facilitate their application in pollutants degradation. J. Colloid Interface Sci. 2014, 420, 158–165. [Google Scholar] [CrossRef]
- Tian, D.; Liu, Z.; Li, D.; Shi, H.; Pan, W.; Cheng, Y. Bimetallic Ni–Fe total-methanation catalyst for the production of substitute natural gas under high pressure. Fuel 2013, 104, 224–229. [Google Scholar] [CrossRef]
- Hu, J.; Guo, Z.; Chu, W.; Li, L.; Lin, T. Carbon dioxide catalytic conversion to nano carbon material on the iron–nickel catalysts using CVD-IP method. J. Energy Chem. 2015, 24, 620–625. [Google Scholar] [CrossRef]
- Wu, M.-S.; Jao, C.-Y.; Chuang, F.-Y.; Chen, F.-Y. Carbon-encapsulated nickel-iron nanoparticles supported on nickel foam as a catalyst electrode for urea electrolysis. Electrochim. Acta 2017, 227, 210–216. [Google Scholar] [CrossRef]
- Singh, V.; Belova, L.; Singh, B.; Sharma, Y.C. Biodiesel production using a novel heterogeneous catalyst, magnesium zirconate (Mg2Zr5O12): Process optimization through response surface methodology (RSM). Energy Convers. Manag. 2018, 174, 198–207. [Google Scholar] [CrossRef]
- Haider, M.; Pakshirajan, K. Screening and optimization of media constituents for enhancing lipolytic activity by a soil microorganism using statistically designed experiments. Appl. Biochem. Biotechnol. 2007, 141, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Abu Hasan, H.; Abdullah, S.R.S.; Kamarudin, S.K.; Kofli, N.T. Response surface methodology for optimization of simultaneous COD, NH4+-N and Mn2+ removal from drinking water by biological aerated filter. Desalination 2011, 275, 50–61. [Google Scholar] [CrossRef]
- Coetzee, J.F. Recommended Methods for Purification of Solvents and Tests for Impurities, 1st ed.; Coetzee, J.F., Ed.; Pergamon: Oxford, UK, 1982; p. 68. [Google Scholar]
- Kustov, A.L.; Frey, A.M.; Larsen, K.E.; Johannessen, T.; Nørskov, J.K.; Christensen, C.H. CO methanation over supported bimetallic Ni–Fe catalysts: From computational studies towards catalyst optimization. Appl. Catal. A Gen. 2007, 320, 98–104. [Google Scholar] [CrossRef]
- Xu, F.; Deng, S.; Xu, J.; Zhang, W.; Wu, M.; Wang, B.; Huang, J.; Yu, G. Highly Active and Stable Ni–Fe Bimetal Prepared by Ball Milling for Catalytic Hydrodechlorination of 4-Chlorophenol. Environ. Sci. Technol. 2012, 46, 4576–4582. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, F.Z.; Zhang, Y.H.; Pan, Z.C.; Lai, B. Catalytic ozonation of N,N-dimethylacetamide (DMAC) in aqueous solution using nanoscaled magnetic CuFe2O4. Sep. Purif. Technol. 2018, 193, 368–377. [Google Scholar] [CrossRef]
- Olszewska, D. Application of XPS method in the research into Ni ion-modified montmorillonite as a SO2 sorbent. Fuel Process. Technol. 2012, 95, 90–95. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Ding, Y.; Xi, J.; Lu, G. Partial Oxidation of Ethanol to Hydrogen over Ni–Fe Catalysts. Catal. Lett. 2002, 81, 63–68. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kendelewicz, T.; Newberg, J.T.; Ketteler, G.; Starr, D.E.; Mysak, E.R.; Andersson, K.J.; Ogasawara, H.; Bluhm, H.; Salmeron, M.; et al. Water Adsorption on α-Fe2O3(0001) at near Ambient Conditions. J. Phys. Chem. C 2010, 114, 2256–2266. [Google Scholar] [CrossRef] [Green Version]
- Qi, F.; Chu, W.; Xu, B. Ozonation of phenacetin in associated with a magnetic catalyst CuFe2O4: The reaction and transformation. Chem. Eng. J. 2015, 262, 552–562. [Google Scholar] [CrossRef]
- Wu, J.; Gao, H.; Yao, S.; Chen, L.; Gao, Y.; Zhang, H. Degradation of Crystal Violet by catalytic ozonation using Fe/activated carbon catalyst. Sep. Purif. Technol. 2015, 147, 179–185. [Google Scholar] [CrossRef]
- Pan, F.; Luo, Y.; Fan, J.-J.; Liu, D.-C.; Fu, J. Degradation of Disperse Blue E-4R in Aqueous Solution by Zero-Valent Iron/Ozone. Clean Soilairwater 2012, 40, 422–427. [Google Scholar] [CrossRef]
- Chiang, H.-L.; Huang, C.P.; Chiang, P.C. The surface characteristics of activated carbon as affected by ozone and alkaline treatment. Chemosphere 2002, 47, 257–265. [Google Scholar] [CrossRef]
- Valdés, H.; Sánchez-Polo, M.; Rivera-Utrilla, J.; Zaror, C.A. Effect of Ozone Treatment on Surface Properties of Activated Carbon. Langmuir 2002, 18, 2111–2116. [Google Scholar] [CrossRef]
- Chiang, H.-L.; Chiang, P.C.; Huang, C.P. Ozonation of activated carbon and its effects on the adsorption of VOCs exemplified by methylethylketone and benzene. Chemosphere 2002, 47, 267–275. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Gómez-Serrano, V.; Álvarez, P.M.; Alvim-Ferraz, M.C.M.; Dias, J.M. Activated carbon modifications to enhance its water treatment applications. An overview. J. Hazard. Mater. 2011, 187, 1–23. [Google Scholar] [CrossRef]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Herath, N.; Ohtani, Y. Color and phenolic compounds reduction of Kraft Pulp Mill effluent by ozonation with some pretreatments. Am. J. Sci. Ind. Res. 2011, 2, 798–806. [Google Scholar] [CrossRef]
- Tu, Y.-J.; Njus, D.; Schlegel, H.B. A theoretical study of ascorbic acid oxidation and HOO˙/O2˙- radical scavenging. Org. Biomol. Chem. 2017, 15, 4417–4431. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ma, J.; Sun, Z.-Z. Oxidation products and pathway of ceramic honeycomb-catalyzed ozonation for the degradation of nitrobenzene in aqueous solution. Appl. Catal. B Environ. 2008, 79, 244–253. [Google Scholar] [CrossRef]
- Wade, A.M.; Tucker, H.N. Antioxidant characteristics of L-histidine 11The work described in this manuscript was partially sponsored and funded by Cytos Pharmaceuticals, LLC. J. Nutr. Biochem. 1998, 9, 308–315. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.; Wang, G.; Jiang, P.; Zhang, J.; Wu, L.; Li, K. Novel magnetically separable nanomaterials for heterogeneous catalytic ozonation of phenol pollutant: NiFe2O4 and their performances. Chem. Eng. J. 2013, 219, 295–302. [Google Scholar] [CrossRef]
- Huang, Y.; Cui, C.; Zhang, D.; Li, L.; Pan, D. Heterogeneous catalytic ozonation of dibutyl phthalate in aqueous solution in the presence of iron-loaded activated carbon. Chemosphere 2015, 119, 295–301. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Zhang, Q.; Gao, L.; Guo, J. Effects of calcination on the photocatalytic properties of nanosized TiO2 powders prepared by TiCl4 hydrolysis. Appl. Catal. B Environ. 2000, 26, 207–215. [Google Scholar] [CrossRef]
- Bader, H.; Hoigné, J. Determination of ozone in water by the indigo method. Water Res. 1981, 15, 449–456. [Google Scholar] [CrossRef]
- Ghaedi, M.; Tavallali, H.; Shokrollahi, A.; Zahedi, M.; Montazerozohori, M.; Soylak, M. Flame atomic absorption spectrometric determination of zinc, nickel, iron and lead in different matrixes after solid phase extraction on sodium dodecyl sulfate (SDS)-coated alumina as their bis (2-hydroxyacetophenone)-1,3-propanediimine chelates. J. Hazard. Mater. 2009, 166, 1441–1448. [Google Scholar] [CrossRef]




| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p > F |
|---|---|---|---|---|---|
| Model | 676.63 | 9 | 75.18 | 147.54 | <0.0001 |
| A | 89.21 | 1 | 89.21 | 175.08 | <0.0001 |
| B | 135.63 | 1 | 135.63 | 266.17 | <0.0001 |
| C | 5.99 | 1 | 5.99 | 11.76 | 0.0110 |
| AB | 13.12 | 1 | 13.12 | 25.74 | 0.0014 |
| AC | 0.4761 | 1 | 0.4761 | 0.9343 | 0.3659 |
| BC | 11.68 | 1 | 11.68 | 22.92 | 0.0020 |
| A2 | 163.34 | 1 | 163.34 | 320.56 | <0.0001 |
| B2 | 56.30 | 1 | 56.30 | 110.50 | <0.0001 |
| C2 | 35.74 | 1 | 35.74 | 70.14 | <0.0001 |
| Residual | 3.57 | 7 | 0.5096 | ||
| Lack of fit | 2.45 | 3 | 0.8172 | 2.93 | 0.1629 |
| Pure error | 1.12 | 4 | 0.2788 | ||
| Total | 680.20 | 16 |
| BET Surface Area (m2/g) | Langmuir Surface Area (m2/g) | Average Pore Volume (cm3/g) | Average Pore Radius (nm) |
|---|---|---|---|
| 502.68 | 887.87 | 0.31 | 2.45 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Shi, X.; Zhao, M.; Chu, S.; Xiao, J. Heterogeneous Catalytic Ozonation of Phenol by a Novel Binary Catalyst of Fe-Ni/MAC. Catalysts 2020, 10, 1123. https://doi.org/10.3390/catal10101123
Yang Y, Shi X, Zhao M, Chu S, Xiao J. Heterogeneous Catalytic Ozonation of Phenol by a Novel Binary Catalyst of Fe-Ni/MAC. Catalysts. 2020; 10(10):1123. https://doi.org/10.3390/catal10101123
Chicago/Turabian StyleYang, Yunlong, Xianwei Shi, Min Zhao, Shuyi Chu, and Jibo Xiao. 2020. "Heterogeneous Catalytic Ozonation of Phenol by a Novel Binary Catalyst of Fe-Ni/MAC" Catalysts 10, no. 10: 1123. https://doi.org/10.3390/catal10101123





