Screening of Activated Carbons for the Treatment of Highly Concentrated Phenol Solutions Using Catalytic Wet Peroxide Oxidation: The Effect of Iron Impurities on the Catalytic Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the AC Samples
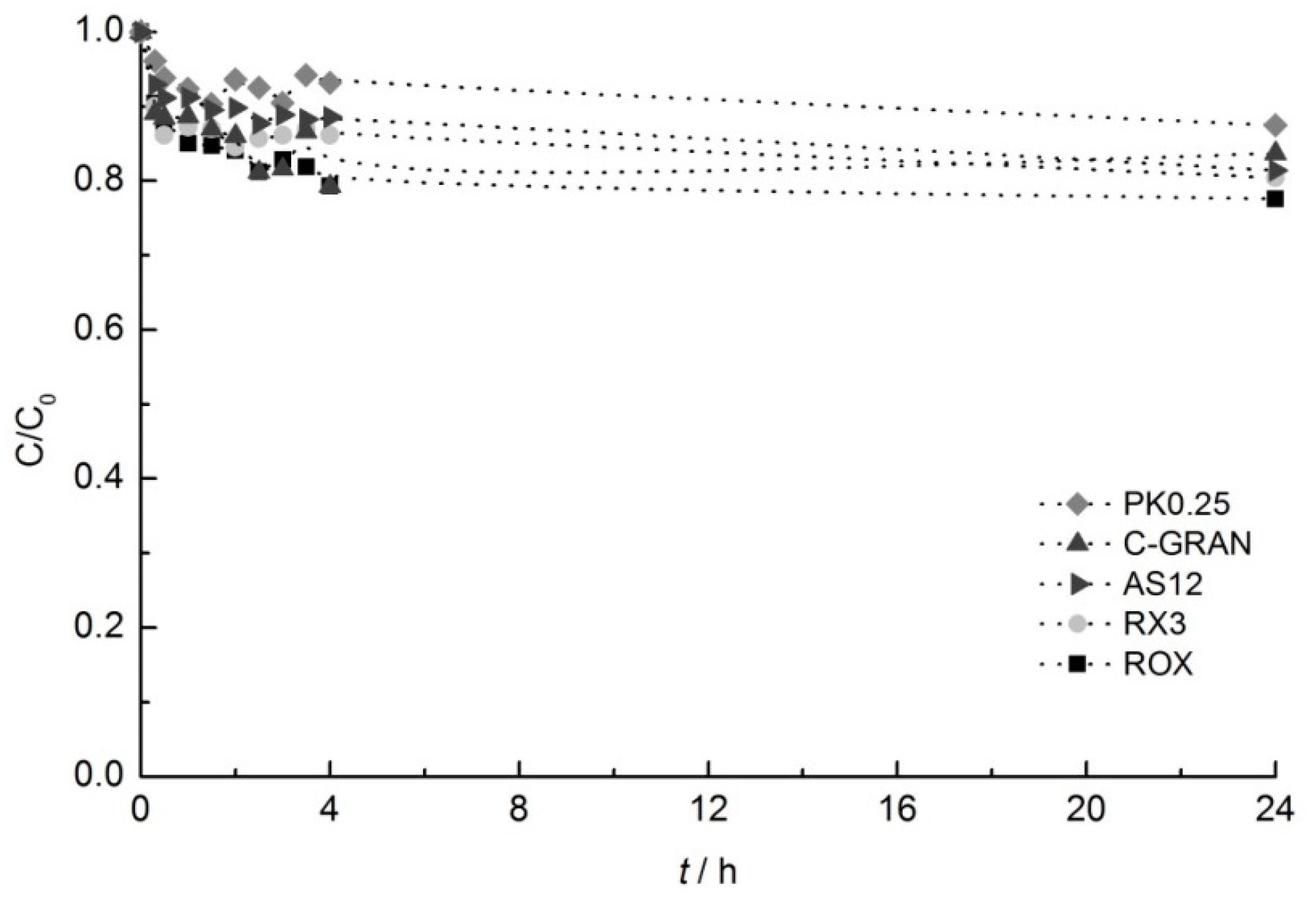
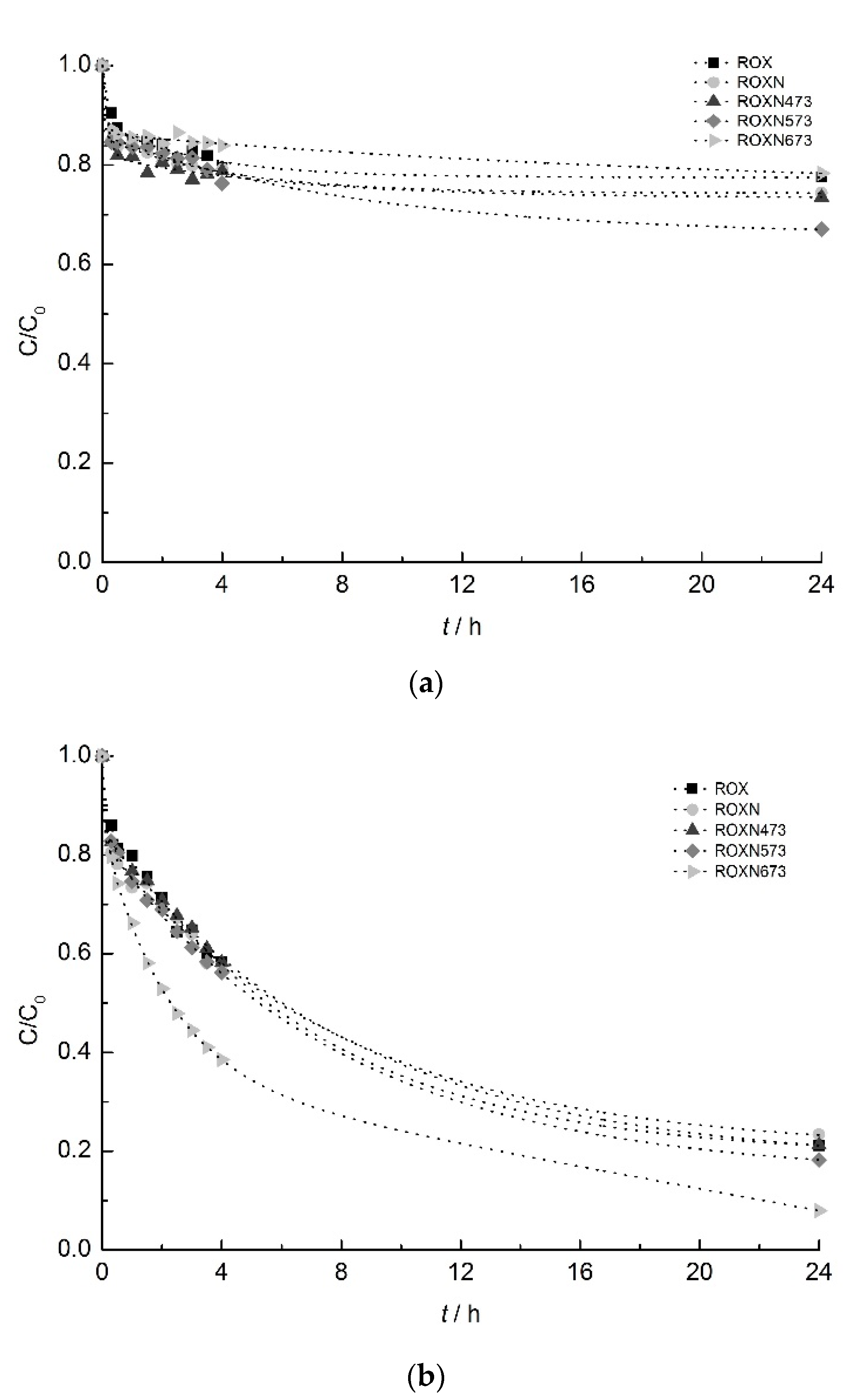
2.2. Adsorption Experiments
2.3. CWPO Experiments
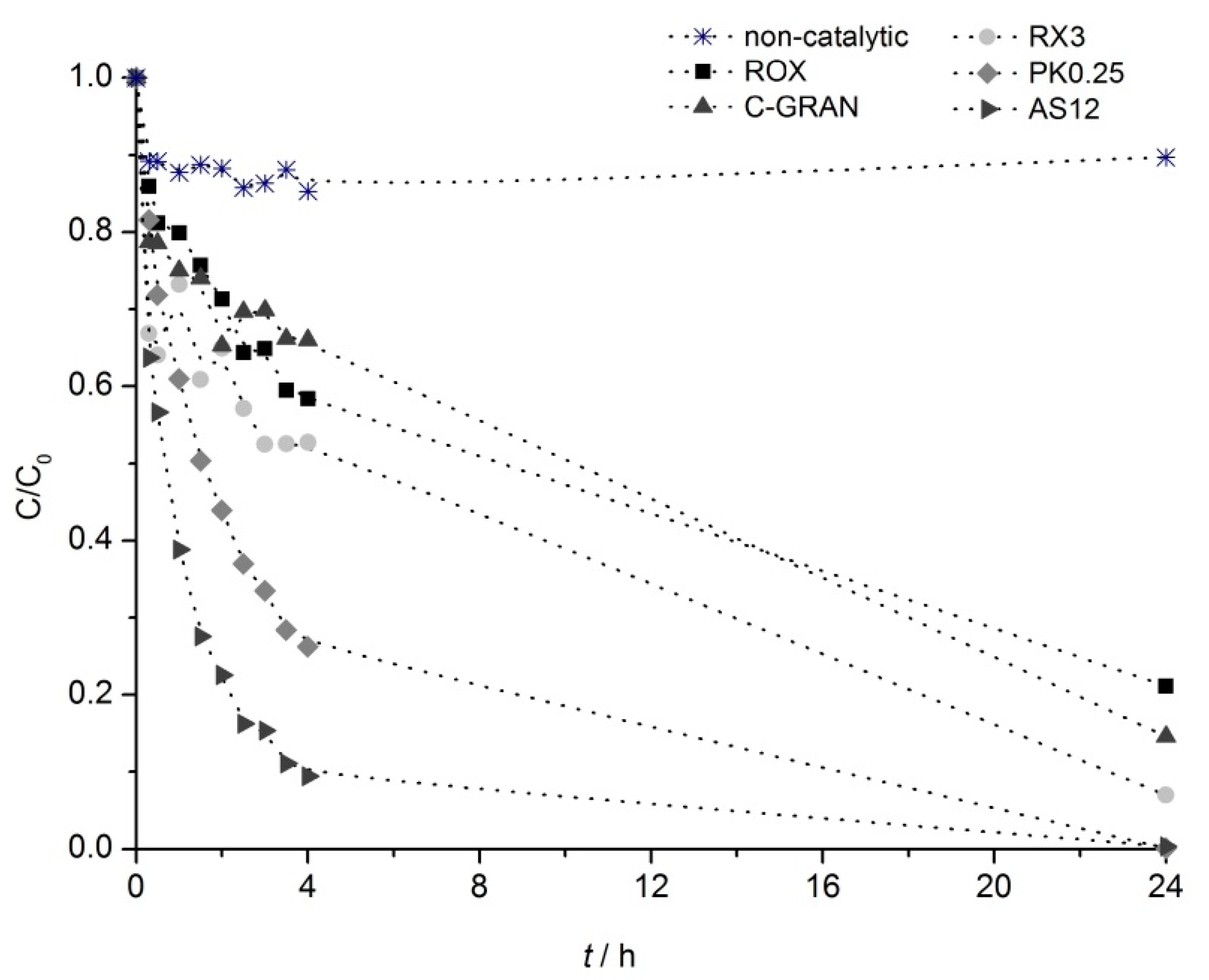
2.3.1. Influence of Surface Chemistry
2.3.2. Reaction Intermediates
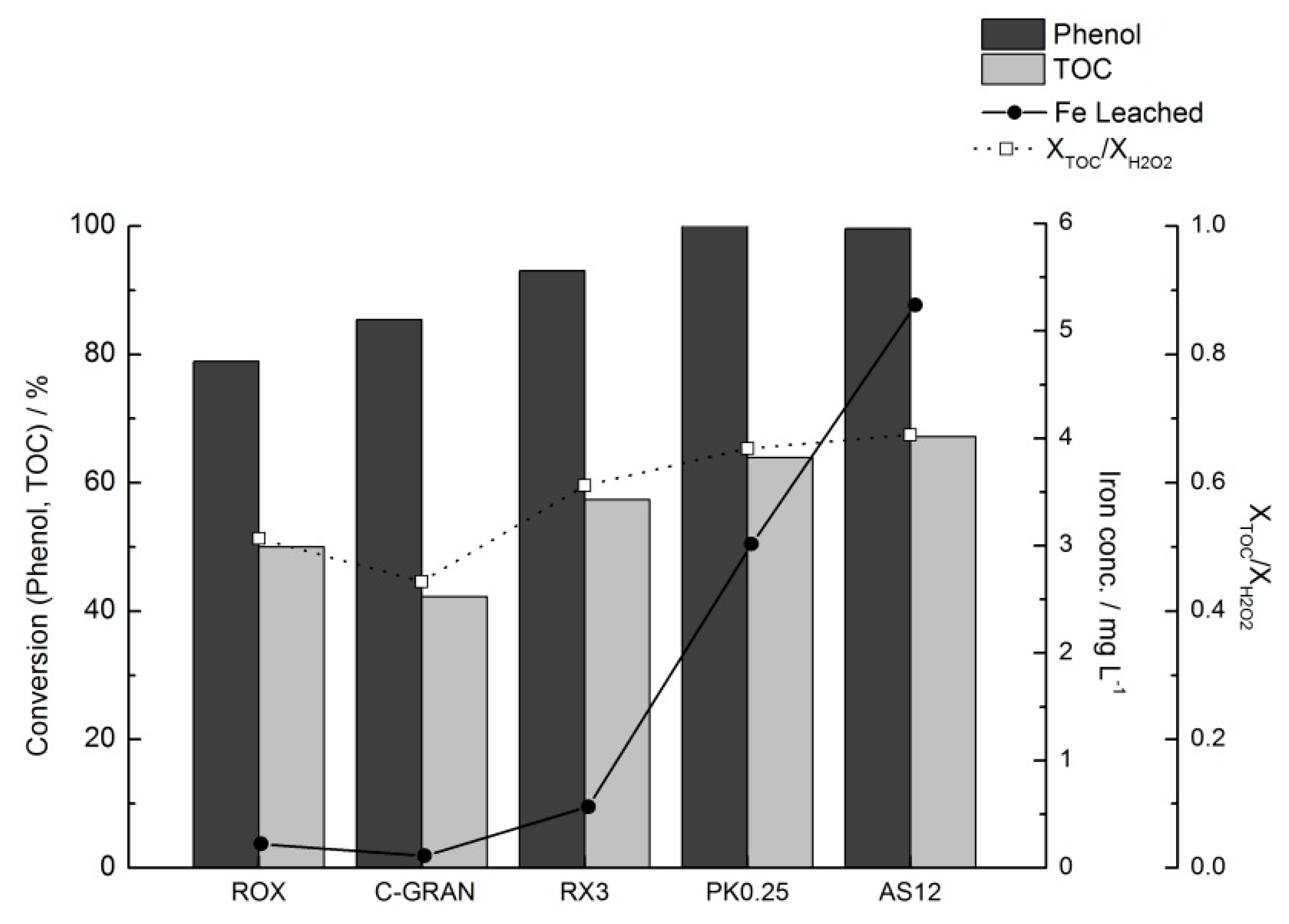
2.3.3. Catalyst Stability and Reusability Studies
3. Materials and Methods
3.1. Reactants
3.2. Activated Carbons
3.3. Characterization Techniques
3.4. Adsorption and CWPO Runs
3.5. Analytical Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rey, A.; Zazo, J.; Casas, J.; Bahamonde, A.; Rodriguez, J. Influence of the structural and surface characteristics of activated carbon on the catalytic decomposition of hydrogen peroxide. Appl. Catal. A Gen. 2011, 402, 146–155. [Google Scholar] [CrossRef]
- Rey, A.; Faraldos, M.; Casas, J.; Zazo, J.; Bahamonde, A.; Rodríguez, J. Catalytic wet peroxide oxidation of phenol over Fe/AC catalysts: Influence of iron precursor and activated carbon surface. Appl. Catal. B Environ. 2009, 86, 69–77. [Google Scholar] [CrossRef]
- Lücking, F.; Köser, H.; Jank, M.; Ritter, A. Iron powder, graphite and activated carbon as catalysts for the oxidation of 4-chlorophenol with hydrogen peroxide in aqueous solution. Water Res. 1998, 32, 2607–2614. [Google Scholar] [CrossRef]
- Khalil, L.B.; Girgis, B.S.; Tawfik, T.A.M. Decomposition of H2O2 on activated carbon obtained from olive stones. J. Chem. Technol. Biotechnol. 2001, 76, 1132–1140. [Google Scholar] [CrossRef]
- Serp, P.; Figueiredo, J.L. Carbon Materials for Catalysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Figueiredo, J.L. Functionalization of porous carbons for catalytic applications. J. Mater. Chem. A 2013, 1, 9351–9364. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.M.; Figueiredo, J.; Faria, J.; Gomes, H.T. Catalytic wet peroxide oxidation: A route towards the application of hybrid magnetic carbon nanocomposites for the degradation of organic pollutants. A review. Appl. Catal. B Environ. 2016, 187, 428–460. [Google Scholar] [CrossRef]
- Munoz, M.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation—A review. Appl. Catal. B 2015, 176–177, 249–265. [Google Scholar] [CrossRef] [Green Version]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Navalón, S.; Alvaro, M.; Garcia, H. Heterogeneous Fenton catalysts based on clays, silicas and zeolites. Appl. Catal. B Environ. 2010, 99, 1–26. [Google Scholar] [CrossRef]
- Dhaouadi, A.; Adhoum, N. Heterogeneous catalytic wet peroxide oxidation of paraquat in the presence of modified activated carbon. Appl. Catal. B Environ. 2010, 97, 227–235. [Google Scholar] [CrossRef]
- Domínguez, C.; Ocón, P.; Quintanilla, A.; Casas, J.; Rodriguez, J. Graphite and carbon black materials as catalysts for wet peroxide oxidation. Appl. Catal. B Environ. 2014, 144, 599–606. [Google Scholar] [CrossRef]
- Pinho, M.T.; Gomes, H.T.; Ribeiro, R.S.; Faria, J.L.; Silva, A.M.T. Carbon nanotubes as catalysts for catalytic wet peroxide oxidation of highly concentrated phenol solutions: Towards process intensification. Appl. Catal. B Environ. 2015, 165, 706–714. [Google Scholar] [CrossRef] [Green Version]
- Domínguez, C.; Ocón, P.; Quintanilla, A.; Casas, J.; Rodriguez, J. Highly efficient application of activated carbon as catalyst for wet peroxide oxidation. Appl. Catal. B Environ. 2013, 663–670. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.M.; Pastrana-Martínez, L.M.; Figueiredo, J.; Faria, J.; Gomes, H.T. Graphene-based materials for the catalytic wet peroxide oxidation of highly concentrated 4-nitrophenol solutions. Catal. Today 2015, 249, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, R.S.; Rodrigues, R.O.; Silva, A.M.; Tavares, P.B.; Carvalho, A.M.; Figueiredo, J.; Faria, J.; Gomes, H.T. Hybrid magnetic graphitic nanocomposites towards catalytic wet peroxide oxidation of the liquid effluent from a mechanical biological treatment plant for municipal solid waste. Appl. Catal. B Environ. 2017, 219, 645–657. [Google Scholar] [CrossRef] [Green Version]
- Gomes, H.T.; Miranda, S.M.; Sampaio, M.J.; Silva, A.M.; Faria, J.L. Activated carbons treated with sulphuric acid: Catalysts for catalytic wet peroxide oxidation. Catal. Today 2010, 151, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, J.L.; Pereira, M.F.R. The role of surface chemistry in catalysis with carbons. Catal. Today 2010, 150, 2–7. [Google Scholar] [CrossRef]
- Figueiredo, J.; Pereira, M.; Freitas, M.; Órfão, J. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.M.; Pinho, M.T.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Development of glycerol-based metal-free carbon materials for environmental catalytic applications. Catal. Today 2015, 240, 61–66. [Google Scholar] [CrossRef]
- Martínez, F.; Pariente, M.I.; Botas, J.A.; Melero, J.A.; Rubalcaba, A. Influence of preoxidizing treatments on the preparation of iron-containing activated carbons for catalytic wet peroxide oxidation of phenol. J. Chem. Technol. Biotechnol. 2012, 87, 880–886. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Characterization of Active Sites on Carbon Catalysts. Ind. Eng. Chem. Res. 2007, 46, 4110–4115. [Google Scholar] [CrossRef] [Green Version]
- Vieira, O.; Ribeiro, R.S.; Pedrosa, M.; Lado Ribeiro, A.R.; Silva, A.M.T. Nitrogen-doped reduced graphene oxide—PVDF nanocomposite membrane for persulfate activation and degradation of water organic micropollutants. Chem. Eng. J. 2020, 402, 126117. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Rivera-Utrilla, J.; López-Ramón, M.V.; Carrasco-Marin, F. Adsorption of some substituted phenols on activated carbons from a bituminous coal. Carbon 1995, 33, 845–851. [Google Scholar] [CrossRef]
- Terzyk, A.P. Further insights into the role of carbon surface functionalities in the mechanism of phenol adsorption. J. Colloid Interface Sci. 2003, 268, 301–329. [Google Scholar] [CrossRef]
- Martin-Martinez, M.; Ribeiro, R.S.; Machado, B.F.; Serp, P.; Morales-Torres, S.; Silva, A.M.; Figueiredo, J.; Faria, J.; Gomes, H.T. Role of Nitrogen Doping on the Performance of Carbon Nanotube Catalysts: A Catalytic Wet Peroxide Oxidation Application. ChemCatChem 2016, 8, 2068–2078. [Google Scholar] [CrossRef]
- Munoz, M.; Domínguez, C.M.; De Pedro, Z.; Quintanilla, A.; Casas, J.; Rodriguez, J.J.; Munoz, M. Degradation of imidazolium-based ionic liquids by catalytic wet peroxide oxidation with carbon and magnetic iron catalysts. J. Chem. Technol. Biotechnol. 2016, 91, 2882–2887. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, R.A.; Wallau, M.; Arends, I.W.C.E.; Schuchardt, U. Heterogeneous Catalysts for Liquid-Phase Oxidations: Philosophers’ Stones or Trojan Horses? Acc. Chem. Res. 1998, 31, 485–493. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Frontistis, Z.; Mantzavinos, D.; Venieri, D.; Antonopoulou, M.; Konstantinou, I.K.; Silva, A.M.; Faria, J.L.; Gomes, H.T. Magnetic carbon xerogels for the catalytic wet peroxide oxidation of sulfamethoxazole in environmentally relevant water matrices. Appl. Catal. B Environ. 2016, 199, 170–186. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Barrault, J.; Tatibouet, J.-M.; Papayannakos, N. Catalytic wet peroxide oxidation of phenol over pillared clays containing iron or copper species. Comptes Rendus de l’Académie des Sciences—Ser. IIC—Chem. 2000, 3, 777–783. [Google Scholar] [CrossRef]
- Pouran, S.R.; Raman, A.A.A.; Daud, W.M.A.W. Review on the application of modified iron oxides as heterogeneous catalysts in Fenton reactions. J. Clean. Prod. 2014, 64, 24–35. [Google Scholar] [CrossRef] [Green Version]



| Material | SBET/ m2 g−1 | Vtotal/ cm3 g−1 | Vmic/ cm3 g−1 | Vmic/Vtotal | pHPZC |
|---|---|---|---|---|---|
| ROX | 1121 | 0.681 | 0.466 | 0.685 | 6.3 |
| RX3 | 1352 | 0.663 | 0.588 | 0.886 | 6.8 |
| C-GRAN | 1273 | 0.918 | 0.499 | 0.543 | 2.8 |
| PK0.25 | 661 | 0.424 | 0.271 | 0.640 | 7.8 |
| AS12 | 1276 | 0.594 | 0.525 | 0.883 | 7.4 |
| ROXN | 1100 | 0.649 | 0.463 | 0.713 | 8.2 |
| ROXN473 | 1063 | 0.627 | 0.436 | 0.695 | 7.4 |
| ROXN573 | 1065 | 0.619 | 0.441 | 0.712 | 7.2 |
| ROXN673 | 1149 | 0.730 | 0.528 | 0.723 | 4.1 |
| Material | [CO]/ µmol g−1 | [CO2]/ µmol g−1 | CO/CO2 | O/ wt % | [Iron]/ wt % |
|---|---|---|---|---|---|
| ROX | 2058 | 1364 | 1.5 | 7.7 | 0.04 |
| RX3 | 816 | 735 | 1.1 | 3.7 | 0.08 |
| C-GRAN | 3128 | 7736 | 0.4 | 29.8 | 0.04 |
| PK0.25 | 516 | 414 | 1.2 | 2.2 | 0.17 |
| AS12 | 2946 | 2394 | 1.2 | 12.4 | 0.29 |
| ROXN | 657 | 606 | 1.1 | 3.0 | 0.06 |
| ROXN473 | 935 | 472 | 2.0 | 3.0 | 0.10 |
| ROXN573 | 1995 | 795 | 2.5 | 5.7 | 0.04 |
| ROXN673 | 1303 | 6266 | 0.2 | 22.1 | 0.06 |
| Material | XPhenol/% | XPhenol/mg g−1 | ||
|---|---|---|---|---|
| Adsorption | CWPO | Adsorption | CWPO | |
| ROX | 22 | 79 | 396 | 1422 |
| RX3 | 20 | 93 | 360 | 1674 |
| C-GRAN | 16 | 85 | 288 | 1530 |
| PK0.25 | 12 | 100 | 216 | 1800 |
| AS12 | 19 | 100 | 342 | 1800 |
| ROXN | 20 | 77 | 360 | 1386 |
| ROXN473 | 19 | 79 | 342 | 1422 |
| ROXN573 | 27 | 82 | 486 | 1476 |
| ROXN673 | 14 | 92 | 252 | 1656 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinho, M.T.; Ribeiro, R.S.; Gomes, H.T.; Faria, J.L.; Silva, A.M.T. Screening of Activated Carbons for the Treatment of Highly Concentrated Phenol Solutions Using Catalytic Wet Peroxide Oxidation: The Effect of Iron Impurities on the Catalytic Activity. Catalysts 2020, 10, 1318. https://doi.org/10.3390/catal10111318
Pinho MT, Ribeiro RS, Gomes HT, Faria JL, Silva AMT. Screening of Activated Carbons for the Treatment of Highly Concentrated Phenol Solutions Using Catalytic Wet Peroxide Oxidation: The Effect of Iron Impurities on the Catalytic Activity. Catalysts. 2020; 10(11):1318. https://doi.org/10.3390/catal10111318
Chicago/Turabian StylePinho, Maria T., Rui S. Ribeiro, Helder T. Gomes, Joaquim L. Faria, and Adrián M. T. Silva. 2020. "Screening of Activated Carbons for the Treatment of Highly Concentrated Phenol Solutions Using Catalytic Wet Peroxide Oxidation: The Effect of Iron Impurities on the Catalytic Activity" Catalysts 10, no. 11: 1318. https://doi.org/10.3390/catal10111318