Hydrothermally Carbonized Waste Biomass as Electrocatalyst Support for α-MnO2 in Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Results and Discussion
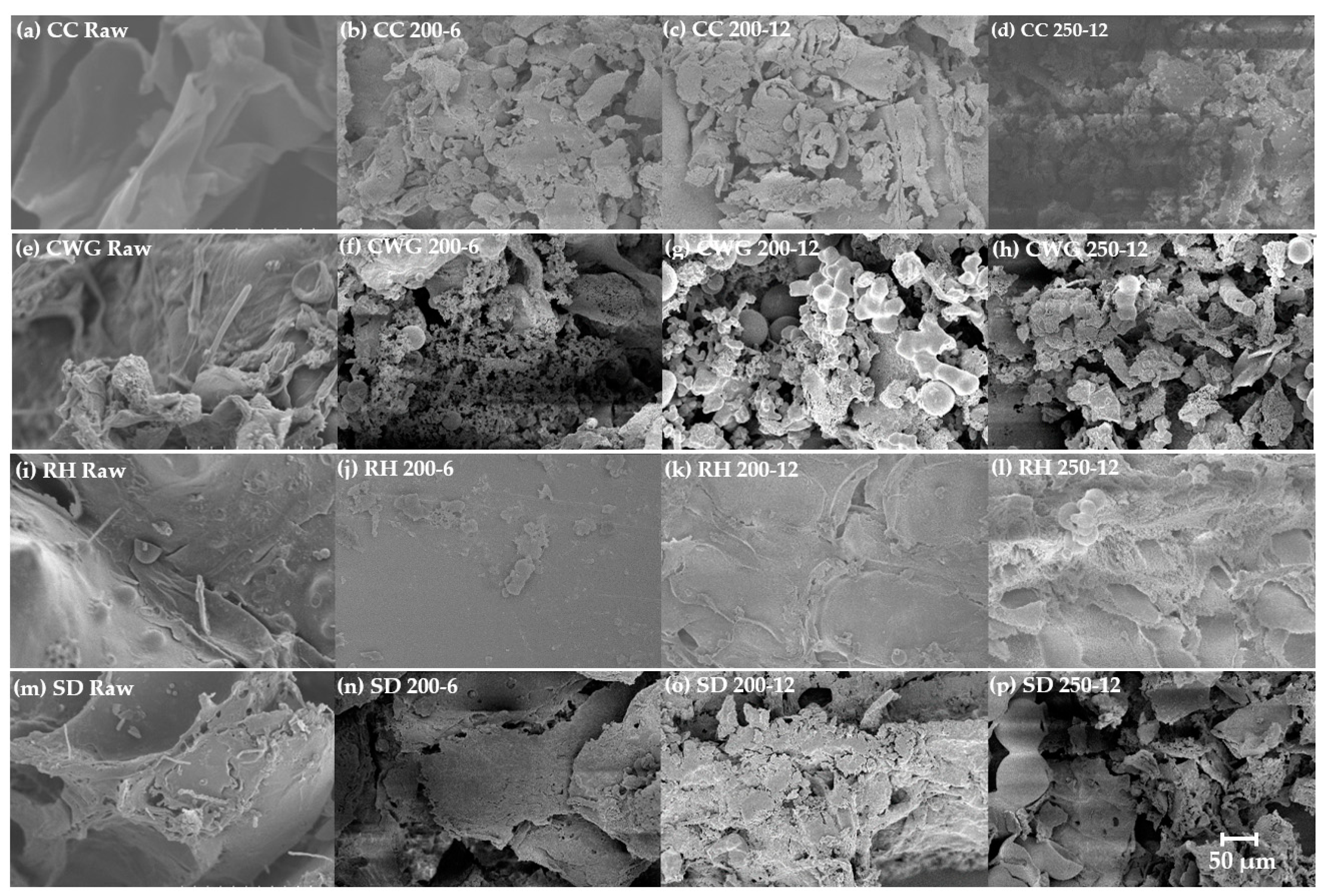
2.1. Hydrothermal Carbonization
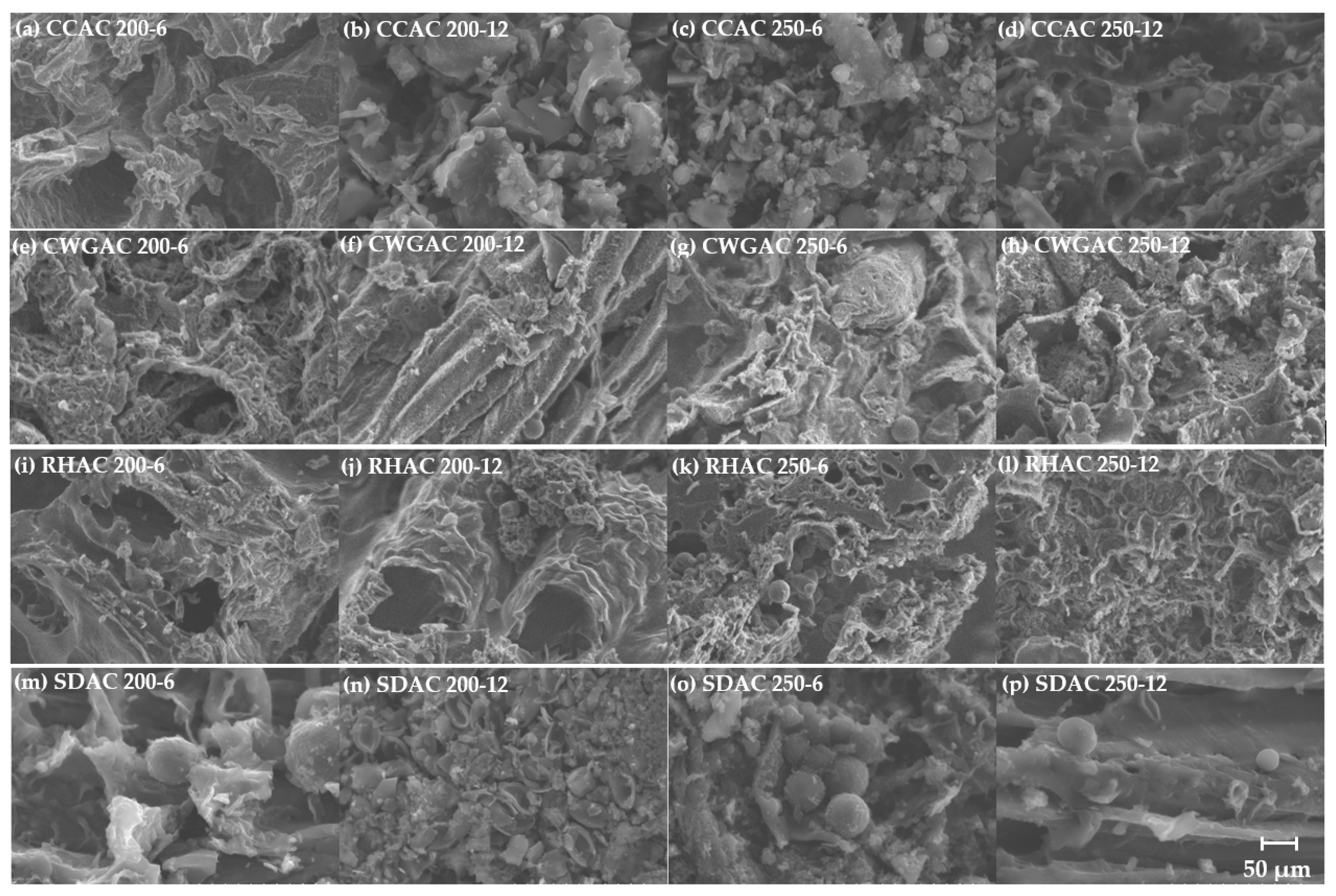
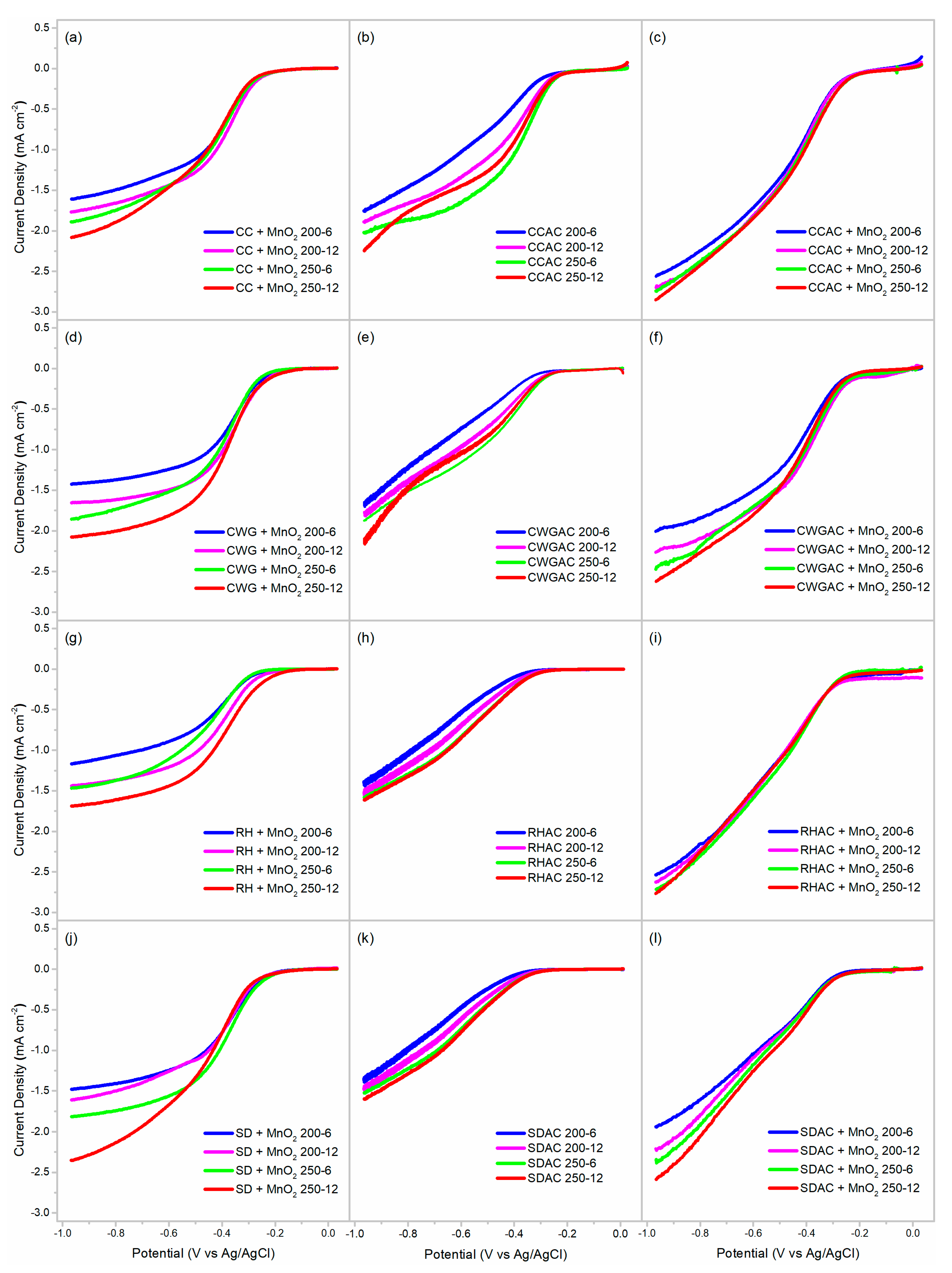
2.2. Activation
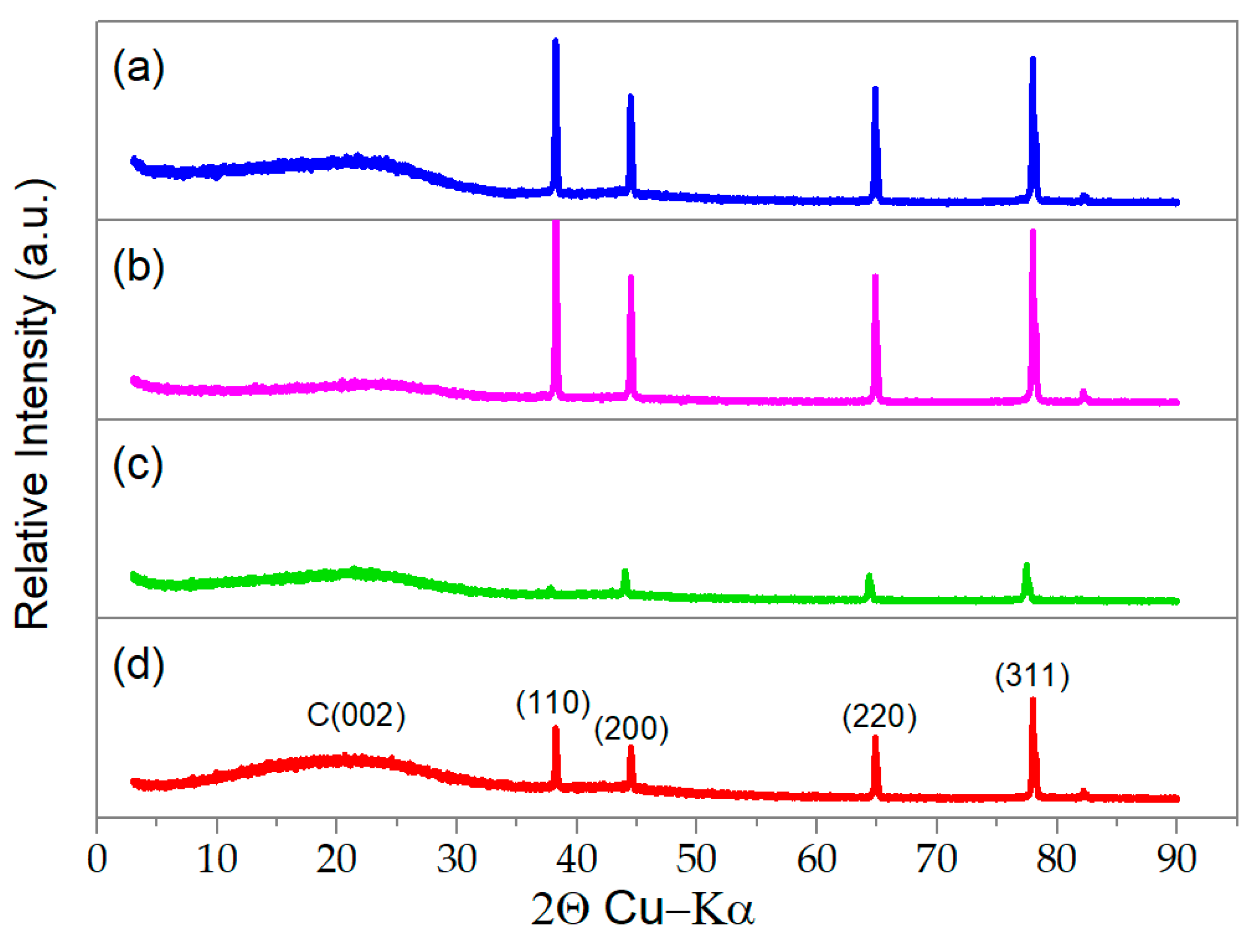
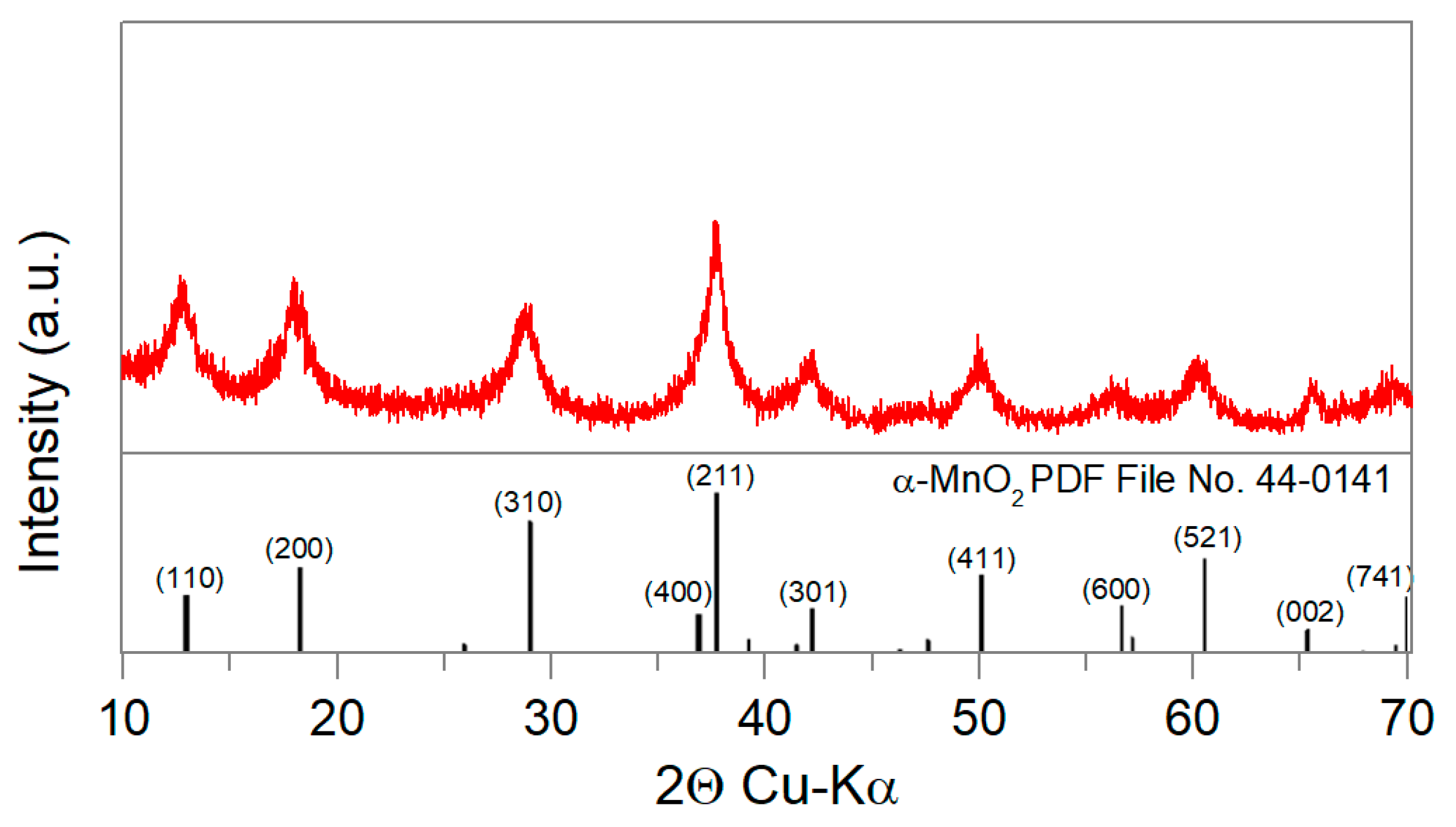
2.3. Incorporation of MnO2
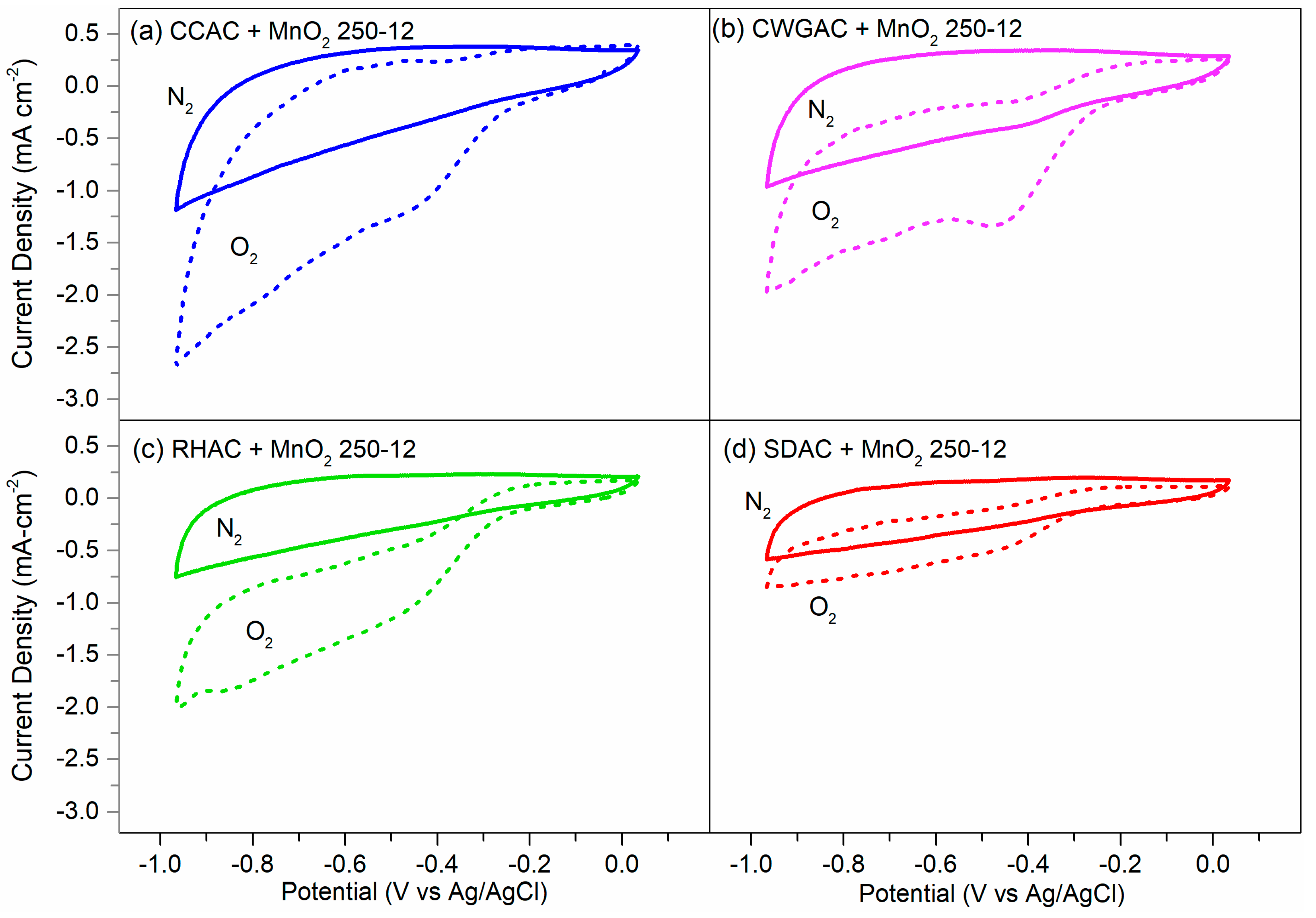
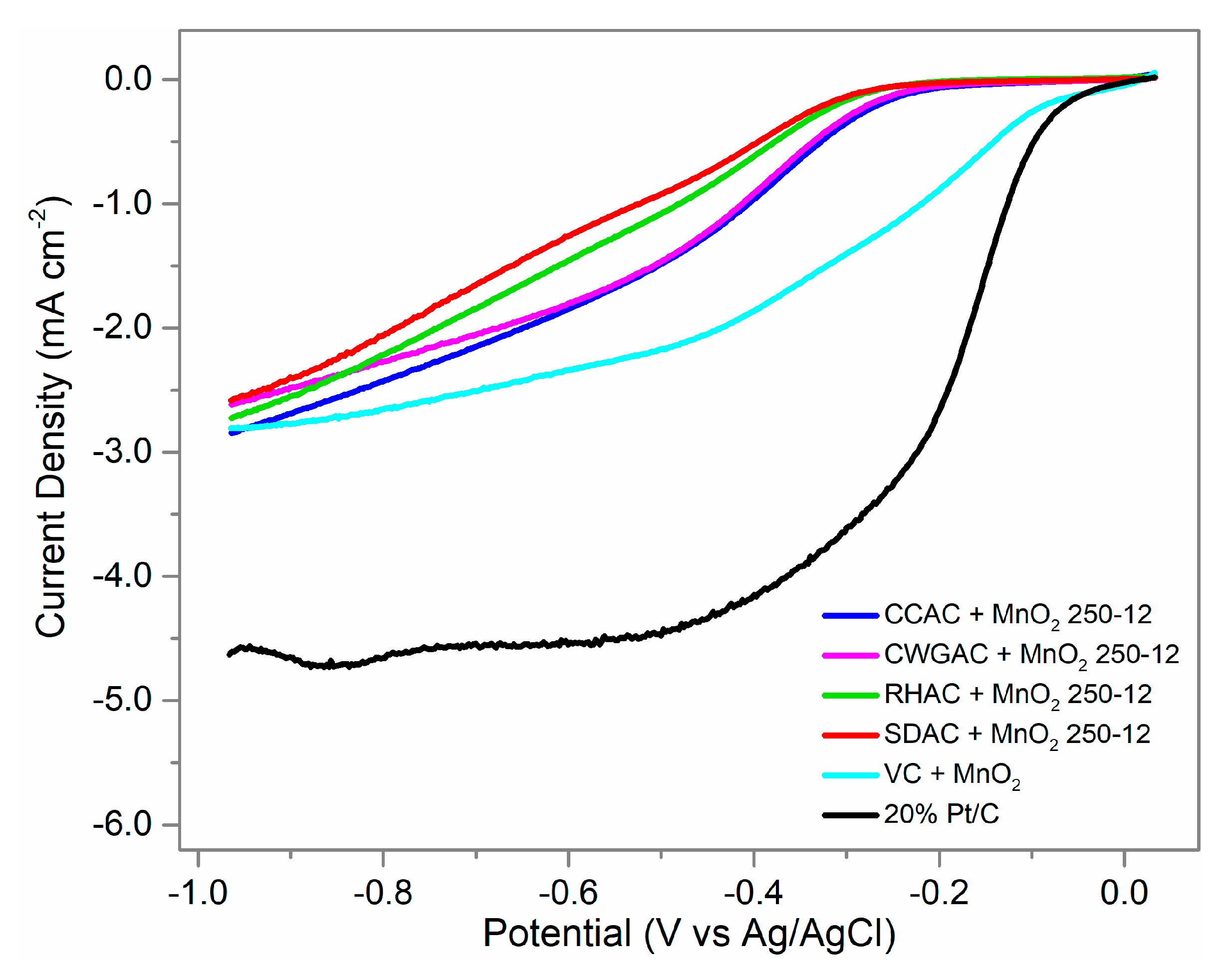
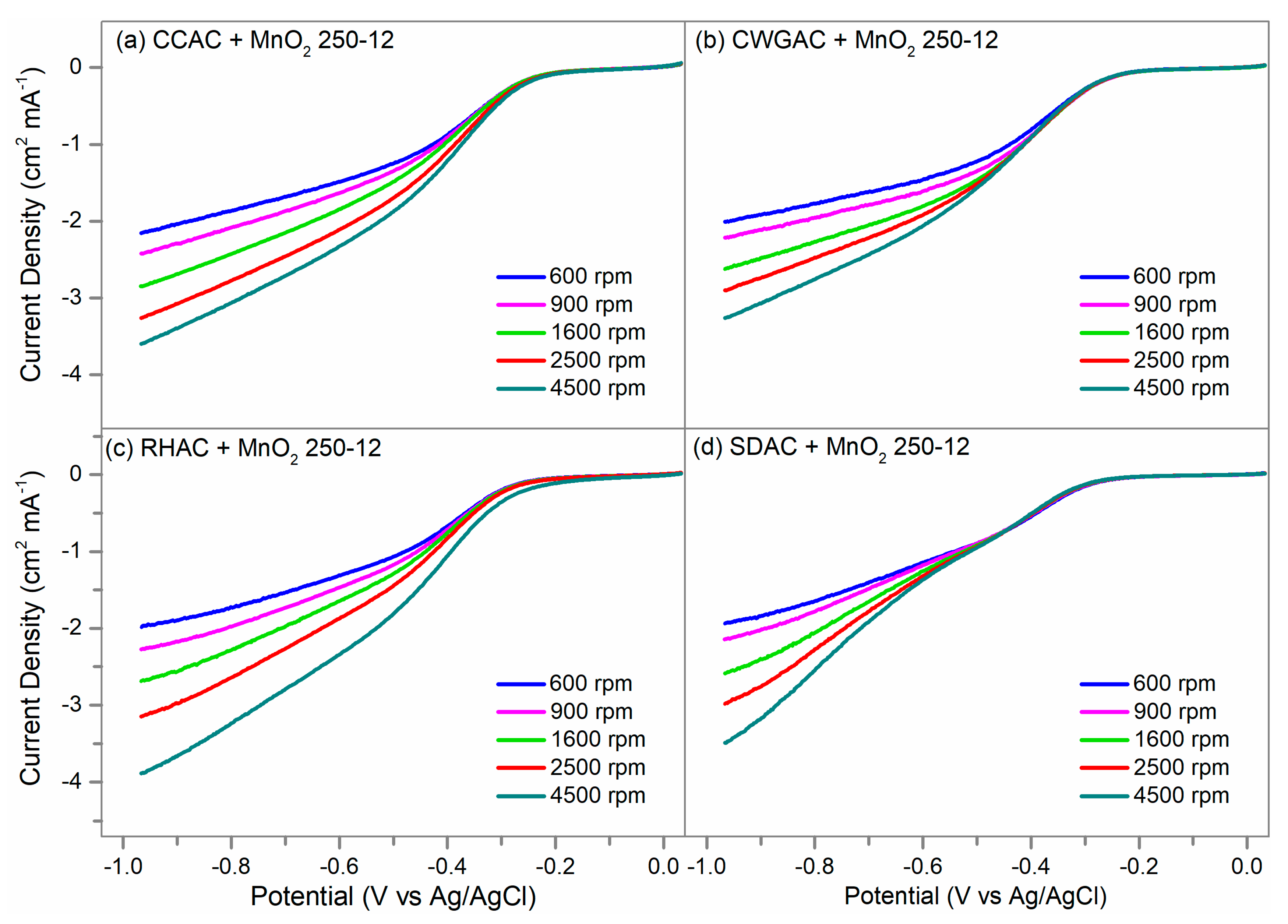
2.4. ORR Activity
3. Materials and Methods
3.1. Hydrothermal Carbonization
3.2. Activation
3.3. Incorporation of MnO2
3.4. Material Characterization
3.5. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davari, E.; Ivey, D.G. Bifunctional electrocatalysts for Zn–air batteries. Sustain. Energ. Fuels 2018, 2, 39–67. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 2058–7546. [Google Scholar] [CrossRef]
- Liu, S.; Han, W.; Cui, B.; Liu, X.; Sun, H.; Zhang, J.; Lefler, M.; Licht, S. Rechargeable zinc air batteries and highly improved performance through potassium hydroxide addition to the molten carbonate eutectic electrolyte. J. Electrochem. Soc. 2018, 165, A149–A154. [Google Scholar] [CrossRef] [Green Version]
- Greeley, J.; Stephens, I.E.L.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Nørskov, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef]
- Osgood, H.; Devaguptapu, S.V.; Xu, H.; Cho, J.P.; Wu, G. Transition metal (Fe, Co, Ni and Mn) oxides for oxygen reduction and evolution bifunctional catalysts in alkaline media. Nano. Today 2016, 11, 601–625. [Google Scholar] [CrossRef]
- Hu, C.; Dai, L. Carbon-based metal-free catalysts for electrocatalysis beyond the ORR. Angew. Chem. Int. Ed. 2016, 55, 11736–11758. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, D.; Xu, J.; Zhang, X. Oxygen electrocatalysts in metal–air batteries: From aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef]
- Li, L. Anchoring Mn3O4 nanoparticles on oxygen functionalized carbon nanotubes as bifunctional catalyst for rechargeable zinc-Air battery. Acs Appl. Energy Mater. 2018, 1, 963–969. [Google Scholar] [CrossRef]
- He, Q.; Li, Q.; Khene, S.; Ren, X.; Lopez-Suarez, F.E.; Lozano-Castello, D.; Bueno-Lopez, A. high-loading cobalt oxide coupled with nitrogen-doped graphene for oxygen reduction in anion-exchange-membrane alkaline fuel cells. J. Phys. Chem. C 2013, 117, 8697–8707. [Google Scholar] [CrossRef]
- Wang, M.; Chen, K.; Liu, J.; He, Q.; Li, G.; Li, F. Efficiently enhancing electrocatalytic activity of α-MnO2 nanorods/N-doped ketjenblack carbon for oxygen reduction reaction and oxygen evolution reaction using facile regulated hydrothermal treatment. Catalysts 2018, 8, 138. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Su, Y.; Liang, J.; Tao, Z.; Chen, J. MnO2-based nanostructures as catalysts for electrochemical oxygen reduction in alkaline media. Chem. Mater. 2010, 22, 898–905. [Google Scholar] [CrossRef]
- Lima, F.H.B.; Calegaro, M.L.; Ticianelli, E.A. Electrocatalytic activity of manganese oxides prepared by thermal decomposition for oxygen reduction. Electrochim. Acta 2007, 52, 3732–3738. [Google Scholar] [CrossRef]
- Moon, J.; Munakata, H.; Kajihara, K.; Nakamura, K. Hydrothermal synthesis of manganese dioxide nanoparticles as cathode material for rechargeable batteries. Electrochemistry 2013, 81, 2–6. [Google Scholar] [CrossRef] [Green Version]
- Valim, R.B.; Santos, C.M.; Lanza, M.; Machado, S.A.; Lima, F.H.B.; Calegaro, M. Oxygen reduction reaction catalyzed by ε-MnO2: Influence of the crystalline structure on the reaction mechanism. Electrochim. Acta 2012, 85, 423–431. [Google Scholar] [CrossRef]
- Xu, N.; Nie, Q.; Luo, L.; Yao, C.Z.; Gong, Q.; Liu, Y.; Zhou, X.; Qiao, J. Controllable hortensia-like MnO2 synergized with carbon nanotubes as an efficient electrocatalyst for long-term metal–air batteries. Acs Appl. Mater. Interfaces 2018, 11, 578–587. [Google Scholar] [CrossRef]
- Smith, M.W.; Shekhawat, D. Fuel Cells: Technologies for Fuel Processing. In Chapter 5—Catalytic Partial Oxidation; Shekhawat, D., Spivey, J.J., Berry, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 73–128. [Google Scholar] [CrossRef]
- Wei, Q.; Fu, Y.; Zhang, G.; Sun, S. Rational design of carbon-based oxygen electrocatalysts for zinc–air batteries. Curr. Opin. Electrochem. 2017, 4, 45–59. [Google Scholar] [CrossRef]
- Fu, G.; Tang, Y.; Lee, J.M. Recent advances in carbon-based bifunctional oxygen electrocatalysts for Zn−air batteries. ChemElectroChem 2018, 5, 1424–1434. [Google Scholar] [CrossRef]
- Kasturi, P.R.; Araunchander, A.; Kalpana, D.; Selvan, R.K. Bio-derived carbon as an efficient supporting electrocatalyst for the oxygen reduction reaction. J. Phys. Chem. Solids 2019, 124, 305–311. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, Y.; Song, N.; Li, X. Biomass-derived renewable carbon materials for electrochemical energy storage. Mater. Res. Lett 2017, 5, 69–88. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A. Agricultural residues as precursors for activated carbon production – A review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Wu, H.; Geng, J.; Ge, H.; Guo, Z.; Wang, Y.; Zheng, G. Egg-derived mesoporous carbon microspheres as bifunctional oxygen evolution and oxygen reduction electrocatalysts. Adv. Energy Mater. 2016, 6, 1600794. [Google Scholar] [CrossRef]
- Li, B.; Geng, D.; Lee, S.; Ge, X.; Chai, J.; Wang, Z.; Zhang, J.; Liu, Z.; Hor, T.; Zong, Y. Eggplant-derived microporous carbon sheets: Towards mass production of efficient bifunctional oxygen electrocatalyst at low cost for rechargeable Zn-Air Batteries. ChemComm 2015, 51, 8841–8844. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Wang, Q.; Zhang, L.; Liang, H.W.; Chen, P.; Yu, S.H. N-, P-and Fe-tridoped nanoporous carbon derived from plant biomass: An excellent oxygen reduction electrocatalyst for zinc–air batteries. J. Mater. Chem. A 2016, 4, 8602–8609. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Zhao, P.; Shen, Y.; Ge, S.; Yoshikawa, K. Energy recycling from sewage sludge by producing solid biofuel with hydrothermal carbonization. Energy Convers Manag 2014, 78, 815–821. [Google Scholar] [CrossRef] [Green Version]
- Knez, Ž.; Markočič, E.; Hrnčič, M.K.; Ravber, M.; Škerget, M. High pressure water reforming of biomass for energy and chemicals: A short review. J. Supercrit Fluids 2015, 96, 46–52. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels. Bioprod. Bioref. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Xu, Q.; Qian, Q.; Quek, A.; Ai, N.; Zeng, G.; Wang, J. Hydrothermal carbonization of macroalgae and the effects of experimental parameters on the properties of hydrochars. Acs Sustain. Chem. Eng. 2013, 1, 1092–1101. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Wang, B.; Wang, Q.; Yang, G.; Chen, J. Effect of residence time on hydrothermal carbonization of corn cob residual. BioResources 2015, 10, 3979–3986. [Google Scholar] [CrossRef] [Green Version]
- Sumtong, P.; Chollacoop, N.; Eiad-ua, A. Effect of temperature and times by hydrothermal carbonization process from sawdust and bagasse for carbon materials supporter. J. Appl. Sci. 2017, 16, 93–97. [Google Scholar] [CrossRef]
- Hoffmann, V.; Jung, D.; Zimmermann, J.; Rodriguez Correa, C.; Elleuch, A.; Halouani, K.; Kruse, A. Conductive carbon materials from the hydrothermal carbonization of vineyard residues for the application in electrochemical double-layer capacitors (EDLCs) and direct carbon fuel cells (DCFCs). Materials 2019, 12, 1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.; Jin, B.; Xiao, R.; Yang, L.; Wu, Y.; Zhong, Z.; Huang, Y. The comparison of two activation techniques to prepare activated carbon from corn cob. Biomass Bioenerg 2013, 48, 250–256. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Li, A. Hydrothermal co-carbonization of sewage sludge with pinewood sawdust for nutrient-rich hydrochar production: Synergistic effects and products characterization. J. Environ. Manag. 2017, 201, 52–62. [Google Scholar] [CrossRef]
- Zbair, M.; Bottlinger, M.; Ainassaari, K.; Ojala, S.; Stein, O.; Keiski, R.; Bensitel, M.; Brahmi, R. Hydrothermal carbonization of argan nut shell: Functional mesoporous carbon with excellent performance in the adsorption of Bisphenol A and Diuron. Waste Biomass Valori 2018, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Reza, M.T.; Wirth, B.; Lüder, U.; Werner, M. Behavior of selected hydrolyzed products during hydrothermal carbonization of biomass. Bioresour. Technol. 2014, 169, 352–361. [Google Scholar] [CrossRef]
- Khataee, A.; Kayan, B.; Kalderis, D.; Karimi, A.; Akay, S.; Konsolakis, M. Ultrasound-assisted removal of Acid Red 17 using nanosized Fe3O4-loaded coffee waste hydrochar. Ultrason. Sonochem. 2017, 35, 72–80. [Google Scholar] [CrossRef]
- Li, X.; Strezov, V.; Kan, T. Energy recovery potential analysis of spent coffee grounds pyrolysis products. J. Anal. Appl. Pyrolysis 2014, 110, 79–86. [Google Scholar] [CrossRef]
- Liu, C.; Huang, X.; Kong, L. Efficient low temperature hydrothermal carbonization of Chinese reed for biochar with high energy density. Energies 2017, 10, 2094. [Google Scholar] [CrossRef] [Green Version]
- Titirici, M.M. Porous Hydrothermal Carbons. In Sustainable Carbon Materials from Hydrothermal Processes; Wiley Editors: School of Engineering and Materials Science, Queen Mary, University of London, UK, 2013; pp. 37–73. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of hydrochars produced by hydrothermal carbonization of lignin, cellulose, D-xylose, and wood meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- Kalderis, D.; Kotti, M.; Méndez, A.; Gascó, G. Characterization of hydrochars produced by hydrothermal carbonization of rice husk. Solid Earth 2014, 6, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Lee, K.; Bae, D.; Park, K.Y. Characterizations of biochar from hydrothermal carbonization of exhausted coffee residue. J. Mater. Cycles Waste Manag. 2017, 19, 1036–1043. [Google Scholar] [CrossRef]
- Lin, G.; Ma, R.; Zhou, Y.; Liu, Q.; Dong, X. KOH activation of biomass-derived nitrogen-doped carbons for supercapacitor and electrocatalytic oxygen reduction. Electrochim. Acta 2017, 261, 49–57. [Google Scholar] [CrossRef]
- Yun, C.; Park, Y.; Park, C. Effects of pre-carbonization on porosity development of activated carbons from rice straw. Carbon 2001, 39, 559–567. [Google Scholar] [CrossRef]
- Xu, K.; Li, Y.; Xiong, J.; Ou, X.; Su, W.; Zhong, G.; Yang, C. Activated amorphous carbon with high-porosity derived from camellia pollen grains as anode materials for lithium/sodium ion batteries. Front. Chem. 2018, 6, 366. [Google Scholar] [CrossRef]
- Saad, M.J.; Chia, C.; Zakaria, S.; Sajab, M.S.; Misran, S.; Bin Abdul Rahman, M.H.; Chin, S.X. Physical and chemical properties of the rice straw activated carbon produced from carbonization and KOH activation processes. Sains Malays. 2019, 48, 385–391. [Google Scholar] [CrossRef]
- Sidik, R.; Anderson, A.; Subramanian, N.; Kumaraguru, S.; Popov, B. O2 Reduction on graphite and nitrogen-doped graphite: Experiment and theory. J. Phys. Chem. B 2006, 110, 1787–1793. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, S.; Stevenson, K. Influence of nitrogen doping on oxygen reduction electrocatalysis at carbon nanofiber electrodes. J. Phys. Chem. B 2005, 109, 4707–4716. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent advances in Zinc-air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Qiao, J.; Zhang, X.; Ma, C.; Jian, S.; Liu, Y.; Pei, P. Morphology controlled La2O3/Co3O4/MnO2–CNTs hybrid nanocomposites with durable bi-functional air electrode in high-performance zinc–air energy storage. Appl. Energy 2016, 175, 495–504. [Google Scholar] [CrossRef]
- Basirun, W.J.; Sookhakian, M.; Baradaran, S.; Endut, Z.; Mahmoudian, M.R.; Ebadi, M.; Yousefi, R.; Ghadimi, H.; Ahmad, S. Graphene oxide electrocatalyst on MnO2 air cathode as an efficient electron pump for enhanced oxygen reduction in alkaline solution. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Zhong, R.S.; Qin, Y.H.; Niu, D.F.; Tian, J.W.; Zhang, X.S.; Zhou, X.G.; Sun, S.G.; Yuan, W.K. Effect of carbon nanofiber surface functional groups on oxygen reduction in alkaline solution. J. Power Sources 2013, 225, 192–199. [Google Scholar] [CrossRef]
- Roche, I.; Chaînet, E.; Chatenet, M.; Vondrák, J. Durability of carbon-supported manganese oxide nanoparticles for the oxygen reduction reaction (ORR) in alkaline medium. J. Appl. Electrochem. 2008, 38, 1195–1201. [Google Scholar] [CrossRef]
- Woon, C.; Islam, A.; Tharan, B.; Ong, H.R.; Cheng, C.K.; Chong, K.F.; Hedge, G.; Khan, M. Carbon nanotube-modified MnO2: An efficient electrocatalyst for oxygen reduction reaction. ChemistrySelect 2017, 2, 7637–7644. [Google Scholar] [CrossRef]
- Karuppiah, S.; Senthil Kumar, S.M.; Rangasamy, T.; Ganesan, K.; Murugan, P.; Rajput, P.; Jha, S.; Bhattacharyya, D. Physiochemical investigation of shape designed MnO2 nanostructures and their influence on oxygen reduction reaction activity in alkaline solution. J. Phys. Chem. C 2015, 119, 6604–6618. [Google Scholar] [CrossRef]
- Gu, P.; Zheng, M.; Zhao, Q.; Xiao, X.; Xue, H.; Pang, H. Rechargeable zinc–air batteries: A promising way to green energy. J. Mater. Chem. A 2017, 5, 7651–7666. [Google Scholar] [CrossRef]
- Molina-García, M.; Rees, N. Effect of catalyst carbon supports on the oxygen reduction reaction in alkaline media: A comparative study. RSC Adv. 2016, 6, 94669–94681. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Hu, A. Recent progress of metal-air batteries—a mini review. Appl. Sci. 2019, 9, 2787. [Google Scholar] [CrossRef] [Green Version]
- Clark, S.; Latz, A.; Horstmann, B. A review of model-based design tools for metal-air batteries. Batteries 2018, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Zheng, Y.; Jaroniec, M.S.; Qiao, Z. Determination of the electron transfer number for the oxygen reduction reaction: From theory to experiment. ACS Catal 2016, 6, 4720–4728. [Google Scholar] [CrossRef]
- Wang, G.; Peng, H.; Qiao, X.; Du, L.; Li, X.; Shu, T.; Liao, S. Biomass-derived porous heteroatom-doped carbon spheres as a high-performance catalyst for the oxygen reduction reaction. Int. J. Hydrog. Energy 2016, 41, 14101–14110. [Google Scholar] [CrossRef]
- Li, X.; Xu, N.; Li, H.; Wang, M.; Zhang, L.; Qiao, J. 3D hollow sphere Co3O4/MnO2-CNTs: Its high-performance bi-functional cathode catalysis and application in rechargeable zinc-air battery. Green Energy Environ. 2017, 2, 316–328. [Google Scholar] [CrossRef]





| Peaks (cm−1) | Functional Group | Reference |
|---|---|---|
| 1655–1700 | C=O and C=C vibration from ketones and aromatic ring structures | [34] |
| 1450–1510 | C=C–C bonds vibration in an aromatic ring | [35] |
| 1310–1410 | O–H bends and aliphatic CH3 and CH2 deformation | [35] |
| 1050–1270 | Aryl–O and C–O stretch from ether compounds | [36] |
| 1000–1055 | C–O bonds in glucose | [36] |
| 900 | C–H groups with aromatic out-of-plane bends | [37,38] |
| Sample | %Weight Concentration | |||||
|---|---|---|---|---|---|---|
| C | O | N | K | Si | Total | |
| CC Raw | 42.36 | 52.66 | 3.95 | 1.02 | 100.00 | |
| CCAC 250-12 | 83.40 | 16.60 | 100.00 | |||
| CWG Raw | 39.01 | 47.90 | 12.02 | 1.08 | 100.00 | |
| CWGAC 250-12 | 76.79 | 23.21 | 100.00 | |||
| RH Raw | 46.06 | 22.70 | 3.56 | 0.00 | 27.68 | 100.00 |
| RHAC 250-12 | 61.92 | 31.71 | 6.38 | 100.00 | ||
| SD Raw | 41.33 | 49.38 | 8.41 | 0.89 | 0.00 | 100.00 |
| SDAC 250-12 | 73.66 | 26.34 | 100.00 | |||
| Sample | jL (mA cm−2) | Eons (V vs. Ag/AgCl) | Overpotential (V) |
|---|---|---|---|
| CCAC + MnO2 250-12 | −2.85 | −0.23 (0.73) 1 | 0.50 |
| CWGAC + MnO2 250-12 | −2.62 | −0.25 (0.71) 1 | 0.52 |
| RHAC + MnO2 250-12 | −2.72 | −0.35 (0.61) 1 | 0.62 |
| SDAC + MnO2 250-12 | −2.58 | −0.36 (0.6) 1 | 0.63 |
| VC + MnO2 | −2.67 | −0.17 (0.79) 1 | 0.44 |
| 20% Pt/C | −4.63 | −0.098 (0.87) 1 | 0.36 |
| Sample | E1/2 (V vs. Ag/AgCl) | jL (mA cm−2) | ||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| CCAC + MnO2 250-12 | −0.32 | −0.39 | 2.85 | 2.62 |
| VC + MnO2 | −0.29 | −0.38 | 2.67 | 1.84 |
| 20% Pt/C | −0.11 | −0.11 | 4.63 | 3.79 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panganoron, H.O.; Pascasio, J.D.A.; Esparcia, E.A., Jr.; del Rosario, J.A.D.; Ocon, J.D. Hydrothermally Carbonized Waste Biomass as Electrocatalyst Support for α-MnO2 in Oxygen Reduction Reaction. Catalysts 2020, 10, 177. https://doi.org/10.3390/catal10020177
Panganoron HO, Pascasio JDA, Esparcia EA Jr., del Rosario JAD, Ocon JD. Hydrothermally Carbonized Waste Biomass as Electrocatalyst Support for α-MnO2 in Oxygen Reduction Reaction. Catalysts. 2020; 10(2):177. https://doi.org/10.3390/catal10020177
Chicago/Turabian StylePanganoron, Harold O., Jethro Daniel A. Pascasio, Eugene A. Esparcia, Jr., Julie Anne D. del Rosario, and Joey D. Ocon. 2020. "Hydrothermally Carbonized Waste Biomass as Electrocatalyst Support for α-MnO2 in Oxygen Reduction Reaction" Catalysts 10, no. 2: 177. https://doi.org/10.3390/catal10020177






