A Novel Reduced Graphene Oxide-Attapulgite (RGO-ATP) Supported Fe2O3 Catalyst for Heterogeneous Fenton-like Oxidation of Ciprofloxacin: Degradation Mechanism and Pathway
Abstract
:1. Introduction
2. Results and Discussion
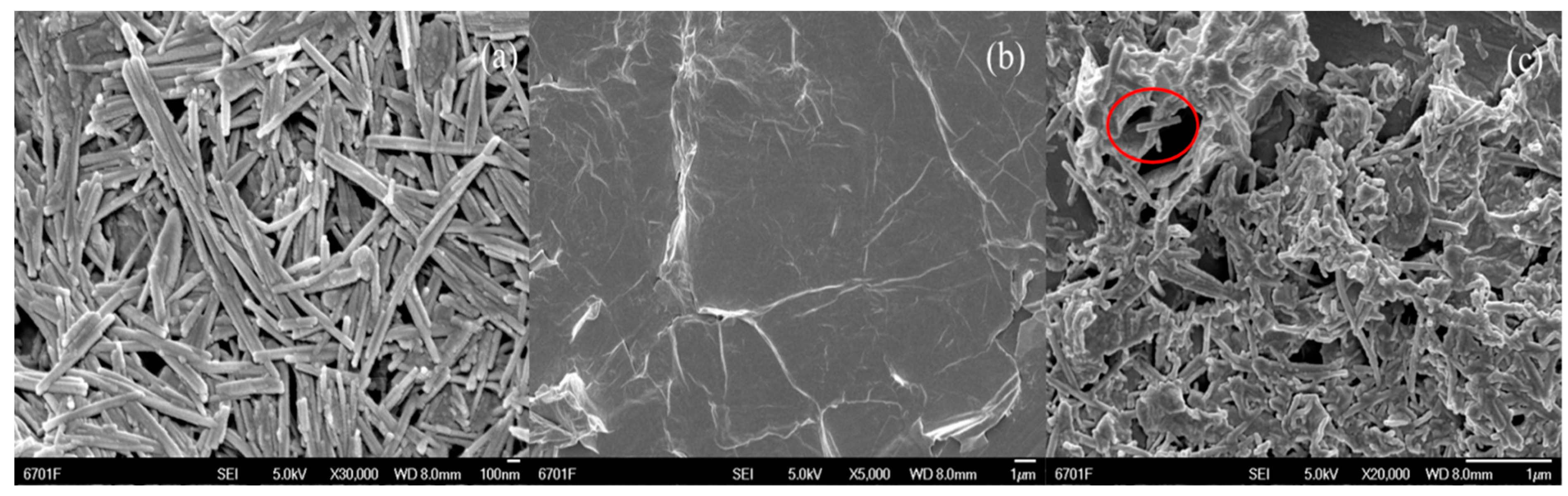
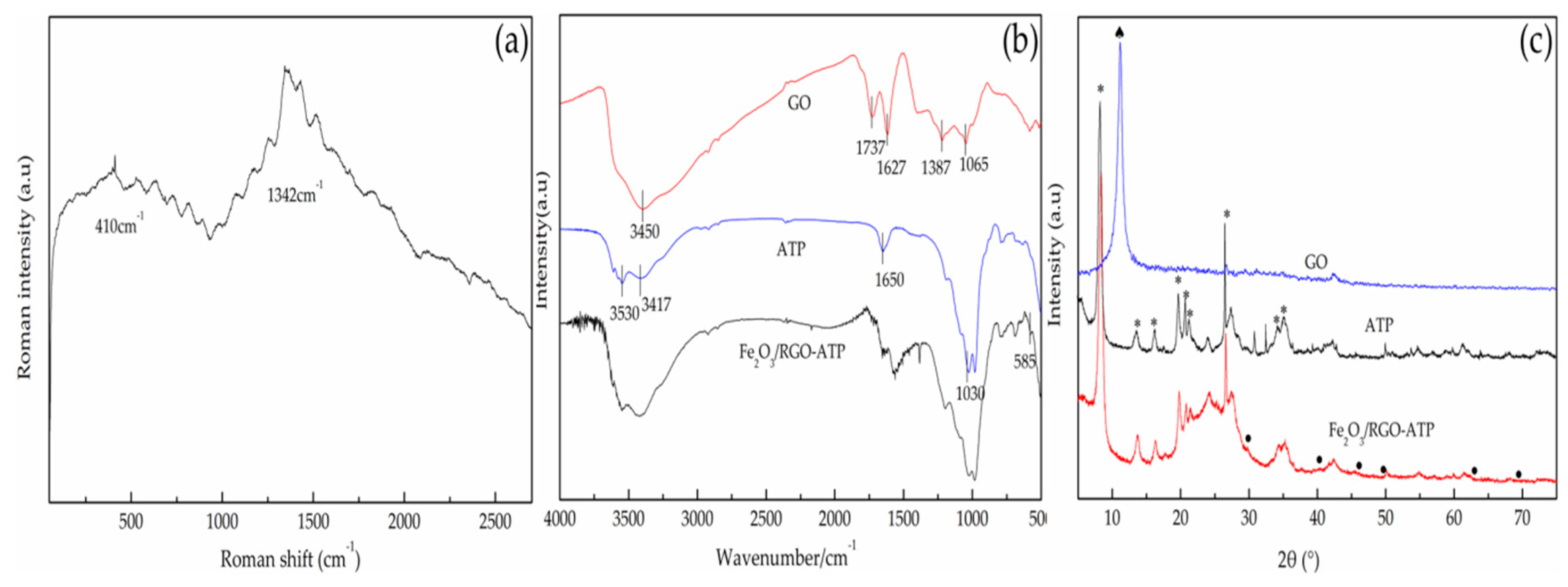
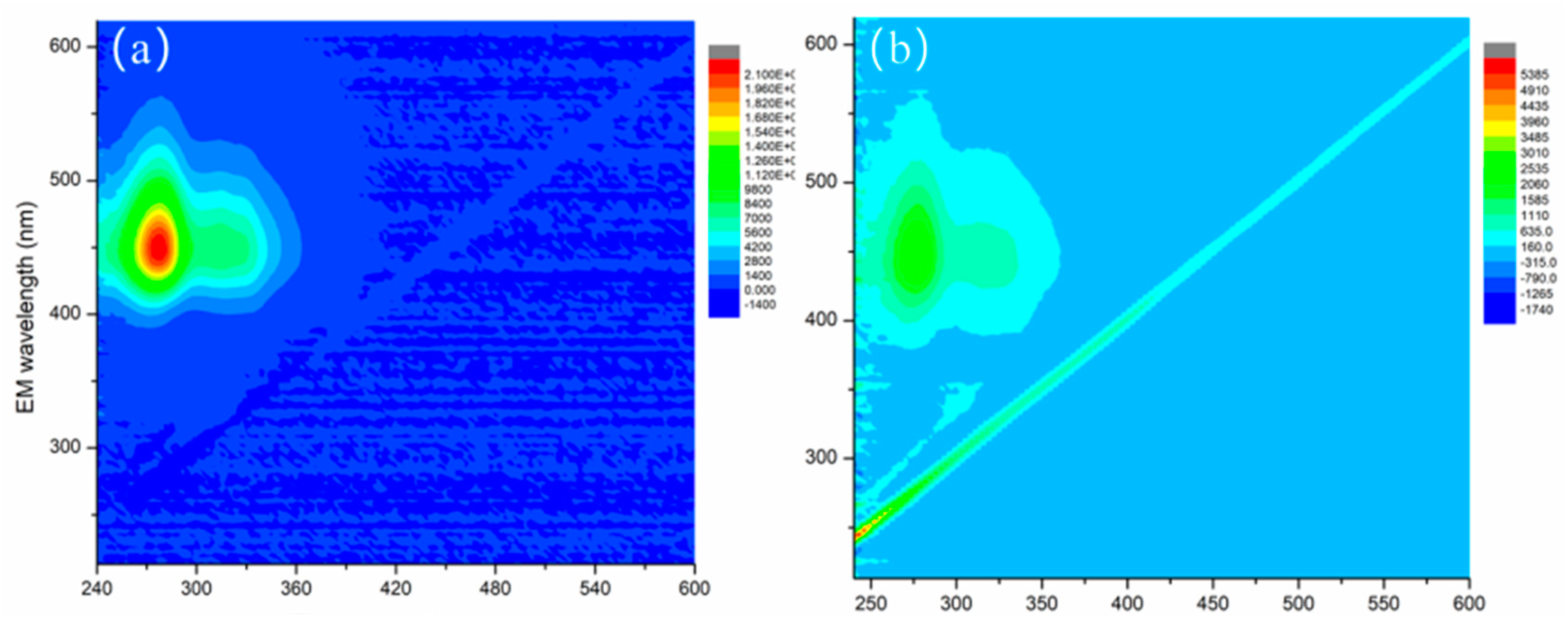
2.1. Characteristics of Catalyst Fe2O3/RGO-ATP
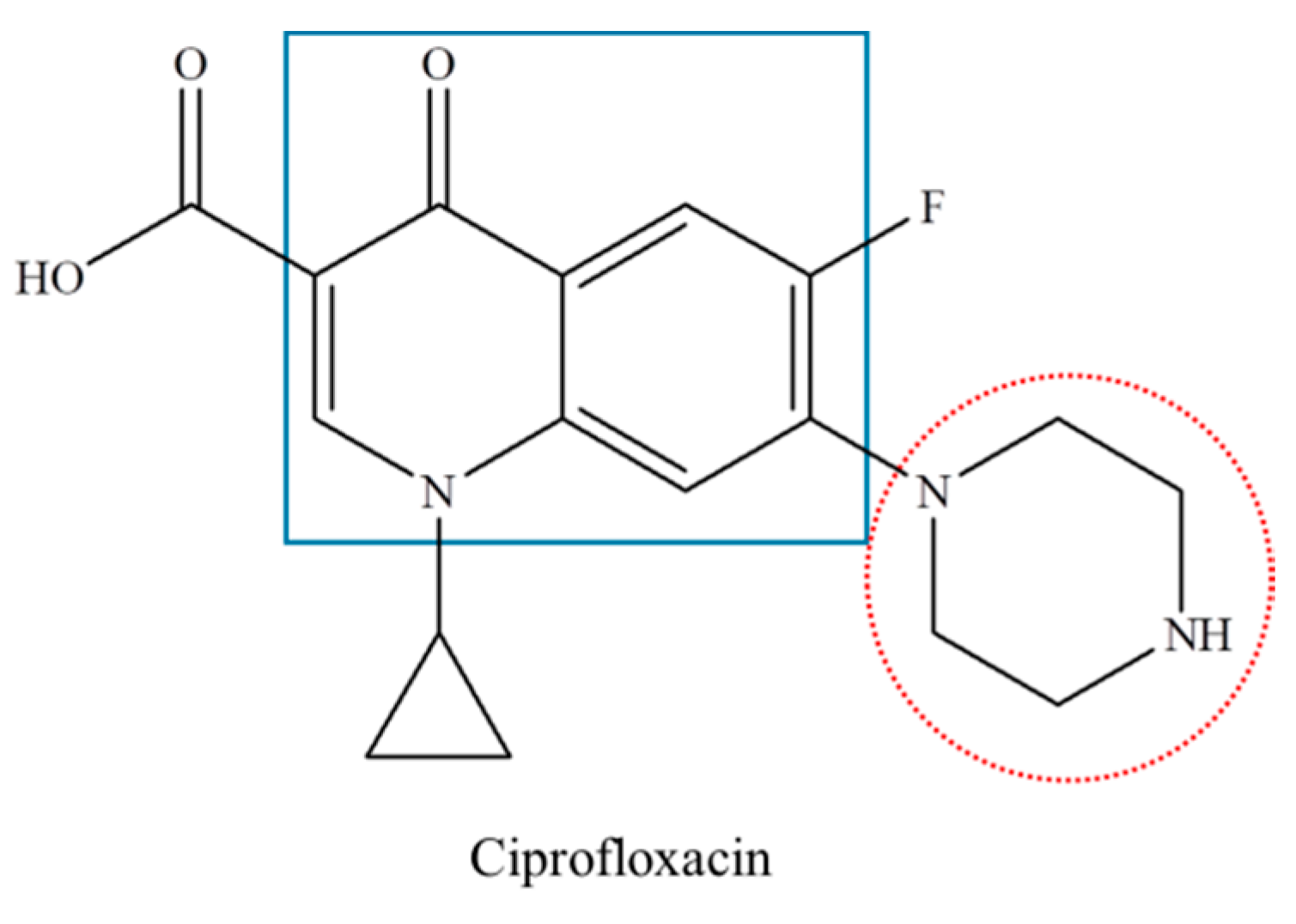
2.2. Ciprofloxacin Degradation
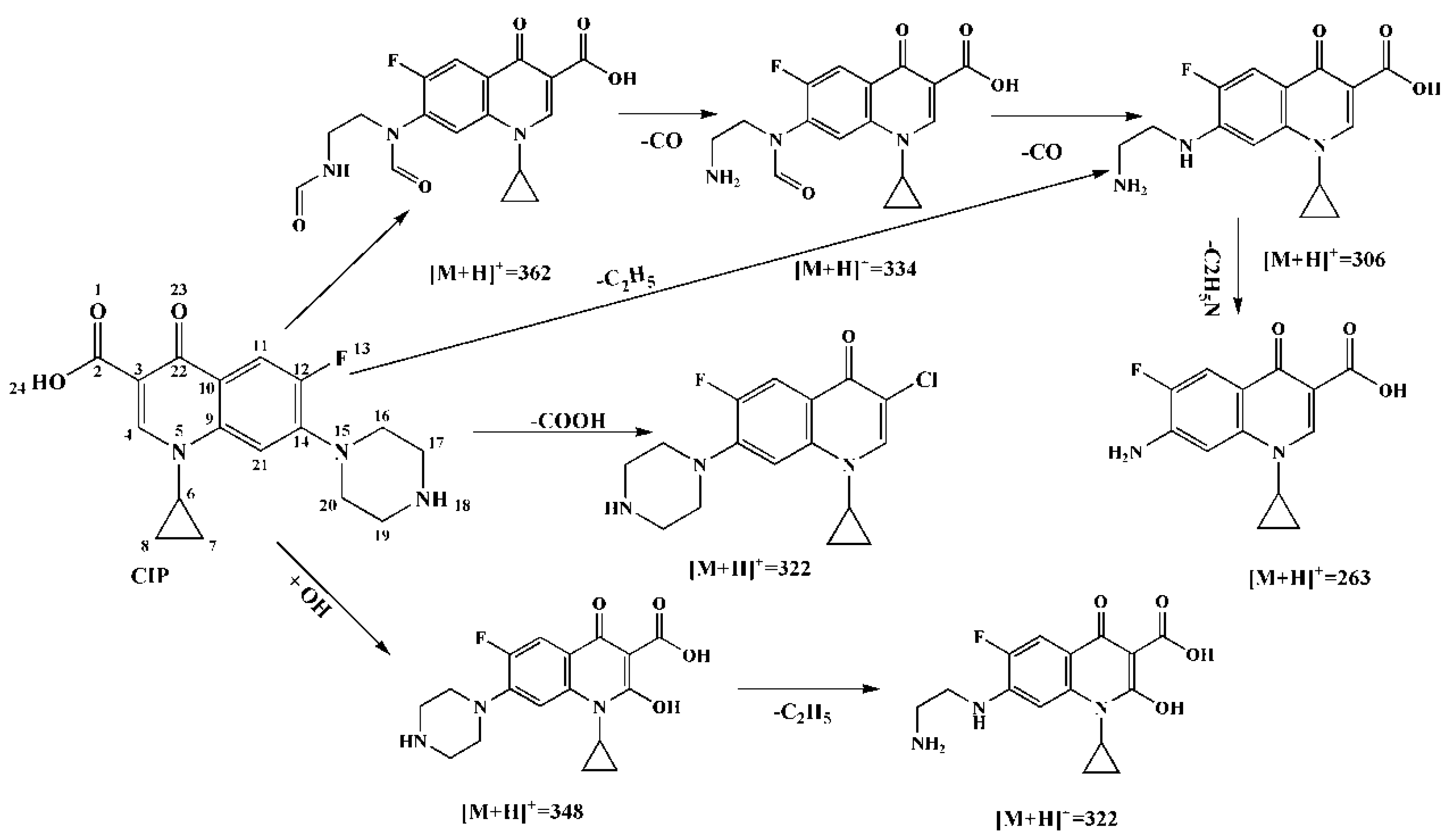
2.3. Mechanism of Ciprofloxacin Degradation by Fe2O3/RGO-ATP Catalyst
3. Materials and Methods
3.1. Preparation of Three-Dimensional Graphene-Based Catalysts
3.2. Methods of Catalytic Degradation by Fe2O3/RGO-ATP Catalyst
3.3. Materials
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuetz, P.; Wirz, Y.; Sager, R.; Christ-Crain, M.; Mueller, B. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect. Dis. 2018, 18, 95–107. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.-G.; Zhu, Y.G.; Johnson, T.A.; Su, J.Q.; Qiao, M.; Guo, G.X.; Stedtfeld, R.D.; Hashsham, S.A.; Tiedje, J.M. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc. Natl. Acad. Sci. USA 2013, 110, 3435–3440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Russo, J.; Fiegel, J.; Brogden, N. Antibiotic Delivery Strategies to Treat Skin Infections When Innate Antimicrobial Defense Fails. Antibiotics. 2020, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Selby, K.; Boxall, A.B. Assessment of the risks of mixtures of major use veterinary antibiotics in European surface waters. Environ. Sci. Technol. 2016, 50, 8282–8289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcoran, J.; Winter, M.J.; Tyler, C.R. Pharmaceuticals in the aquatic environment: A critical review of the evidence for health effects in fish. Crit. Rev. Toxicol. 2010, 40, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.A.; Lester, J.N.; Voulvoulis, N. Pharmaceuticals: A threat to drinking water? Trends Biotechnol. 2005, 23, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.F.; Liu, X.; Zhang, S.; Pan, B.; Liu, M.L. Ciprofloxacin derivatives and their antibacterial activities. Eur. J. Med. Chem. 2018, 146, 599–612. [Google Scholar] [CrossRef]
- Hassani, A.; Khataee, A.; Karaca, S.; Karaca, C.; Gholami, P. Sonocatalytic degradation of ciprofloxacin using synthesized TiO2 nanoparticles omontmorillonite. Ultrason. Sonochem. 2017, 35, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Salma, A.; Thor E-Boveleth, S.; Schmidt, T.C.; Tuerk, J. Dependence of transformation product formation on pH during photolytic and photocatalytic degradation of ciprofloxacin. J. Hazard. Mater. 2016, 313, 49–59. [Google Scholar] [CrossRef]
- Xiong, J.Q.; Kurade, M.B.; Kim, J.R.; Roh, H.S.; Jeon, B.H. Ciprofloxacin toxicity and its co-metabolic removal by a freshwater microalga Chlamydomonas mexicana. J. Hazard. Mater. 2017, 323, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Yang, Q.; Pang, Y.; Wang, D.B.; Li, X.; Lei, M.; Huang, Q. Unveiling the mechanism of biochar-activated hydrogen peroxide on the degradation of ciprofloxacin. Chem. Eng. J. 2019, 374, 520–530. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Huan, Y. Zeolitic imidazolate framework-8 derived nanoporous carbon as an effective and recyclable adsorbent for removal of ciprofloxacin antibiotics from water. J. Hazard. Mater. 2017, 321, 711–719. [Google Scholar] [CrossRef]
- Sun, Y.; Yue, Q.; Gao, B.; Gao, Y.; Wang, Y. Adsorption and cosorption of ciprofloxacin and Ni (II) on activated arbon-mechanism study. J. Taiwan Inst. Chem. Eng. 2014, 45, 681–688. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Nghiem, L.D.; Seungdae, O.H. Aerobic biotransformation of the antibiotic ciprofloxacin by Bradyrhizobium sp. isolated from activated sludge. Chemosphere 2018, 211, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, M.; Wu, M.; Li, Z.; Liu, X. Occurrences and regional distributions of 20 antibiotics in water bodies during groundwater recharge. Sci. Total Environ. 2015, 518, 498–506. [Google Scholar] [CrossRef]
- Miao, X.S.; Bishay, F.; Chen, M.; Metcalfe, C.D. Occurrence of antimicrobials in the final effluents of wastewater treatment plants in Canada. Environ. Sci. Technol. 2004, 38, 3533–3541. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Soares, A.D.; Adejumo, H.; McDiarmid, M.; Squibb, K.; Blaney, L. Detection of a wide variety of human and veterinary fluoroquinolone antibiotics in municipal wastewater and wastewater-impacted surface water. J. Pharm. Biomed. Anal. 2015, 106, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Wolfgang, K.; Bernd, J.; Ursula, O. Detection of antibiotic-resistant bacteria and their resistance genes in wastewater, surface water, and drinking water biofilms. FEMS Microbiol. Ecol. 2003, 43, 325–335. [Google Scholar]
- Wei, Z.; Liu, J.; Fang, W.; Guo, W.; Zhu, Y.; Xu, M.Q.; Jiang, Z.; Shangguan, W.F. Photocatalytic hydrogen energy evolution from antibiotic wastewater via metallic bi nanosphere doped g-c3n4: Performances and mechanisms. Catal. Sci. Technol. 2019, 9. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.J.; Ma, J.X.; Xia, P.; Zhao, J.F. Removal of cadmium (II) from aqueous solution: A comparative study of raw attapulgite clay and a reusable waste–struvite/attapulgite obtained from nutrient-rich wastewater. J. Hazard. Mater. 2017, 329, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Tian, A.; You, J.H.; Yang, H.; Wang, Y.Z.; Xue, X.X. Degradation of organic dyes by a new heterogeneous Fenton reagent-Fe2GeS4 nanoparticle. J. Hazard. Mater. 2018, 353, 182–189. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, S.; Basu, S. Synthesis of Fe2O3/TiO2 monoliths for the enhanced degradation of industrial dye and pesticide via photo-Fenton catalysis. J. Photochem. Photobiol. A Chem. 2019, 376, 32–42. [Google Scholar] [CrossRef]
- Cheng, M.; Lai, C.; Liu, Y.; Zeng, G.; Huang, D.; Zhang, C.; Qin, L.; Hu, L.; Zhou, C.Y.; Xiong, W.P. Metal-organic frameworks for highly efficient heterogeneous Fenton-like catalysis. Coord. Chem. Rev. 2018, 368, 80–92. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.S.; Liu, B.; Sun, L.Y. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Wang, X.; Guo, B.; Fu, W.; Yang, H. Triethylenetetramine promoted rGO-Fe3O4 nanocomposites for highly efficient Fenton-like reaction. J. Water Process Eng. 2019, 31, 100814. [Google Scholar] [CrossRef]
- Gu, M.; Farooq, U.; Lu, S.; Zhang, X.; Qiu, Z.; Sui, Q. Degradation of trichloroethylene in aqueous solution by rGO supported nZVI catalyst under several oxic environments. J. Hazard. Mater. 2018, 349, 35–44. [Google Scholar] [CrossRef]
- Trapalis, A.; Todorova, N.; Giannakopoulou, T.; Boukos, N.; Speliotis, T.; Dimotikali, D.; Yu, J. TiO2/graphene composite photocatalysts for NOx removal: A comparison of surfactant-stabilized graphene and reduced graphene oxide. Appl. Catal. B Environ. 2016, 180, 637–647. [Google Scholar] [CrossRef]
- Hung, C.M.; Van Duy, N.; Van Quang, V.; Van Toan, N.; Van Hieu, N.; Hoa, N.D. Facile synthesis of ultrafine rGO/WO3 nanowire nanocomposites for highly sensitive toxic NH3 gas sensors. Mater. Res. Bull. 2020, 110810. [Google Scholar] [CrossRef]
- Vellaichamy, B.; Prakash, P.; Thomas, J. Synthesis of AuNPs@ RGO nanosheets for sustainable catalysis toward nitrophenols reduction. Ultrason. Sonochem. 2018, 48, 362–369. [Google Scholar] [PubMed]
- Xu, X.; Qin, J.; Wei, Y.; Ye, S.; Shen, J.; Yao, Y.; Ding, B.; Shu, Y.R.; He, G.Y.; Chen, H. Heterogeneous activation of persulfate by NiFe2− xCoxO4-RGO for oxidative degradation of bisphenol A in water. Chem. Eng. J. 2019, 365, 259–269. [Google Scholar] [CrossRef]
- MA, J.; Zou, J.; Li, L.L.; Yao, C.; Kong, Y.; Cui, B.Y.; Zhu, R.L.; Li, D.L. Nanocomposite of attapulgite–Ag3PO4 for Orange II photodegradation. Appl. Catal. B Environ. 2014, 144, 36–40. [Google Scholar] [CrossRef]
- Cai, J.; Lei, M.; He, J.R.Z.; Chen, T.; Fei, P. Electrospun composite nanofiber mats of Cellulose@ Organically modified montmorillonite for heavy metal ion removal: Design, characterization, evaluation of absorption performance. Compos. Part A Appl. Sci. Manuf. 2017, 92, 10–16. [Google Scholar] [CrossRef]
- Ahmad, T.; Guria, C.; Mandal, A. Synthesis, characterization and performance studies of mixed-matrix poly (vinyl chloride)-bentonite ultrafiltration membrane for the treatment of saline oily wastewater. Process Saf. Environ. Prot. 2018, 116, 703–717. [Google Scholar] [CrossRef]
- Tang, J.; Mu, B.; Zong, L.; Wang, A. From waste hot-pot oil as carbon precursor to development of recyclable attapulgite/carbon composites for wastewater treatment. J. Environ. Sci. 2019, 75, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Takase, M.; Pappoe, A.N.M.; Afrifa, E.A.; Miyittah, M. High performance heterogeneous catalyst for biodiesel production from non-edible oil. Renew. Energy Focus 2018, 25, 24–30. [Google Scholar] [CrossRef]
- Zhu, Z.; Fan, W.Q.; Liu, Z.; Dong, H.J.; Yan, Y.S.; Huo, P.W. Construction of an attapulgite intercalated mesoporous g-C3N4 with enhanced photocatalytic activity for antibiotic degradation. J. Photochem. Photobiol. A Chem. 2018, 359, 102–110. [Google Scholar]
- Ren, B.; Xu, Y.L.; Zhang, C.H.; Zhang, L.H.; Zhao, J.P.; Liu, Z.F. Degradation of methylene blue by a heterogeneous Fenton reaction using an octahedron-like, high-graphitization, carbon-doped Fe2O3 catalyst. J. Taiwan Inst. Chem. Eng. 2019, 97, 170–177. [Google Scholar] [CrossRef]
- Vu, A.-T.; Xuan, T.N.; Lee, C.-H. Preparation of mesoporous Fe2O3· SiO2 composite from rice husk as an efficient heterogeneous Fenton-like catalyst for degradation of organic dyes. J. Water Process Eng. 2019, 28, 169–180. [Google Scholar] [CrossRef]
- Liang, H.; Zhou, S.; Chen, Y.T.; Zhou, F.; Yan, C.J. Diatomite coated with Fe2O3 as an efficient heterogeneous catalyst for degradation of organic pollutant. J. Taiwan Inst. Chem. Eng. 2015, 49, 105–112. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, G.; Wang, J. Photo-Fenton degradation of rhodamine B using Fe2O3–Kaolin as heterogeneous catalyst: Characterization, process optimization and mechanism. J. Colloid Interface Sci. 2014, 433, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Nan, Z.-R. Decolorization of Methylene Blue and Congo Red by attapulgite-based heterogeneous Fenton catalyst. Desalin. Water Treat. 2016, 57, 4633–4640. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, M.; Yu, S. Decolorization of methylene blue by an attapulgite-based heterogeneous Fenton catalyst: Process optimization. Desalin. Water Treat. 2017, 63, 275–282. [Google Scholar] [CrossRef]
- Ting, Z. Sodium dodecyl benzene sulfonate (SDBS) degradation by heterogeneous Fenton-like reactions on two types of catalysts: Experimental and comparison. Res. J. Chem. Environ. 2013, 17, 32–39. [Google Scholar]
- Shang, E.; Li, Y.; Niu, J.; Li, S.; Zhang, G.; Wang, X. Photocatalytic degradation of perfluorooctanoic acid over Pb-BiFeO3/rGO catalyst: Kinetics and mechanism. Chemosphere 2018, 211, 34–43. [Google Scholar] [CrossRef]
- Li, W.; Yu, R.; Li, M.; Guo, N.; Yu, H.; Yu, Y. Photocatalytical degradation of diclofenac by Ag-BiOI-rGO: Kinetics, mechanisms and pathways. Chemosphere 2019, 218, 966–973. [Google Scholar] [CrossRef]
- Zhao, Y.; Kuai, L.; Liu, Y.G.; Wang, P.P.; Arandiyan, H.; Cao, S.F.; Zhang, J.; Li, F.Y.; Wang, Q.; Geng, B.Y.; et al. Well-constructed single-layer molybdenum disulfide nanorose cross-linked by three dimensional-reduced graphene oxide network for superior water splitting and lithium storage property. Sci. Rep. 2015, 5, 8722. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.H.; Yang, H.F.; Zhang, Z.R. Effect of TiO2 on the Raman scattering effect of α-Fe2O3 nanoparticles. J. Light Scatt. 2004, 16, 15–20. [Google Scholar]
- Boucherit, N.; Hugot-LE Goff, A.; Joire, S. Raman studies of corrosion films grown on Fe and Fe-6Mo in pitting conditions. Corros. Sci. 1991, 32, 497–507. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Mahesh, K.; Le, N.H.; Kemp, K.C.; Timilsina, R.; Tiwari, R.N.; Kim, K.S. Reduced graphene oxide-based hydrogels for the efficient capture of dye pollutants from aqueous solutions. Carbon 2013, 56, 173–182. [Google Scholar] [CrossRef]
- Cheng, G.; Liu, Y.L.; Wang, Z.G.; Zhang, J.L.; Sun, D.H.; Ni, J.Z. The GO/rGO-Fe3O4 composites with good water-dispersibility and fast magnetic response for effective immobilization and enrichment of biomolecules. J. Mater. Chem. 2012, 22, 21998–22004. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Yao, C.; Lu, X.; Zhao, X.; Ni, C. Attapulgite-CeO2/MoS2 ternary nanocomposite for photocatalytic oxidative desulfurization. Appl. Surf. Sci. 2016, 364, 589–596. [Google Scholar] [CrossRef]
- Cheng, W.; Ding, C.; Sun, Y.; Wang, X. Fabrication of fungus/attapulgite composites and their removal of U (VI) from aqueous solution. Chem. Eng. J. 2015, 269, 1–8. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Lu, X.; Zuo, S.; Yao, C.; Ni, C. Integrated nanostructures of CeO2/attapulgite/g-C3N4 as efficient catalyst for photocatalytic desulfurization: Mechanism, kinetics and influencing factors. Chem. Eng. J. 2017, 326, 87–98. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, J.; Chen, M.; Wang, Y. Preparation of heterogeneous Fenton catalyst Fe/organo-attapulgite and its performance in sodium humate degradation. Desalin. Water Treat. 2018, 107, 91–99. [Google Scholar] [CrossRef]
- Kurt, B.Z.; Durmus, Z.; Sevgi, E. In situ reduction of graphene oxide by different plant extracts as a green catalyst for selective hydrogenation of nitroarenes. Int. J. Hydrog. Energy. 2019, 44, 26322–26337. [Google Scholar] [CrossRef]
- Yan, Y.; Yan, Y.; Sun, S.; Song, Y.; Yan, X.; Guan, W.; Liu, X.; Shi, W. Microwave-assisted in situ synthesis of reduced graphene oxide-BiVO4 composite photocatalysts and their enhanced photocatalytic performance for the degradation of ciprofloxacin. J. Hazard. Mater. 2013, 250, 106–114. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Thang, G.; Jiang, Z.G.; Li, X.; Zhang, H.B.; Dasari, A.; Yu, Z.Z. Three dimensional graphene aerogels and their electrically conductive composites. Carbon 2014, 77, 592–599. [Google Scholar] [CrossRef]
- Li, J.-S.; Wang, Y.; Liu, C.H.; Li, S.L.; Wang, Y.G.; Dong, L.Z.; Dai, Z.H.; Li, Y.F.; Lan, Y.Q. Coupled molybdenum carbide and reduced graphene oxide electrocatalysts for efficient hydrogen evolution. Nat. Commun. 2016, 7, 11204. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; He, P.; Huang, T.; Li, J.; Hu, X.; Xiang, P.; Chen, D.; Yang, S.; Wang, G.; Ding, G. Selective supramolecular interaction of ethylenediamine functionalized graphene quantum dots: Ultra-sensitive photoluminescence detection for nickel ion in vitro. Synth. Met. 2018, 244, 106–112. [Google Scholar] [CrossRef]
- Ortiz, B.; Saby, C.; Champagne, G.Y.; Bélanger, D. Electrochemical modification of a carbon electrode using aromatic diazonium salts. 2. Electrochemistry of 4-nitrophenyl modified glassy carbon electrodes in aqueous media. J. Electroanal. Chem. 1998, 455, 75–81. [Google Scholar] [CrossRef]
- Ling, L.-L.; Liu, W.J.; Zhang, S.; Jiang, H. Magnesium oxide embedded nitrogen self-doped biochar composites: Fast and high-efficiency adsorption of heavy metals in an aqueous solution. Environ. Sci. Technol. 2017, 51, 10081–10089. [Google Scholar] [CrossRef] [PubMed]
- Dedryvere, R.; Maccario, M.; Croguennec, L.; Cras, F.L.; Gonbeau, D. X-Ray Photoelectron Spectroscopy Investigations of Carbon-Coated Li x FePO4 Materials. Chem. Mater. 2008, 20, 7164–7170. [Google Scholar] [CrossRef]
- YamashitaA, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Liming, D.; Jingping, W.; Yanhong, L. Study on Fluorescence Characteristics of Ciprofloxacin Based on Charge Transfer Reaction. Chin. J. Anal. Chem. 2002, 30, 658–660. [Google Scholar]
- Inagawa, H.; Ishizawa, K.; Mitsuhashi, T.; Shimizu, M.; Adachi, J.; Nishikawa, R.; Matsutani, M.; Hirose, T. Giant invasive pituitary adenoma extending into the sphenoid sinus and nasopharynx. Acta Cytol. 2005, 49, 452–456. [Google Scholar] [CrossRef]
- Li, N.; Zhang, J.; Yu, T.; Zhao, J.; Jian, Z.; Wei, Z. Precisely controlled fabrication of magnetic 3D γ-Fe2O3@ ZnO core-shell photocatalyst with enhanced activity: Ciprofloxacin degradation and mechanism insight. Chem. Eng. J. 2017, 308, 377–385. [Google Scholar] [CrossRef]
- Giri, A.S.; Golder, A.K. Ciprofloxacin degradation from aqueous solution by Fenton oxidation: Reaction kinetics and degradation mechanisms. RSC Adv. 2014, 4, 6738–6745. [Google Scholar] [CrossRef]
- Wajahat, R.; Yasar, A.; Khan, A.M.; Tabinda, A.B.; Bhatti, S.G. Ozonation and photo-driven oxidation of ciprofloxacin in pharmaceutical wastewater: Degradation kinetics and energy requirements. Pol. J. Environ. Stud. 2019, 28, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, C.C.; Zhang, M.M.; Zhang, B.T.; Yu, Y.G. The electrochemical degradation of ciprofloxacin using a SnO2-Sb/Ti anode: Influencing factors, reaction pathways and energy demand. Chem. Eng. J. 2016, 296, 79–89. [Google Scholar] [CrossRef]
- Chen, M.; Yao, J.; Huang, Y.; Gong, H.; Chu, W. Enhanced photocatalytic degradation of ciprofloxacin over Bi2O3/(BiO) 2CO3 heterojunctions: Efficiency, kinetics, pathways, mechanisms and toxicity evaluation. Chem. Eng. J. 2018, 334, 453–461. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, R.; Jia, M.; Wang, S.; Huang, Y.; Chen, C.C. Degradation of ciprofloxacin in aqueous bismuth oxybromide (BiOBr) suspensions under visible light irradiation: A direct hole oxidation pathway. Chem. Eng. J. 2015, 274, 290–297. [Google Scholar] [CrossRef]
- Dewitie, B.; Dewulf, J.; Demeestere, K.; Van De Vyvere, V.; De Wispelaere, P.; Van Langenhove, H. Ozonation of ciprofloxacin in water: HRMS identification of reaction products and pathways. Environ. Sci. Technol. 2008, 42, 4889–4895. [Google Scholar] [CrossRef]
- An, T.; Yang, H.; Li, G.Y.; Song, W.H.; Nie, X.P. Kinetics and mechanism of advanced oxidation processes (AOPs) in degradation of ciprofloxacin in water. Appl. Catal. B Environ. 2010, 94, 288–294. [Google Scholar] [CrossRef]
- Wei, H.; Yang, H.; Li, K.B.; Ma, H.; Zhang, Y.H.; Li, W.Y. Effect and path analysis of CCl4 enhanced ciprofloxacin degradation by ultrasound. J. Chem. Eng. Chin. Univ. 2015, 29, 703–708. [Google Scholar]
- Chen, J.; Yao, B.; Li, C.; Shi, G.Q. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Botas, C.; Álvarez, P.; Blanco, P.; Granda, M.; Blanco, C.; Santamaría, R.; Romasanta, L.J.; Verdejo, R.; López-Manchado, M.A.; Menéndez, R. Graphene materials with different structures prepared from the same graphite by the Hummers and Brodie methods. Carbon 2013, 65, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Shahriary, L.; Athwalf, A.A. Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]


| Samples | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| Fe2O3/RGO-ATP | 168.0802 | 0.446792 | 9.13365 |
| ATP | 123.7691 | 0.315779837 | 4.43788 |
| Methods | Process or Catalyst | Optimal Degradation Conditions | Degradation Ratio | Literature |
|---|---|---|---|---|
| Photocatalytic | 3D γ-Fe2O3@ZnO | Surroundings: dark time = 30 min | 18.30% | [69] |
| Fenton oxidation | Fe2+/H2O2 | Initial concentration = 15 mg/L molar ratio (Fe2+/H2O2) = 0.125, pH = 3.5 time = 45 min, | 74.40% | [70] |
| Ozonation | Ozone | Initial concentration = 7.91 mg/L, time = 30 min, pH = 9 | 98.7% | [71] |
| Heterogeneous Fenton method | Fe2O3/RGO-ATP | Initial concentration = 50 mg/L, time = 60 min, pH = 5, dosage of hydrogen peroxide = 0.2477 mmol/L | 88.27% | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Qian, C.; Guo, P.; Gan, S.; Dong, L.; Bai, G.; Guo, Q. A Novel Reduced Graphene Oxide-Attapulgite (RGO-ATP) Supported Fe2O3 Catalyst for Heterogeneous Fenton-like Oxidation of Ciprofloxacin: Degradation Mechanism and Pathway. Catalysts 2020, 10, 189. https://doi.org/10.3390/catal10020189
Zhang T, Qian C, Guo P, Gan S, Dong L, Bai G, Guo Q. A Novel Reduced Graphene Oxide-Attapulgite (RGO-ATP) Supported Fe2O3 Catalyst for Heterogeneous Fenton-like Oxidation of Ciprofloxacin: Degradation Mechanism and Pathway. Catalysts. 2020; 10(2):189. https://doi.org/10.3390/catal10020189
Chicago/Turabian StyleZhang, Ting, Chunyuan Qian, Pengran Guo, Shuchai Gan, Lingyu Dong, Ge Bai, and Qiyang Guo. 2020. "A Novel Reduced Graphene Oxide-Attapulgite (RGO-ATP) Supported Fe2O3 Catalyst for Heterogeneous Fenton-like Oxidation of Ciprofloxacin: Degradation Mechanism and Pathway" Catalysts 10, no. 2: 189. https://doi.org/10.3390/catal10020189




