Propane Oxidative Dehydrogenation on Vanadium-Based Catalysts under Oxygen-Free Atmospheres
Abstract
:1. Introduction
2. Propane Dehydrogenation (DH) and Oxidative Dehydrogenation (PODH) Reactions: Stoichiometry and Thermodynamics
3. Conversion, Selectivity and Yield Calculations
4. Vanadium-Based Alkane ODH Catalysts
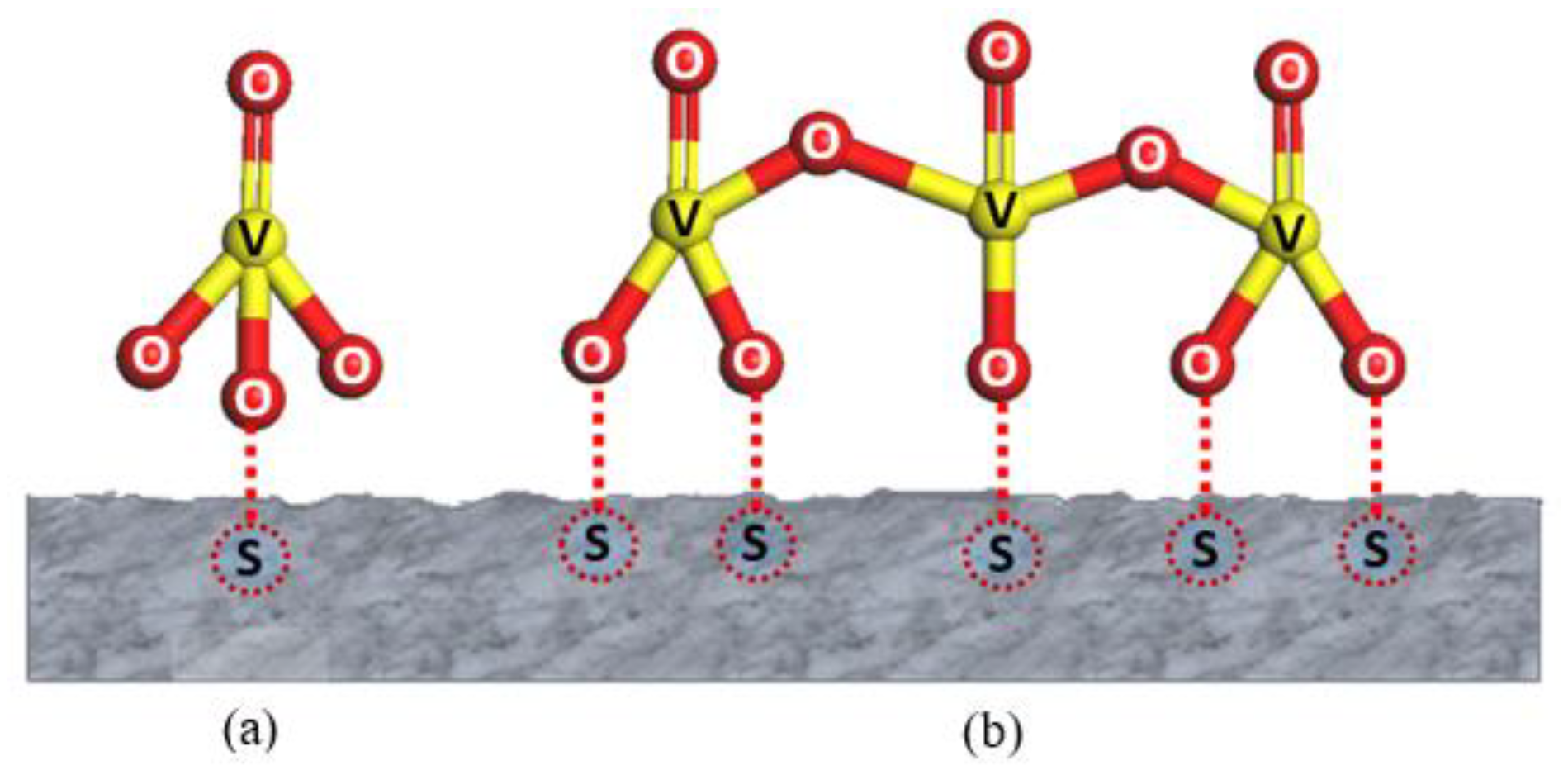
4.1. VOx Surface Coverage
4.2. Active Lattice Oxygen Species
4.3. Effect of Support (Acid/Base Properties)
4.4. Redox Properties of Supported VOx Catalysts
4.5. Propane ODH Catalysts
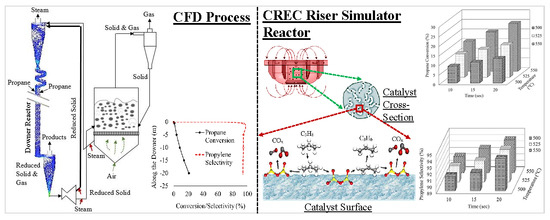
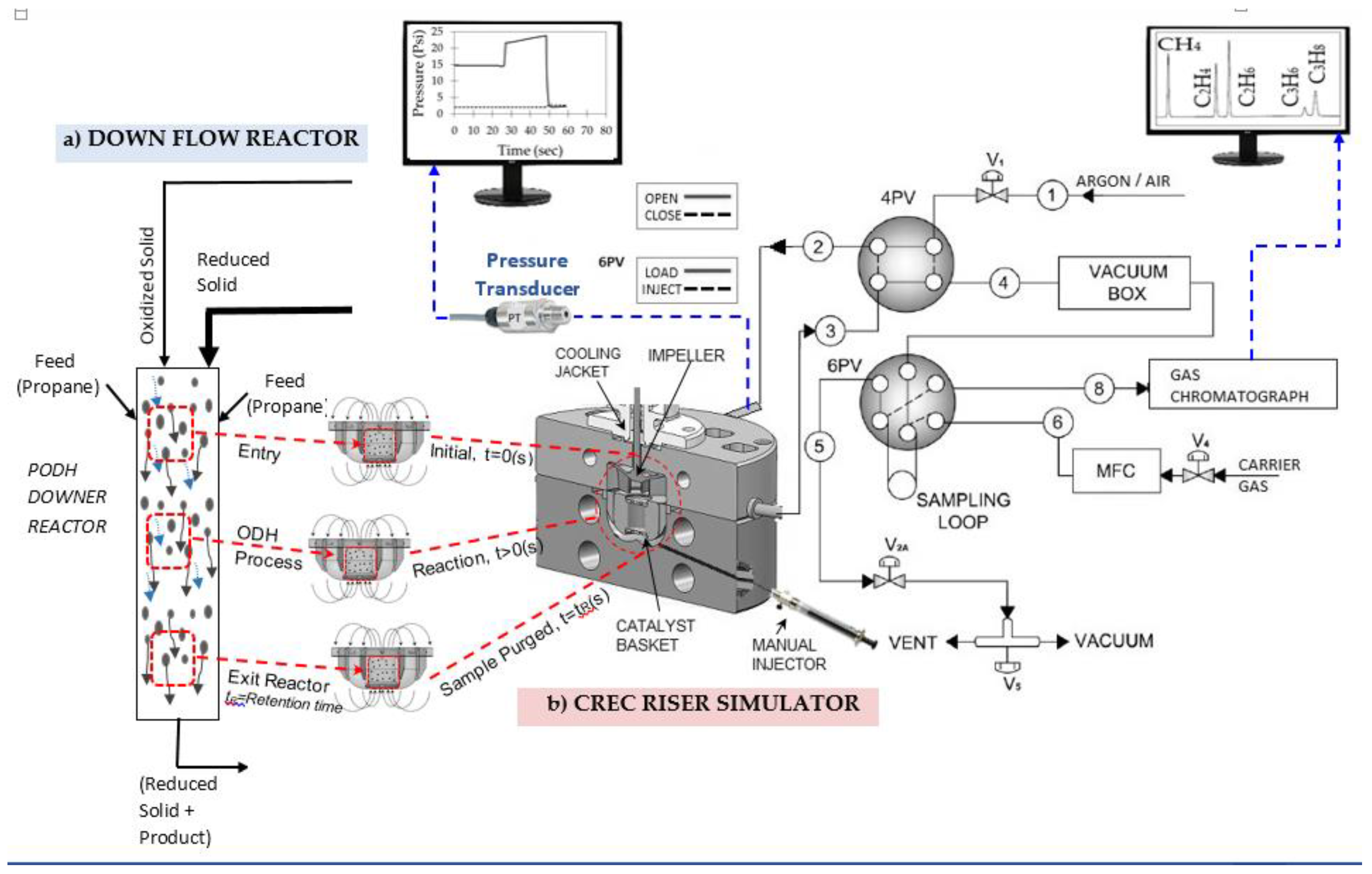
5. Experimental Laboratory Reactors for PODH
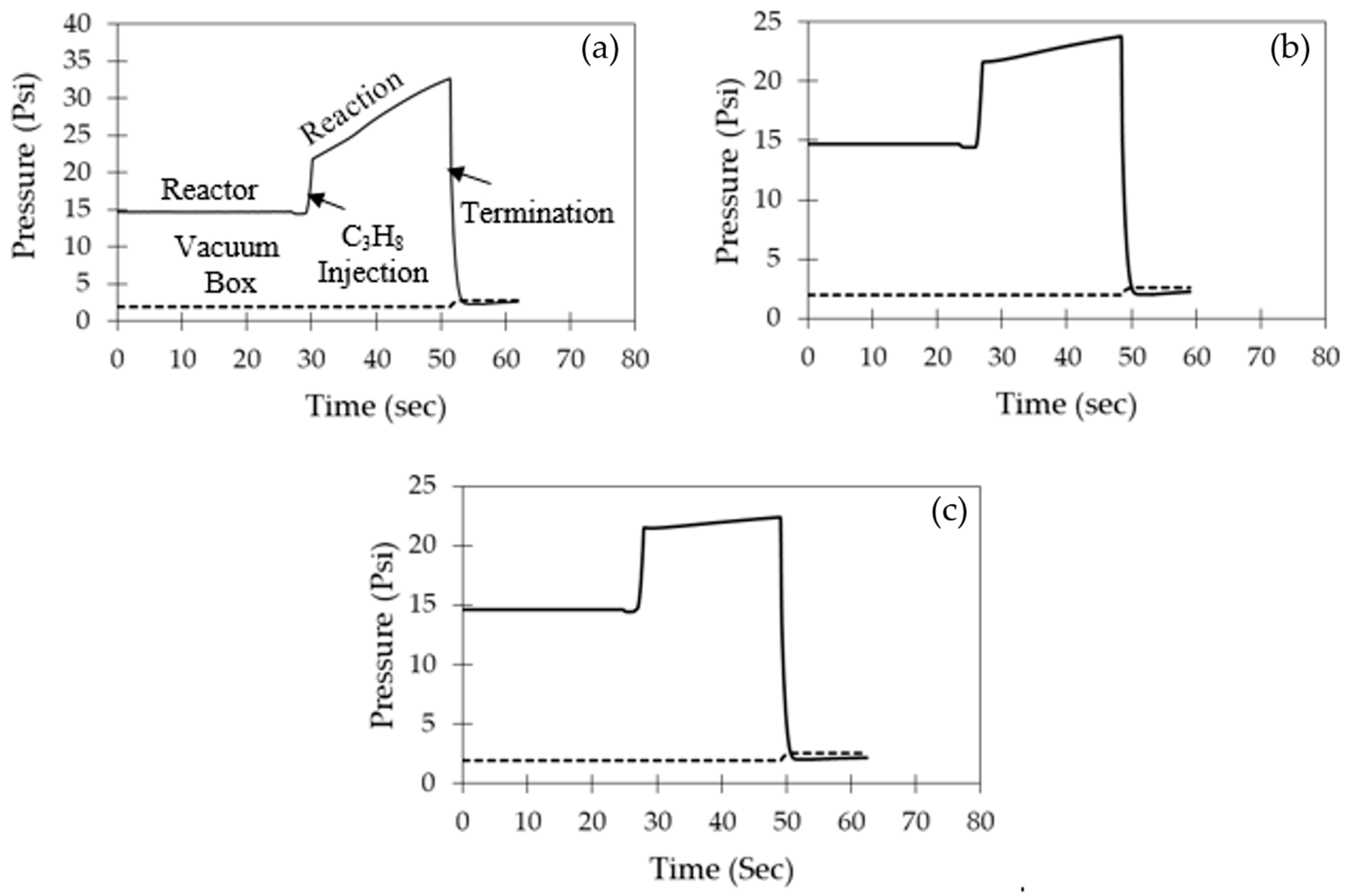
5.1. Single Propane Injection
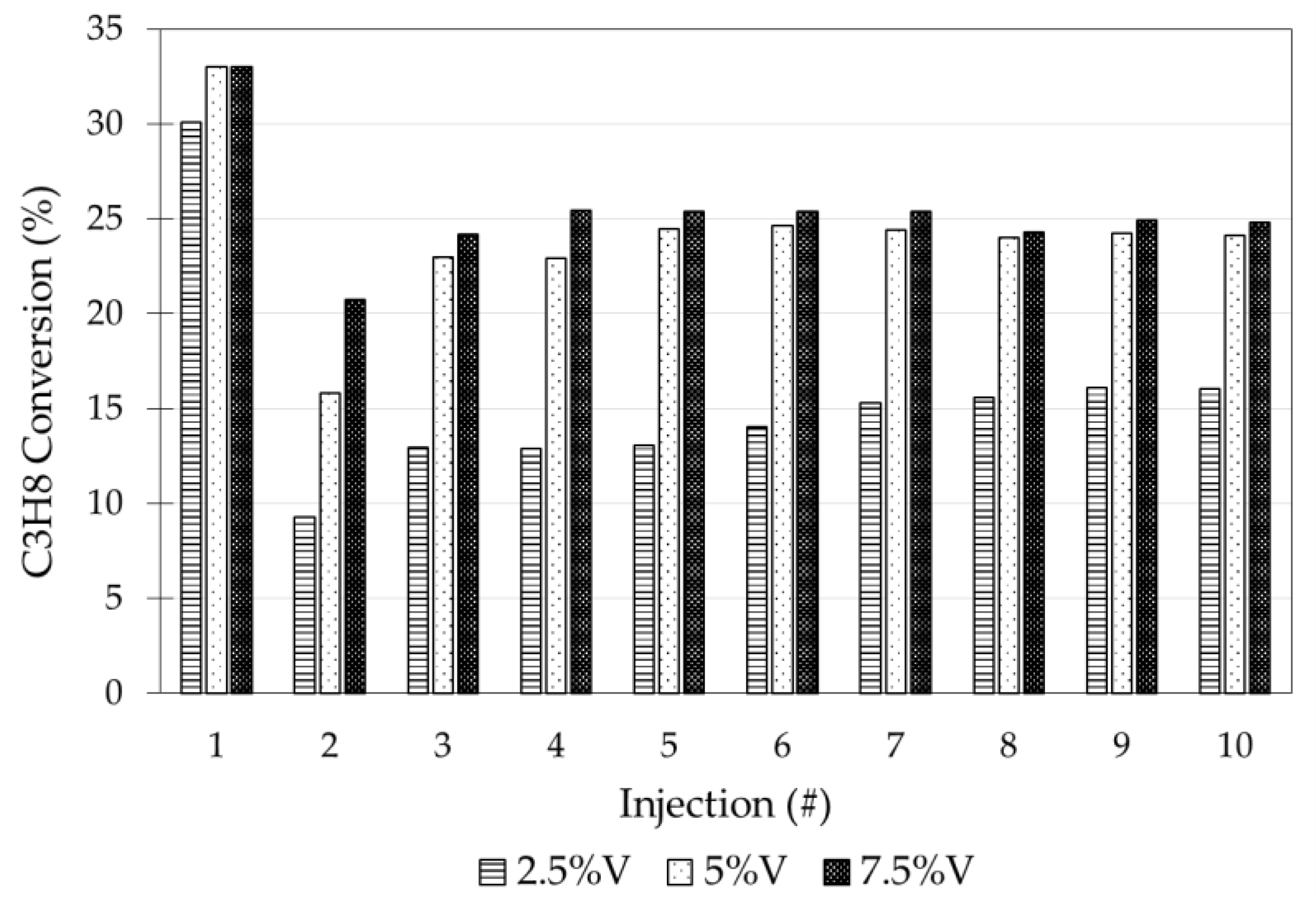
5.2. Successive Propane Injections
6. Kinetics and Reaction Mechanisms of Propane ODH over Vanadium-Based Catalysts
7. Parameters that Affect ODH Reactions
7.1. Effect of Reaction Temperature
7.2. Effect of Reaction Time
8. Reactor Concepts for PODH
8.1. Fixed-Bed Reactors
8.2. Twin Circulating Fluidized Bed Reactors for PODH
8.3. Circulating Fluidized Bed Reactor Models for PODH
9. PODH Industrial Prospects
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A.—Oxygen (O–S) Conversion during Consecutive Runs with the 7.5 wt. % V/ZrO2-γAl2O3 Catalyst

References
- Bai, P.T.; Manokaran, V.; Saiprasad, P.S.; Srinath, S. Studies on Heat and Mass Transfer Limitations in Oxidative Dehydrogenation of Ethane Over Cr2O3 /Al2O3 Catalyst. Procedia Eng. 2015, 127, 1338–1345. [Google Scholar] [CrossRef] [Green Version]
- Khadzhiev, S.N.; Usachev, N.Y.; Gerzeliev, I.M.; Belanova, E.P.; Kalinin, V.P.; Kharlamov, V.V.; Kazakov, A.V.; Kanaev, S.A.; Starostina, T.S.; Popov, A.Y. Oxidative dehydrogenation of ethane to ethylene in a system with circulating microspherical metal oxide oxygen carrier: 1. Synthesis and study of the catalytic system. Pet. Chem. 2015, 55, 651–654. [Google Scholar] [CrossRef]
- Bakare, I.A.; Mohamed, S.A.; Al-Ghamdi, S.; Razzak, S.A.; Hossain, M.M.; de Lasa, H.I. Fluidized bed ODH of ethane to ethylene over VOx-MoOx/γ-Al2O3 catalyst: Desorption kinetics and catalytic activity. Chem. Eng. J. 2015, 278, 207–216. [Google Scholar] [CrossRef]
- Zhai, Z.; Wang, X.; Licht, R.; Bell, A.T. Selective oxidation and oxidative dehydrogenation of hydrocarbons on bismuth vanadium molybdenum oxide. J. Catal. 2015, 325, 87–100. [Google Scholar] [CrossRef] [Green Version]
- H.Zea, L.C. Oxidative dehydrogenation of propane on Pd-Mo/gamma-Al2O3 catalyst: A kinetic study. Aust. J. Basic Appl. Sci. 2015, 9, 78–83. [Google Scholar]
- Khalil, Y.P. Propylene in Demand: Roadblocks and Opportunities. 2015. Available online: https://insights.globalspec.com/article/473/propylene-in-demand-roadblocks-and-opportunities (accessed on 9 April 2020).
- Rebsdat, S.; Mayer, D. Ethylene Glycol. Ullmann’s Encycl. Ind. Chem. 2012. [Google Scholar] [CrossRef]
- Darvishi, A.; Davand, R.; Khorasheh, F.; Fattahi, M. Modeling-based optimization of a fixed-bed industrial reactor for oxidative dehydrogenation of propane. Chin. J. Chem. Eng. 2016, 24, 612–622. [Google Scholar] [CrossRef]
- Elbadawi, A.H.; Osman, M.S.; Razzak, S.A.; Hossain, M.M. VOx-Nb/La-γAl2O3 catalysts for oxidative dehydrogenation of ethane to ethylene. J. Taiwan Inst. Chem. Eng. 2016, 61, 106–116. [Google Scholar] [CrossRef]
- Ayandiran, A.A.; Bakare, I.A.; Binous, H.; Al-Ghamdi, S.; Razzak, S.A.; Hossain, M.M. Oxidative dehydrogenation of propane to propylene over VOx/CaO–γ-Al2O3 using lattice oxygen. Catal. Sci. Technol. 2016, 6, 5154–5167. [Google Scholar] [CrossRef]
- Bhasin, M.M. Is true ethane oxydehydrogenation feasible? Top. Catal. 2003, 23, 145–149. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.A.; de Lasa, H.I. Propylene production via propane oxidative dehydrogenation over VOx/γ-Al2O3 catalyst. Fuel 2014, 128, 120–140. [Google Scholar] [CrossRef]
- Gao, Y.; Neal, L.M.; Li, F. Li-Promoted LaxSr2-xFeO4-δ Core—Shell Redox Catalysts for Oxidative Dehydrogenation of Ethane under a Cyclic Redox Scheme. ACS Catal. 2016, 6, 7293–7302. [Google Scholar] [CrossRef]
- Setnička, M.; Tišler, Z.; Kubička, D.; Bulánek, R. Activity of Molybdenum Oxide Catalyst Supported on Al2O3, TiO2, and SiO2 Matrix in the Oxidative Dehydrogenation of n-Butane. Top. Catal. 2015, 58, 866–876. [Google Scholar] [CrossRef]
- Koirala, R.; Buechel, R.; Pratsinis, S.E.; Baiker, A. Silica is preferred over various single and mixed oxides as support for CO2-assisted cobalt-catalyzed oxidative dehydrogenation of ethane. Appl. Catal. A Gen. 2016, 527, 96–108. [Google Scholar] [CrossRef]
- Ren, T.; Patel, M.; Blok, K. Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy 2006, 31, 425–451. [Google Scholar] [CrossRef] [Green Version]
- Barghi, B.; Fattahi, M.; Khorasheh, F. The Modeling of Kinetics and Catalyst Deactivation in Propane Dehydrogenation Over Pt-Sn/γ-Al2O3 in Presence of Water as an Oxygenated Additive. Pet. Sci. Technol. 2014, 32, 1139–1149. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F. The oxidative dehydrogenation of ethane and propane as an alternative way for the production of light olefins. Catal. Today 1995, 24, 307–313. [Google Scholar] [CrossRef]
- Sanfilippo, D. Dehydrogenation of Paraffins; Key Technology for Petrochemicals and Fuels. Cattech 2000, 4, 56–73. [Google Scholar] [CrossRef]
- Cavani, F.; Ballarini, N.; Cericola, A. Oxidative dehydrogenation of ethane and propane: How far from commercial implementation? Catal. Today 2007, 127, 113–131. [Google Scholar] [CrossRef]
- Chu, B.; Truter, L.; Nijhuis, T.A.; Cheng, Y. Oxidative dehydrogenation of ethane to ethylene over phase-pure M1 MoVNbTeOx catalysts in a micro-channel reactor. Catal. Sci. Technol. 2015, 5, 2807–2813. [Google Scholar] [CrossRef]
- Botková, Š.; Čapek, L.; Setnička, M.; Bulánek, R.; Čičmanec, P.; Kalužová, A.; Pastva, J.; Zukal, A. VOx species supported on Al2O3-SBA-15 prepared by the grafting of alumina onto SBA-15: Structure and activity in the oxidative dehydrogenation of ethane. React. Kinet Mech. Catal. 2016, 119, 319–333. [Google Scholar] [CrossRef]
- Koc, S.N.; Dayioglu, K.; Ozdemir, H. Oxidative dehydrogenation of propane with K-MoO3/MgAl2O4 catalysts. J. Chem. Sci. 2016, 128, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Li, J.; Zhao, Z.; Liu, Q.; Sun, Q.; Liu, J.; Wei, Y. Oxidative dehydrogenation of ethane to ethylene over Mo-incorporated mesoporous SBA-16 catalysts: The effect of MoOx dispersion. Appl. Catal. A Gen. 2016, 510, 84–97. [Google Scholar] [CrossRef]
- Jermy, B.R.; Ajayi, B.P.; Abussaud, B.A.; Asaoka, S.; Al-Khattaf, S. Oxidative dehydrogenation of n-butane to butadiene over Bi-Ni-O/γ-alumina catalyst. J. Mol. Catal. A Chem. 2015, 400, 121–131. [Google Scholar] [CrossRef]
- Gascón, J.; Valenciano, R.; Téllez, C.; Herguido, J.; Menéndez, M. A generalized kinetic model for the partial oxidation of n-butane to maleic anhydride under aerobic and anaerobic conditions. Chem. Eng. Sci. 2006, 61, 6385–6394. [Google Scholar] [CrossRef]
- Rubio, O.; Herguido, J.; Menéndez, M. Oxidative dehydrogenation of n-butane on V/MgO catalysts-kinetic study in anaerobic conditions. Chem. Eng. Sci. 2003, 58, 4619–4627. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, J.; He, Y.; Wu, T. Preparation and characterization of Ni-Zr-O nanoparticles and its catalytic behavior for ethane oxidative dehydrogenation. Appl. Surf. Sci. 2012, 258, 4922–4928. [Google Scholar] [CrossRef]
- Fattahi, M.; Kazemeini, M.; Khorasheh, F.; Rashidi, A. An investigation of the oxidative dehydrogenation of propane kinetics over a vanadium-graphene catalyst aiming at minimizing of the COx species. Chem. Eng. J. 2014, 250, 14–24. [Google Scholar] [CrossRef]
- Mukherjee, D.; Park, S.-E.; Reddy, B.M. CO2 as a soft oxidant for oxidative dehydrogenation reaction: An eco benign process for industry. J. CO2 Util. 2016, 16, 301–312. [Google Scholar] [CrossRef]
- Frank, B.; Dinse, A.; Ovsitser, O.; Kondratenko, E.V.; Schomäcker, R. Mass and heat transfer effects on the oxidative dehydrogenation of propane (ODP) over a low loaded VOx/Al2O3 catalyst. Appl. Catal. A Gen. 2007, 323, 66–76. [Google Scholar] [CrossRef]
- Valverde, J.A.; Echavarría, A.; Eon, J.-G.; Faro, A.C.; Palacio, L.A. V-Mg-Al catalyst from hydrotalcite for the oxidative dehydrogenation of propane. React. Kinet. Mech. Catal. 2014, 111, 679–696. [Google Scholar] [CrossRef]
- Fukudome, K.; Suzuki, T. Highly Selective Oxidative Dehydrogenation of Propane to Propylene over VOx-SiO2 Catalysts. Catal. Surv. Asia 2015, 19, 172–187. [Google Scholar] [CrossRef]
- Siahvashi, A.; Chesterfield, D.; Adesina, A.A. Nonoxidative and Oxidative Propane Dehydrogenation over Bimetallic Mo-Ni/Al2O3 Catalyst. Ind. Eng. Chem. Res. 2013, 52, 4017–4026. [Google Scholar] [CrossRef]
- Sinev, M.Y.; Fattakhova, Z.T.; Tulenin, Y.P.; Stennikov, P.S.; Vislovskii, V.P. Hydrogen formation during dehydrogenation of C2-C4 alkanes in the presence of oxygen: Oxidative or non-oxidative? Catal. Today 2003, 81, 107–116. [Google Scholar] [CrossRef]
- Fu, B.; Lu, J.; Stair, P.C.; Xiao, G.; Kung, M.C.; Kung, H.H. Oxidative dehydrogenation of ethane over alumina-supported Pd catalysts. Effect of alumina overlayer. J. Catal. 2013, 297, 289–295. [Google Scholar] [CrossRef]
- Carrero, C.; Kauer, M.; Dinse, A.; Wolfram, T.; Hamilton, N.; Trunschke, A.; Schlögl, R.; Schomäcker, R. High performance (VOx)n-(TiOx)m/SBA-15 catalysts for the oxidative dehydrogenation of propane. Catal. Sci. Technol. 2014, 4, 786. [Google Scholar] [CrossRef] [Green Version]
- Carrero, C.A.; Schlögl, R.; Wachs, I.E.; Schomaecker, R. Critical literature review of the kinetics for the oxidative dehydrogenation of propane over well-defined supported vanadium oxide catalysts. ACS Catal. 2014, 4, 3357–3380. [Google Scholar] [CrossRef]
- Dar, H.J.; Nanot, S.U.; Jens, K.J.; Jakobsen, H.A.; Tangstad, E.; Chen, D. Kinetic Analysis and Upper Bound of Ethylene Yield of Gas Phase Oxidative Dehydrogenation of Ethane to Ethylene. Ind. Eng. Chem. Res. 2012, 51, 10571–10585. [Google Scholar] [CrossRef]
- Ahmed, S.; Rahman, F.; Al-Amer, A.M.J.; Al-Mutairi, E.M.; Baduruthamal, U.; Alam, K. Oxidative dehydrogenation of lower alkanes over metal incorporated MCM-41 catalysts. React. Kinet. Mech. Catal. 2012, 105, 483–493. [Google Scholar] [CrossRef]
- Schwarz, O.; Habel, D.; Ovsitser, O.; Kondratenko, E.V.; Hess, C.; Schomäcker, R.; Schubert, H. Impact of preparation method on physico-chemical and catalytic properties of VOx/γ-Al2O3 materials. J. Mol. Catal. A Chem. 2008, 293, 45–52. [Google Scholar] [CrossRef]
- Mishanin, I.I.; Kalenchuk, A.N.; Maslakov, K.I.; Lunin, V.V.; Koklin, A.E.; Finashina, E.D.; Bogdan, V.I. Deactivation of a mixed oxide catalyst of Mo-V-Te-Nb-O composition in the reaction of oxidative ethane dehydrogenation. Russ. J. Phys. Chem. A 2016, 90, 1132–1136. [Google Scholar] [CrossRef]
- Ajayi, B.P.; Rabindran Jermy, B.; Abussaud, B.A.; Al-Khattaf, S. Oxidative dehydrogenation of n-butane over bimetallic mesoporous and microporous zeolites with CO2 as mild oxidant. J. Porous Mater. 2013, 20, 1257–1270. [Google Scholar] [CrossRef]
- Koirala, R.; Buechel, R.; Krumeich, F.; Pratsinis, S.E.; Baiker, A. Oxidative Dehydrogenation of Ethane with CO2 over Flame-Made Ga-Loaded TiO2. ACS Catal. 2015, 5, 690–702. [Google Scholar] [CrossRef]
- Kowalska-Kuś, J.; Held, A.; Nowińska, K. Oxydehydrogenation of C2-C4 hydrocarbons over Fe-ZSM-5 zeolites with N2O as an oxidant. Catal. Sci. Technol. 2013, 3, 508–518. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Cherian, M.; Baerns, M.; Su, D.; Schlogl, R.; Wang, X.; Wachs, I.E. Oxidative dehydrogenation of propane over V/MCM-41 catalysts: Comparison of O2 and N2O as oxidants. J. Catal. 2005, 234, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Védrine, J. Heterogeneous Partial (amm) Oxidation and Oxidative Dehydrogenation Catalysis on Mixed Metal Oxides. Catalysts 2016, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Talati, A.; Haghighi, M.; Rahmani, F. Impregnation vs. coprecipitation dispersion of Cr over TiO2 and ZrO2 used as active and stable nanocatalysts in oxidative dehydrogenation of ethane to ethylene by carbon dioxide. RSC Adv. 2016, 6, 44195–44204. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Li, H.; Huang, Q. Intrinsic kinetics of oxidative dehydrogenation of propane in the presence of CO2 over Cr/MSU-1 catalyst. J. Nat. Gas Chem. 2011, 20, 311–317. [Google Scholar] [CrossRef]
- Qiao, A.; Kalevaru, V.N.; Radnik, J.; Martin, A. Oxidative dehydrogenation of ethane to ethylene over Ni-Nb-M-O catalysts: Effect of promoter metal and CO2-admixture on the performance. Catal. Today 2016, 264, 144–151. [Google Scholar] [CrossRef]
- Elfadly, A.M.; Badawi, A.M.; Yehia, F.Z.; Mohamed, Y.A.; Betiha, M.A.; Rabie, A.M. Selective nano alumina supported vanadium oxide catalysts for oxidative dehydrogenation of ethylbenzene to styrene using CO2 as soft oxidant. Egypt. J. Pet. 2013, 22, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, Y.; Thirumala Bai, P.; Hari Babu, B.; Lingaiah, N.; Rama Rao, K.S.; Prasad, P.S.S. Oxidative dehydrogenation of ethane to ethylene on Cr2O3/Al2O3-ZrO2 catalysts: The influence of oxidizing agent on ethylene selectivity. Appl. Petrochem. Res. 2014, 4, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Raju, G.; Reddy, B.M.; Abhishek, B.; Mo, Y.; Park, S. Synthesis of C4 olefins from n-butane over a novel VOx/SnO2-ZrO2 catalyst using CO2 as soft oxidant. Appl. Catal. A Gen. 2012, 423–424, 168–175. [Google Scholar] [CrossRef]
- Elbadawi, A.H.; Ba-Shammakh, M.S.; Al-Ghamdi, S.; Razzak, S.A.; Hossain, M.M.; de Lasa, H.I. Phenomenologically based kinetics of ODH of ethane to ethylene using lattice oxygen of VOx/Al2O3-ZrO2 catalyst. Chem. Eng. Res. Des. 2017, 117, 733–745. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.; Volpe, M.; Hossain, M.M.; de Lasa, H. VOx/c-Al2O3 catalyst for oxidative dehydrogenation of ethane to ethylene: Desorption kinetics and catalytic activity. Appl. Catal. A Gen. 2013, 450, 120–130. [Google Scholar] [CrossRef]
- Elbadawi, A.H.; Ba-Shammakh, M.S.; Al-Ghamdi, S.; Razzak, S.A.; Hossain, M.M.; de Lasa, H.I. A fluidizable VOx/γ-Al2O3-ZrO2 catalyst for the ODH of ethane to ethylene operating in a gas phase oxygen free environment. Chem. Eng. Sci. 2016, 145, 59–70. [Google Scholar] [CrossRef]
- Sedor, K.E.; Hossain, M.M.; De Lasa, H.I. Reduction kinetics of a fluidizable nickel-alumina oxygen carrier for chemical-looping combustion. Can. J. Chem. Eng. 2008, 86, 323–334. [Google Scholar] [CrossRef]
- Khan, M.Y.; Al-Ghamdi, S.; Razzak, S.A.; Hossain, M.M.; de Lasa, H. Fluidized bed oxidative dehydrogenation of ethane to ethylene over VOx/Ce-γAl2O3catalysts: Reduction kinetics and catalyst activity. Mol. Catal. 2017, 443, 78–91. [Google Scholar] [CrossRef]
- Rostom, S.; de Lasa, H.I. Propane Oxidative Dehydrogenation Using Consecutive Feed Injections and Fluidizable VOx/γAl2O3 and VOx/ZrO2-γAl2O3 Catalysts. Ind. Eng. Chem. Res. 2017, 56, 13109–13124. [Google Scholar] [CrossRef]
- Rostom, S.; Lasa, H. De High Propylene Selectivity via Propane Oxidative Dehydrogenation Using a Novel Fluidizable Catalyst: Kinetic Modeling. Ind. Eng. Chem. Res. 2018, 57, 10251–10260. [Google Scholar] [CrossRef]
- Hossain, M.M. Kinetics of Oxidative Dehydrogenation of Propane to Propylene Using Lattice Oxygen of VOx/CaO/γAl2O3 Catalysts. Ind. Eng. Chem. Res. 2017, 56, 4309–4318. [Google Scholar] [CrossRef]
- Fukudome, K.; Ikenaga, N.O.; Miyake, T.; Suzuki, T. Oxidative dehydrogenation of alkanes over vanadium oxide prepared with V(t-BuO)3O and Si(OEt)4 in the presence of polyethyleneglycol. Catal. Today 2013, 203, 10–16. [Google Scholar] [CrossRef]
- Fukudome, K.; Ikenaga, N.; Miyake, T.; Suzuki, T. Oxidative dehydrogenation of propane using lattice oxygen of vanadium oxides on silica. Catal. Sci. Technol. 2011, 1, 987. [Google Scholar] [CrossRef]
- Rischard, J.; Antinori, C.; Maier, L.; Deutschmann, O. Oxidative dehydrogenation of n-butane to butadiene with Mo-V-MgO catalysts in a two-zone fluidized bed reactor. Appl. Catal. A Gen. 2016, 511, 23–30. [Google Scholar] [CrossRef]
- Rodríguez, M.L.; Ardissone, D.E.; López, E.; Pedernera, M.N.; Borio, D.O. Reactor designs for ethylene production via ethane ODH: Comparison of performance. Engineering 2010, 2010, 45–46. [Google Scholar]
- Chalakov, L.; Rihko-Struckmann, L.K.; Munder, B.; Sundmacher, K. Oxidative dehydrogenation of ethane in an electrochemical packed-bed membrane reactor: Model and experimental validation. Chem. Eng. J. 2009, 145, 385–392. [Google Scholar] [CrossRef]
- Zaynali, Y.; Alavi-Amleshi, S.M. Comparative study of propane oxidative dehydrogenation in fluidized and fixed bed reactor. Part. Sci. Technol. 2016, 6351, 1–7. [Google Scholar] [CrossRef]
- Torabi, A.; Kazemeini, M.; Fattahi, M. Developing a mathematical model for the oxidative dehydrogenation of propane in a fluidized bed reactor. Asia-Pac. J. Chem. Eng. 2016, 11, 448–459. [Google Scholar] [CrossRef]
- Smith, J.M.; Van Ness, H.C.; Abbott, M.M. Introduction to Chemical Engineering Thermodynamics; McGraw-Hill Education: New York, NY, USA, 2005; Volume 27. [Google Scholar]
- Hossain, M.M. Chemical-Looping Combustion with Gaseous Fuels: Thermodynamic Parametric Modeling. Arab. J. Sci. Eng. 2014, 39, 3415–3421. [Google Scholar] [CrossRef]
- De Lasa, H.; Salaices, E.; Mazumder, J.; Lucky, R. Catalytic steam gasification of biomass: Catalysts, thermodynamics and kinetics. Chem. Rev. 2011, 111, 5404–5433. [Google Scholar] [CrossRef]
- Grant, J.T.; Love, A.M.; Carrero, C.A.; Huang, F.; Panger, J.; Verel, R.; Hermans, I. Improved Supported Metal Oxides for the Oxidative Dehydrogenation of Propane. Top. Catal. 2016, 59, 1545–1553. [Google Scholar] [CrossRef]
- Zea, H.; Carballo, L.M. Kinetic evaluation of Pd alumina supported catalyst for the reaction of oxidative dehydrogenation of propane. ARPN J. Eng. Appl. Sci. 2015, 10, 896–900. [Google Scholar]
- Qiao, A.; Kalevaru, V.N.; Radnik, J.; Düvel, A.; Heitjans, P.; Kumar, A.S.H.; Prasad, P.S.S.; Lingaiah, N.; Martin, A. Oxidative Dehydrogenation of Ethane to Ethylene over V2O5/Al2O3 Catalysts: Effect of Source of Alumina on the Catalytic Performance. Ind. Eng. Chem. Res. 2014, 53, 18711–18721. [Google Scholar] [CrossRef]
- Elbadawi, A.H.; Ba-Shammakh, M.S.; Al-Ghamdi, S.; Razzak, S.A.; Hossain, M.M. Reduction kinetics and catalytic activity of VOx/γ-Al2O3-ZrO2 for gas phase oxygen free ODH of ethane. Chem. Eng. J. 2016, 284, 448–457. [Google Scholar] [CrossRef]
- Zaynali, Y.; Alavi, S. Higher propene yield by tailoring operating conditions of propane oxidative dehydrogenation over V2O5/γ-Al2O3. J. Serb. Chem. Soc. 2015, 80, 355–366. [Google Scholar] [CrossRef]
- Fattahi, M.; Kazemeini, M.; Khorasheh, F.; Rashidi, A.M. Vanadium Pentoxide Catalyst over Carbon-Based Nanomaterials for the Oxidative Dehydrogenation of Propane. Ind. Eng. Chem. Res. 2013, 52, 16128–16141. [Google Scholar] [CrossRef]
- Kazemeini, M. Physicochemical Properties and Catalytic Performances of Nanostructured V2O5 over TiO2 and γ-Al2O3 for Oxidative Dehydrogenation of Propane. Chem. Biochem. Eng. Q. J. 2016, 30, 9–18. [Google Scholar] [CrossRef]
- Oyama, S.T.; Somorjai, G.A. Effect of structure in selective oxide catalysis: Oxidation reactions of ethanol and ethane on vanadium oxide. J. Phys. Chem. 1990, 94, 5022–5028. [Google Scholar] [CrossRef]
- Ciambelli, P.; Galli, P.; Lisi, L.; Massucci, M.A.; Patrono, P.; Pirone, R.; Ruoppolo, G.; Russo, G. TiO2 supported vanadyl phosphate as catalyst for oxidative dehydrogenation of ethane to ethylene. Appl. Catal. A Gen. 2000, 203, 133–142. [Google Scholar] [CrossRef]
- Dinse, A.; Schomäcker, R.; Bell, A.T. The role of lattice oxygen in the oxidative dehydrogenation of ethane on alumina-supported vanadium oxide. Phys. Chem. Chem. Phys. 2009, 11, 6119. [Google Scholar] [CrossRef]
- Xu, B.; Zhu, X.; Cao, Z.; Yang, L.; Yang, W. Catalytic oxidative dehydrogenation of n-butane over V2O5/MO-Al2O3 (M = Mg, Ca, Sr, Ba) catalysts. Chin. J. Catal. 2015, 36, 1060–1067. [Google Scholar] [CrossRef]
- Martínez-Huerta, M.V.; Gao, X.; Tian, H.; Wachs, I.E.; Fierro, J.L.G.; Bañares, M.A. Oxidative dehydrogenation of ethane to ethylene over alumina-supported vanadium oxide catalysts: Relationship between molecular structures and chemical reactivity. Catal. Today 2006, 118, 279–287. [Google Scholar] [CrossRef]
- Enache, D.I.; Bordes-Richard, E.; Ensuque, A.; Bozon-Verduraz, F. Vanadium oxide catalysts supported on zirconia and titania I. Preparation and characterization. Appl. Catal. A Gen. 2004, 278, 93–102. [Google Scholar]
- Gao, X. In Situ UV-vis-NIR Diffuse Reflectance and Raman Spectroscopic Studies of Propane Oxidation over ZrO2-Supported Vanadium Oxide Catalysts. J. Catal. 2002, 209, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Rossetti, I.; Mancini, G.F.; Ghigna, P.; Scavini, M.; Piumetti, M.; Bonelli, B.; Cavani, F.; Comite, A. Spectroscopic enlightening of the local structure of VOx active sites in catalysts for the ODH of propane. J. Phys. Chem. C 2012, 116, 22386–22398. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Keller, D.E. Chemistry, spectroscopy and the role of supported vanadium oxides in heterogeneous catalysis. Catal. Today 2003, 78, 25–46. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Kim, H.S.; Stair, P.C.; Rugmini, S.; Jackson, S.D. On the structure of vanadium oxide supported on aluminas: UV and visible Raman spectroscopy, UV-visible diffuse reflectance spectroscopy, and temperature-programmed reduction studies. J. Phys. Chem. B 2005, 109, 2793–2800. [Google Scholar] [CrossRef]
- Argyle, M.D.; Chen, K.; Bell, A.T.; Iglesia, E. Effect of catalyst structure on oxidative dehydrogenation of ethane and propane on alumina-supported vanadia. J. Catal. 2002, 208, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Klose, F.; Wolff, T.; Lorenz, H.; Seidelmorgenstern, A.; Suchorski, Y.; Piorkowska, M.; Weiss, H. Active species on γ-alumina-supported vanadia catalysts: Nature and reducibility. J. Catal. 2007, 247, 176–193. [Google Scholar] [CrossRef]
- Murgia, V.; Sham, E.; Gottifredi, J.C.; Torres, E.M.F. Oxidative dehydrogenation of propane and n-butane over alumina supported vanadium catalysts. Lat. Am. Appl. Res. 2004, 34, 75–82. [Google Scholar]
- Wachs, I.E.; Jehng, J.-M.; Deo, G.; Weckhuysen, B.M.; Guliants, V.V.; Benziger, J.B. In situ Raman spectroscopy studies of bulk and surface metal oxide phases during oxidation reactions. Catal. Today 1996, 32, 47–55. [Google Scholar] [CrossRef]
- Wachs, I.E.; Weckhuysen, B.M. Structure and reactivity of surface vanadium oxide species on oxide supports. Appl. Catal. A Gen. 1997, 157, 67–90. [Google Scholar] [CrossRef] [Green Version]
- Deo, G.; Wachs, I.E. Reactivity of Supported Vanadium Oxide Catalysts: The Partial Oxidation of Methanol. J. Catal. 1994, 146, 323–334. [Google Scholar] [CrossRef]
- Blasco, T.; Nieto, J.M.L.; Dejoz, A.; Vázquez, M.I. Influence of the acid-base character of supported vanadium catalysts on their catalytic properties for the oxidative dehydrogenation of n-butane. J. Catal. 1995, 157, 271–282. [Google Scholar] [CrossRef]
- Nieto, J.L. The selective oxidative activation of light alkanes. From supported vanadia to multicomponent bulk V-containing catalysts. Top. Catal. 2006, 41, 3–15. [Google Scholar] [CrossRef]
- Blasco, T.; Lopez-Nieto, J.M. Oxidative dyhydrogenation of short chain alkanes on supported vanadium oxide catalysts. Appl. Catal. A Gen. 1997, 157, 117–142. [Google Scholar] [CrossRef]
- Galli, A.; López Nieto, J.M.; Dejoz, A.; Vazquez, M.I. The effect of potassium on the selective oxidation of n-butane and ethane over Al2O3-supported vanadia catalysts. Catal. Lett. 1995, 34, 51–58. [Google Scholar] [CrossRef]
- Chen, K.; Bell, A.T.; Iglesia, E. The Relationship between the Electronic and Redox Properties of Dispersed Metal Oxides and Their Turnover Rates in Oxidative Dehydrogenation Reactions. J. Catal. 2002, 209, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Lemonidou, A.A.; Nalbandian, L.; Vasalos, I.A. Oxidative Dehydrogenation of Propane over Vanadium Oxide Based Catalysts: Effect of Support and Alkali Promoter. Catal. Today 2000, 61, 333–341. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.; Moreira, J.; de Lasa, H. Kinetic Modeling of Propane Oxidative Dehydrogenation over VOx/γ-Al2 O3 Catalysts in the Chemical Reactor Engineering Center Riser Reactor Simulator. Ind. Eng. Chem. Res. 2014, 53, 15317–15332. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.A.; Hossain, M.M.; de Lasa, H.I. Kinetic Modeling of Ethane Oxidative Dehydrogenation over VOx/Al2O3 Catalyst in a Fluidized-Bed Riser Simulator. Ind. Eng. Chem. Res. 2013, 52, 5235–5244. [Google Scholar] [CrossRef]
- De Lasa, H.I. Riser Simulator. U.S. Patent 5,102,628, 7 April 1992. [Google Scholar]
- De Lasa, H. Reactor and Multifunctional Riser and Downer Simulator. U.S. Patent 10,220,363, 5 March 2019. [Google Scholar]
- Baerns, M.; Buyevskaya, O.V. Simple Chemical Processes Based on Low Molecular-Mass Alkanes as Chemical Feedstocks. Catal. Today 1998, 45, 13–22. [Google Scholar] [CrossRef]
- Dinse, A.; Frank, B.; Hess, C.; Habel, D.; Schomäcker, R. Oxidative dehydrogenation of propane over low-loaded vanadia catalysts: Impact of the support material on kinetics and selectivity. J. Mol. Catal. A Chem. 2008, 289, 28–37. [Google Scholar] [CrossRef]
- Daniell, W.; Ponchel, A.; Kuba, S.; Anderle, F.; Weingand, T.; Gregory, D.H.; Kno, H. Characterization and catalytic behavior of VOx-CeO2 catalysts for the oxidative dehydrogenation of propane. Top. Catal. 2002, 20, 65–74. [Google Scholar] [CrossRef]
- Yang, S.; Iglesia, E.; Bell, A.T. Oxidative dehydrogenation of propane over V2O5/MoO3/Al2O3 and V2O5/Cr2O3/Al2O3: Structural characterization and catalytic function. J. Phys. Chem. B 2005, 109, 8987–9000. [Google Scholar] [CrossRef] [Green Version]
- Grabowski, R.; Słoczyński, J. Kinetics of oxidative dehydrogenation of propane and ethane on VOx/SiO2 pure and with potassium additive. Chem. Eng. Process. Process Intensif. 2005, 44, 1082–1093. [Google Scholar] [CrossRef]
- Chen, K.; Iglesia, E.; Bell, A.T. Kinetic isotopic effects in oxidative dehydrogenation of propane on vanadium oxide catalysts. J. Catal. 2000, 192, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Balcaen, V.; Sack, I.; Olea, M.; Marin, G.B. Transient kinetic modeling of the oxidative dehydrogenation of propane over a vanadia-based catalyst in the absence of O2. Appl. Catal. A Gen. 2009, 371, 31–42. [Google Scholar] [CrossRef]
- Farjoo, A.; Khorasheh, F.; Niknaddaf, S.; Soltani, M. Kinetic modeling of side reactions in propane dehydrogenation over Pt-Sn/γ-Al2O3 catalyst. Sci. Iran. 2011, 18, 458–464. [Google Scholar] [CrossRef]
- Fattahi, M.; Kazemeini, M.; Khorasheh, F.; Rashidi, A. Kinetic modeling of oxidative dehydrogenation of propane (ODHP) over a vanadium–graphene catalyst: Application of the DOE and ANN methodologies. J. Ind. Eng. Chem. 2014, 20, 2236–2247. [Google Scholar] [CrossRef]
- You, R.; Zhang, X.; Luo, L.; Pan, Y.; Pan, H.; Yang, J.; Wu, L.; Zheng, X.; Jin, Y.; Huang, W. NbOx/CeO2-rods catalysts for oxidative dehydrogenation of propane: Nb-CeO2 interaction and reaction mechanism. J. Catal. 2017, 348, 189–199. [Google Scholar] [CrossRef]
- Wang, L.; Chu, W.; Jiang, C.; Liu, Y.; Wen, J.; Xie, Z. Oxidative dehydrogenation of propane over Ni-Mo-Mg-O catalysts. J. Nat. Gas Chem. 2012, 21, 43–48. [Google Scholar] [CrossRef]
- Fattahi, M.; Kazemeini, M.; Khorasheh, F.; Darvishi, A.; Rashidi, A.M. Fixed-bed multi-tubular reactors for oxidative dehydrogenation in ethylene process. Chem. Eng. Technol. 2013, 36, 1691–1700. [Google Scholar] [CrossRef]
- Kotanjac, Ž.S.; van Sint Annaland, M.; Kuipers, J.A.M. A packed bed membrane reactor for the oxidative dehydrogenation of propane on a Ga2O3/MoO3 based catalyst. Chem. Eng. Sci. 2010, 65, 441–445. [Google Scholar] [CrossRef]
- Che-Galicia, G.; Ruiz-Martínez, R.S.; López-Isunza, F.; Castillo-Araiza, C.O. Modeling of oxidative dehydrogenation of ethane to ethylene on a MoVTeNbO/TiO2 catalyst in an industrial-scale packed bed catalytic reactor. Chem. Eng. J. 2015, 280, 682–694. [Google Scholar] [CrossRef]
- Elbadawi, A.H.; Khan, M.Y.; Quddus, M.R.; Razzak, S.A.; Hossain, M.M. Kinetics of oxidative cracking of n-hexane to olefins over VOx/Ce-Al2O3 under gas phase oxygen-free environment. AIChE J. 2017, 63, 130–138. [Google Scholar] [CrossRef]
- Argyle, M.; Bartholomew, C. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Rostom, S.; Lasa, H. De Chemical Engineering & Processing: Process Intensification Downer fluidized bed reactor modeling for catalytic propane oxidative dehydrogenation with high propylene selectivity. Chem. Eng. Process. Process Intensif. 2019, 137, 87–99. [Google Scholar]
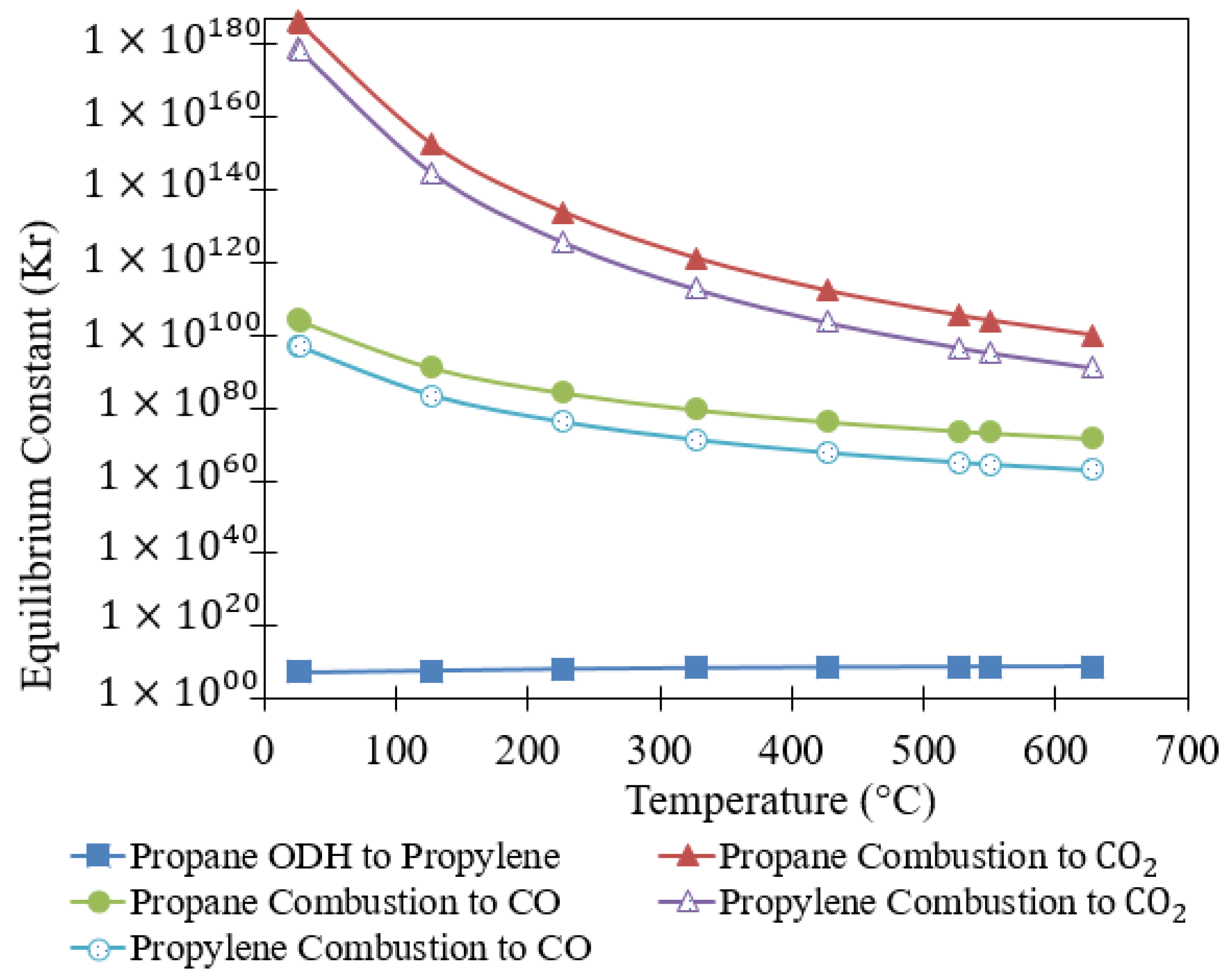



| Possible Reactions | Chemical Reactions | K(823K) | ||
|---|---|---|---|---|
| Dehydrogenation (DH) [72] | 86.2 | 1.11 × 10−1 | 124.3 | |
| 41.0 | 7.47 × 101 | 81.4 | ||
| −101.3 | 1.31 × 102 | −137.2 | ||
| −60.2 | 9.80 × 103 | −55.7 | ||
| 23.4 | 3.69 × 108 | 103.9 | ||
| O2-Rich Atmosphere [5,8,68,72,73] | −142.4 | 7.72 × 1011 | −117.6 | |
| −2074.2 | 1.83 × 10135 | −2044.0 | ||
| −1302.7 | 1.20 × 1095 | −1195.1 | ||
| −1931.8 | 2.62 × 10122 | −1926.4 | ||
| −1160.2 | 1.56 × 1083 | −1077.5 | ||
| −257.2 | 2.47 × 1013 | −283.0 | ||
| −502.6 | 1.99 × 1039 | −437.9 | ||
| −759.8 | 4.93 × 1052 | −720.8 | ||
| −360.2 | 2.58 × 1027 | −320.3 | ||
| −617.4 | 6.39 × 1040 | −603.3 | ||
| O2-Free Atmosphere [10,12] | −41.5 | 5.88 × 108 | 5.9 | |
| −1065.2 | 1.20 × 10104 | −809.7 | ||
| −596.3 | 1.79 × 1073 | −331.1 | ||
| −1023.6 | 2.04 × 1095 | −815.6 | ||
| −554.8 | 3.05 × 1064 | −337.0 | ||
| Catalyst Regeneration [10] | −201.8 | 1.72 × 106 | −246.9 |
| Conversion | |
| where, nC3H8,in = moles of propane in; nC3H8,out = moles of propane out; νi = number of carbon atoms in gaseous carbon containing product i; ni = moles of gaseous carbon containing product i. | |
| Selectivity | |
| where, nii = moles of product i; nT = total products moles. | |
| Yield | |
| ODHP Catalyst | Feed | Reactor | Method | T (°C) | Reaction Time | XC3H8 | Selectivity (%) | YC3H6 | Year | |
|---|---|---|---|---|---|---|---|---|---|---|
| SC3H6 | SCOx | |||||||||
| 7.5 wt.% V/ZrO2-γAl2O3 | C3H8 | Fluidized bed | Successive | 550 | 20 s | 25.00 | 94.00 | 2.10 | 23.50 | 2017 [59] |
| 7.5 wt.% V/γAl2O3 | C3H8 | Fluidized bed | Successive | 550 | 20 s | 25.70 | 89.30 | 3.70 | 22.90 | 2017 [59] |
| VOx/CaO-γAl2O3 | C3H8 | Fluidized bed | Successive | 550–640 | 10–31 s | 10.30–25.50 | 78.30–94.20 | 5.80–21.70 | 8.10–24.00 | 2017 [61] |
| 5% VOx/γAl2O3 | C3H8 | Fluidized bed | Successive | 475–550 | 5–20 s | 2.35–11.73 | 70.89–85.94 | 86.49–96.90 | 2.76–5.41 | 2014 [12] |
| 7% VOx/γAl2O3 | C3H8 | Fluidized bed | Successive | 475–550 | 5–20 s | 3.24–13.36 | 60.73–75.34 | 10.43–35.81 | 2.57–7.21 | 2014 [12] |
| 10% VOx/γAl2O3 | C3H8 | Fluidized bed | Successive | 475–550 | 5–20 s | 3.73–15.05 | 55.12–67.77 | 15.01–41.52 | 2.48–8.72 | 2014 [12] |
| V(1.0)-PEG25 | C3H8 | Fixed-bed | Single | 450 | 8 min | 2.00 | 94.80 | 1.90 | 1.90 | 2013 [62] |
| VOx/SiO2 | C3H8 | Fixed-bed | Single | 450 | 8 min | 3.00 | 88.30 | 7.00 | 23.90 | 2011 [63] |
| Catalyst | Activation Energy of Formation (kJ/mol) | Decay Parameter (λ) | Year | ||
|---|---|---|---|---|---|
| C3H6 | Carbon Oxides | ||||
| 7.5 wt.% V/ZrO2-γAl2O3 | 55.7 | (COx) 33.3 a | (COx) 98.5 a | 0 | 2018 [60] |
| 10% VOx/CaO-γAl2O3 (1:1) | 120.3 | (CO2) 55.1 a | (CO2) 53.7 b | 1.6×10−3 ± 0.6×10−3 | 2017 [61] |
| 5% VOx/γAl2O3 | 124.92 | (COx) 52.81 a | (COx) 52.54 b | 0.01−0.053 | 2014 [101] |
| 7% VOx/γAl2O3 | 115.08 | (COx) 51.07 a | (COx) 52.73 b | 0.017–0.056 | 2014 [101] |
| 10% VOx/γAl2O3 | 109.42 | (COx) 45.58 a | (COx) 53.75 b | 0.015–0.047 | 2014 [101] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostom, S.; de Lasa, H. Propane Oxidative Dehydrogenation on Vanadium-Based Catalysts under Oxygen-Free Atmospheres. Catalysts 2020, 10, 418. https://doi.org/10.3390/catal10040418
Rostom S, de Lasa H. Propane Oxidative Dehydrogenation on Vanadium-Based Catalysts under Oxygen-Free Atmospheres. Catalysts. 2020; 10(4):418. https://doi.org/10.3390/catal10040418
Chicago/Turabian StyleRostom, Samira, and Hugo de Lasa. 2020. "Propane Oxidative Dehydrogenation on Vanadium-Based Catalysts under Oxygen-Free Atmospheres" Catalysts 10, no. 4: 418. https://doi.org/10.3390/catal10040418