On the Activity and Selectivity of CoAl and CoAlCe Mixed Oxides in Formaldehyde Production from Pulp Mill Emissions
Abstract
:1. Introduction
2. Results and Discussion
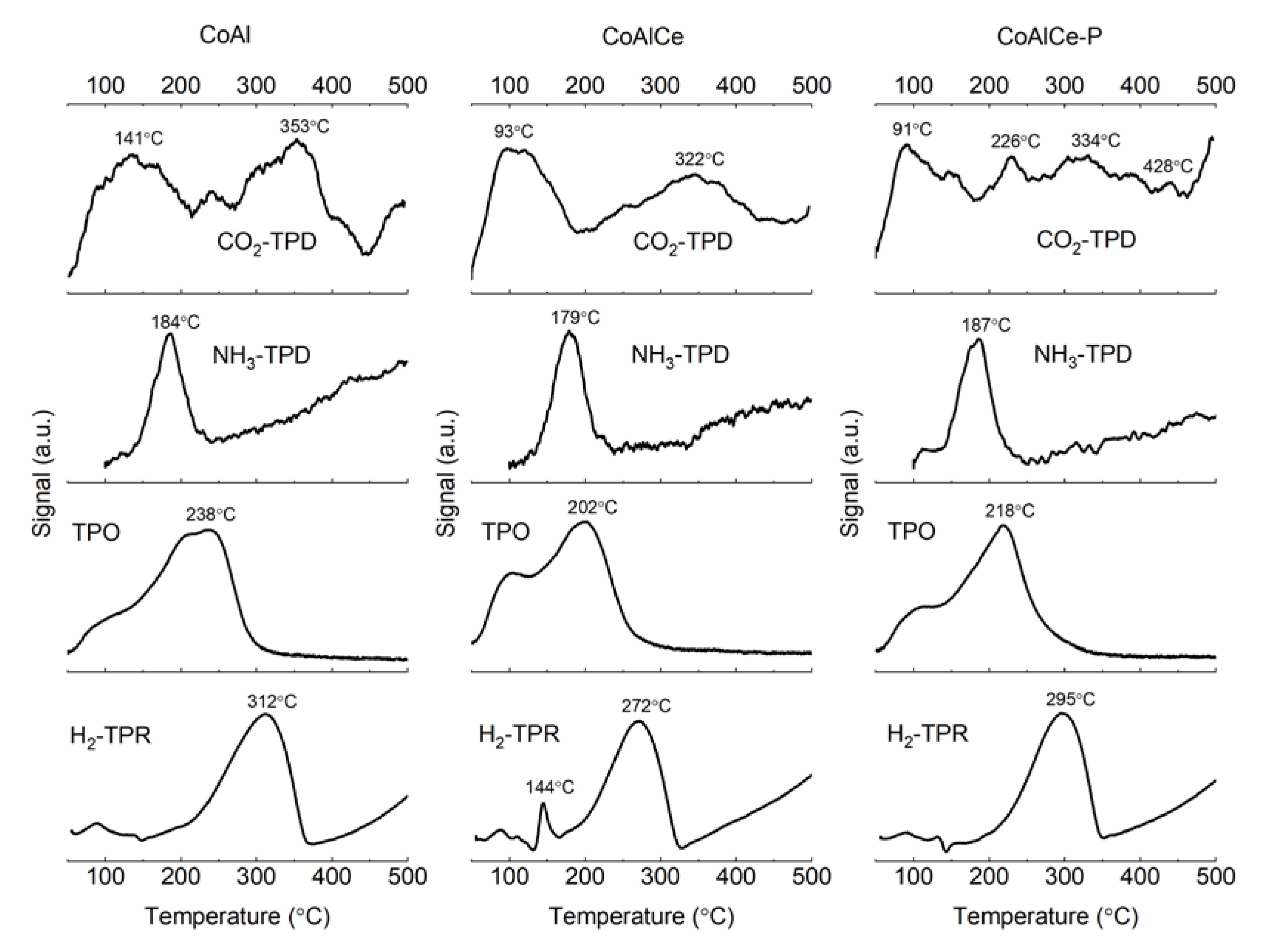
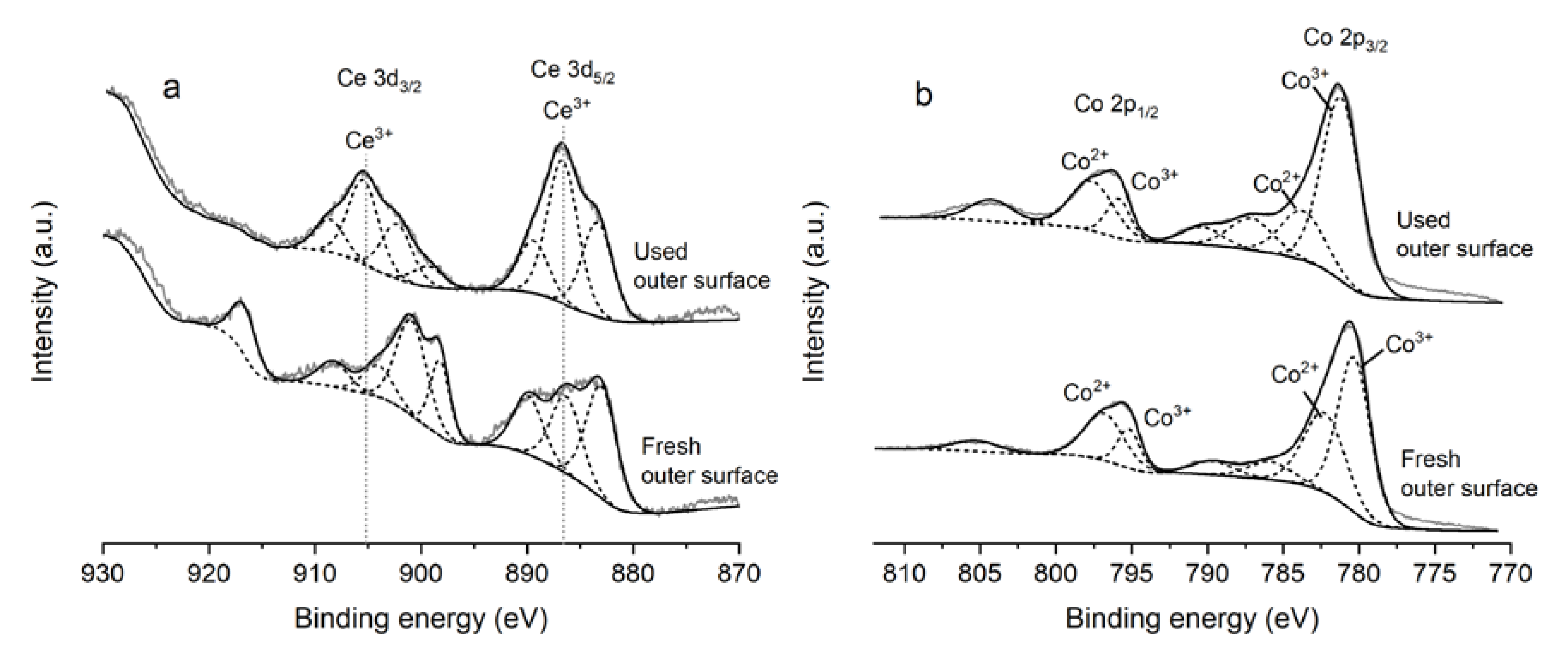
2.1. Characterization
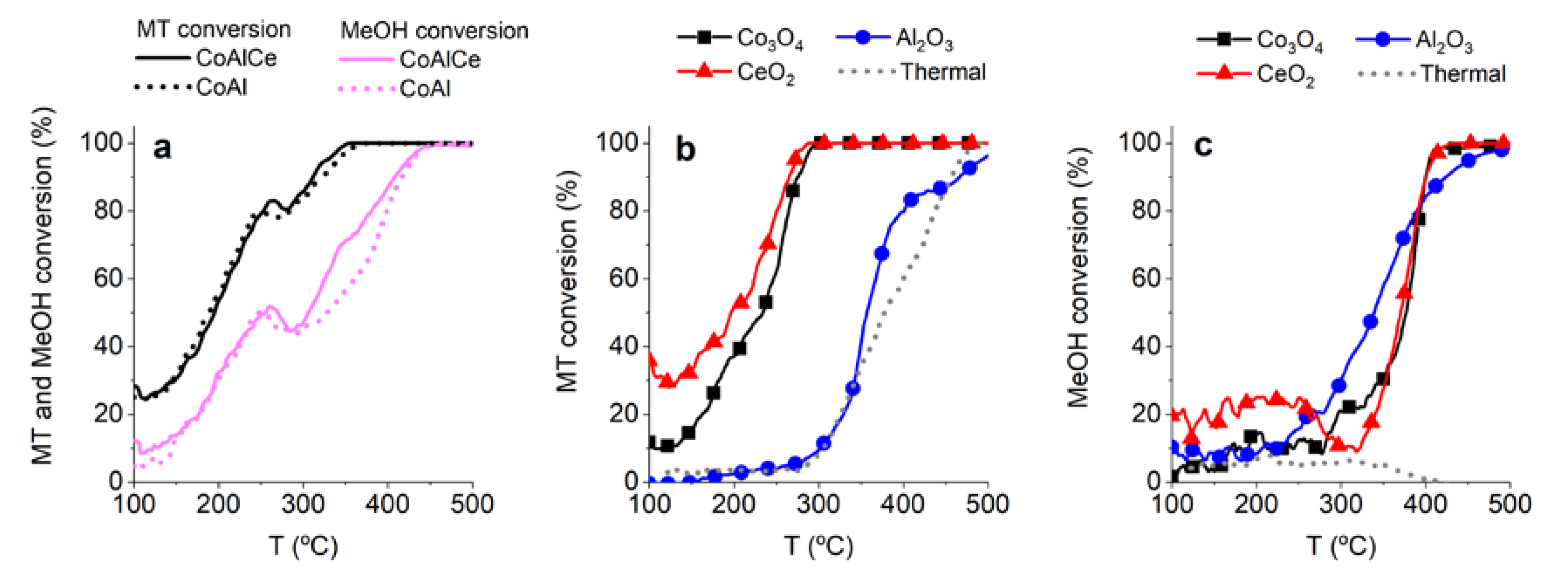
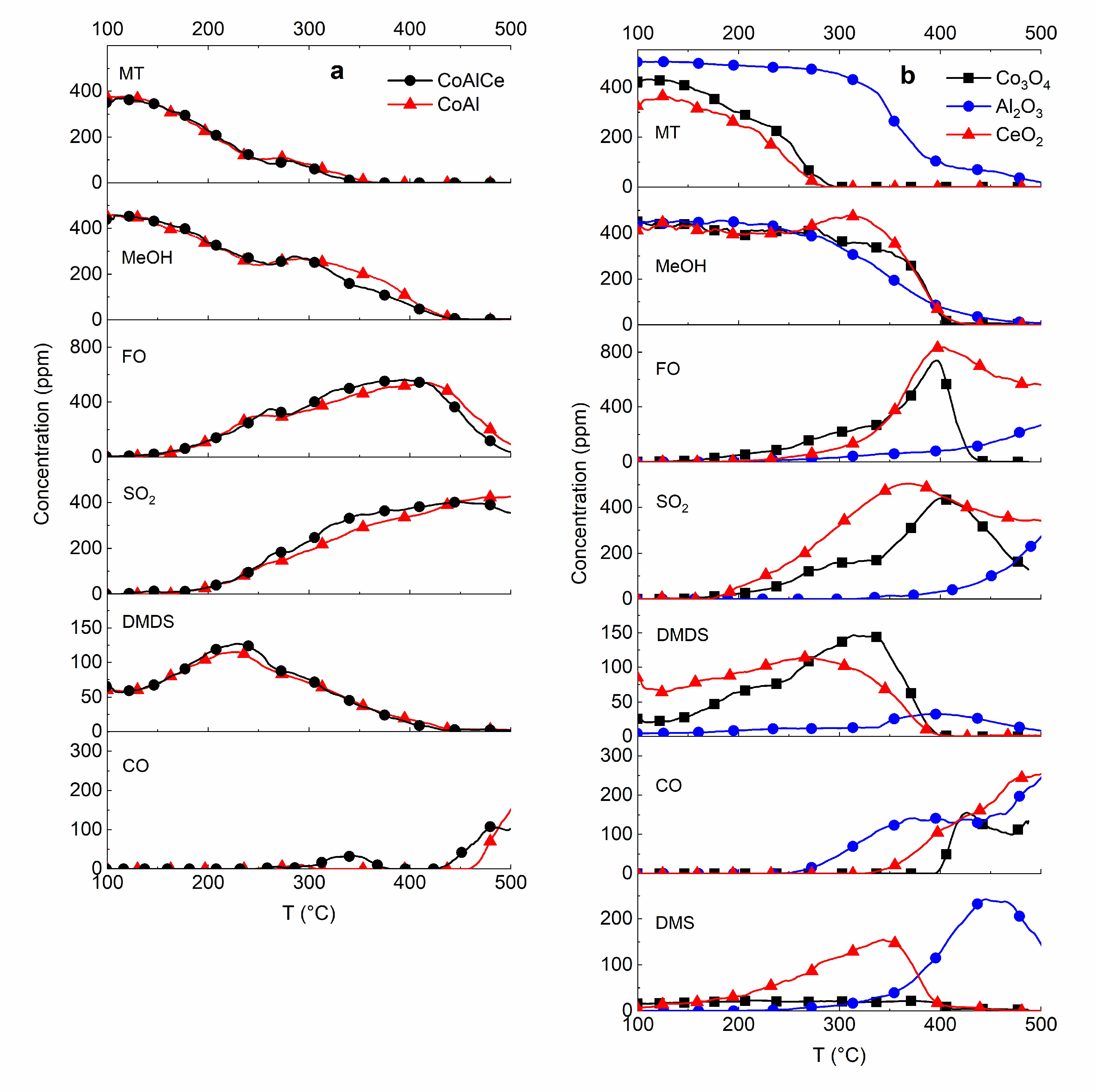
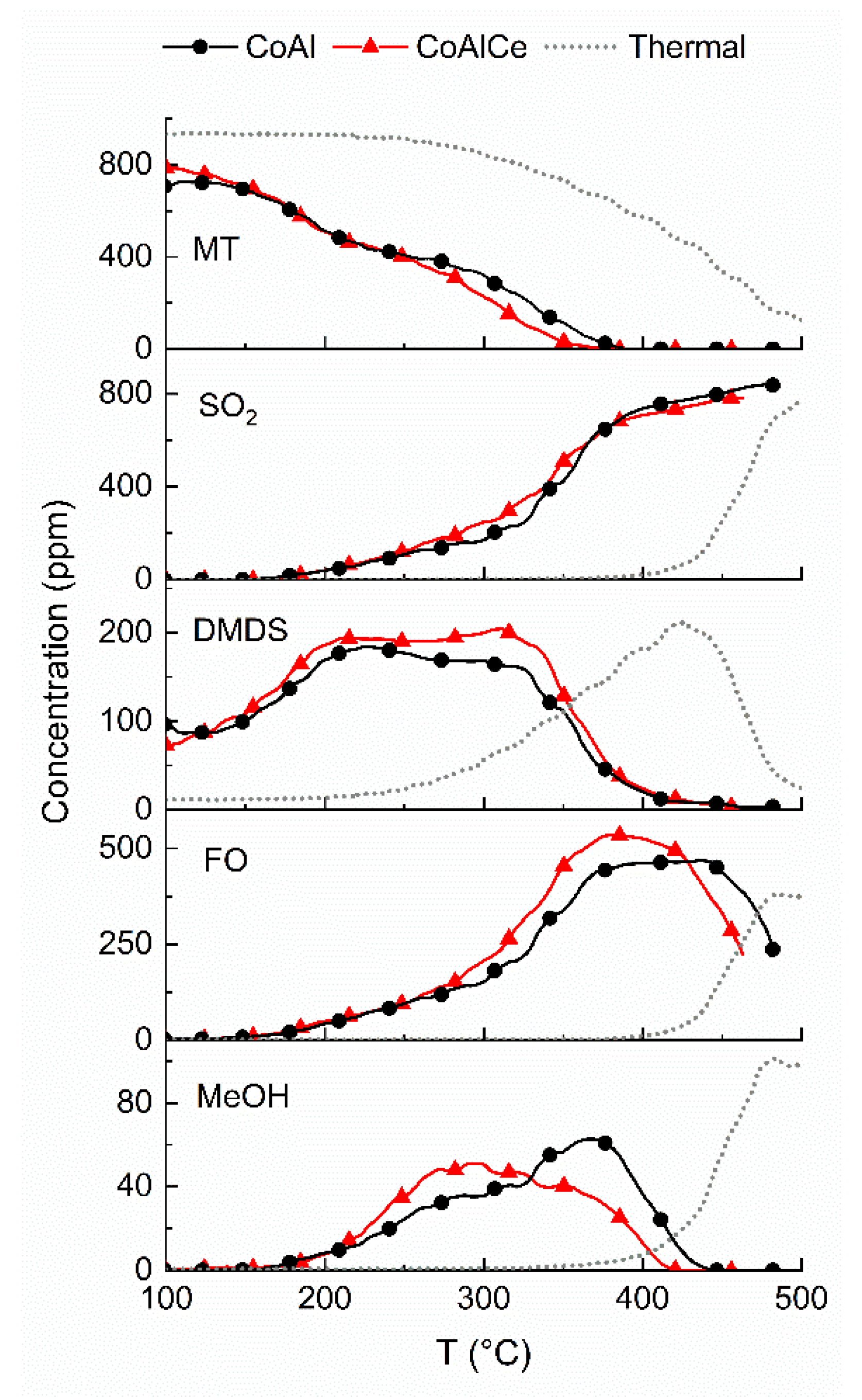
2.2. Production of Formaldehyde from Methanol and Methanethiol Mixture
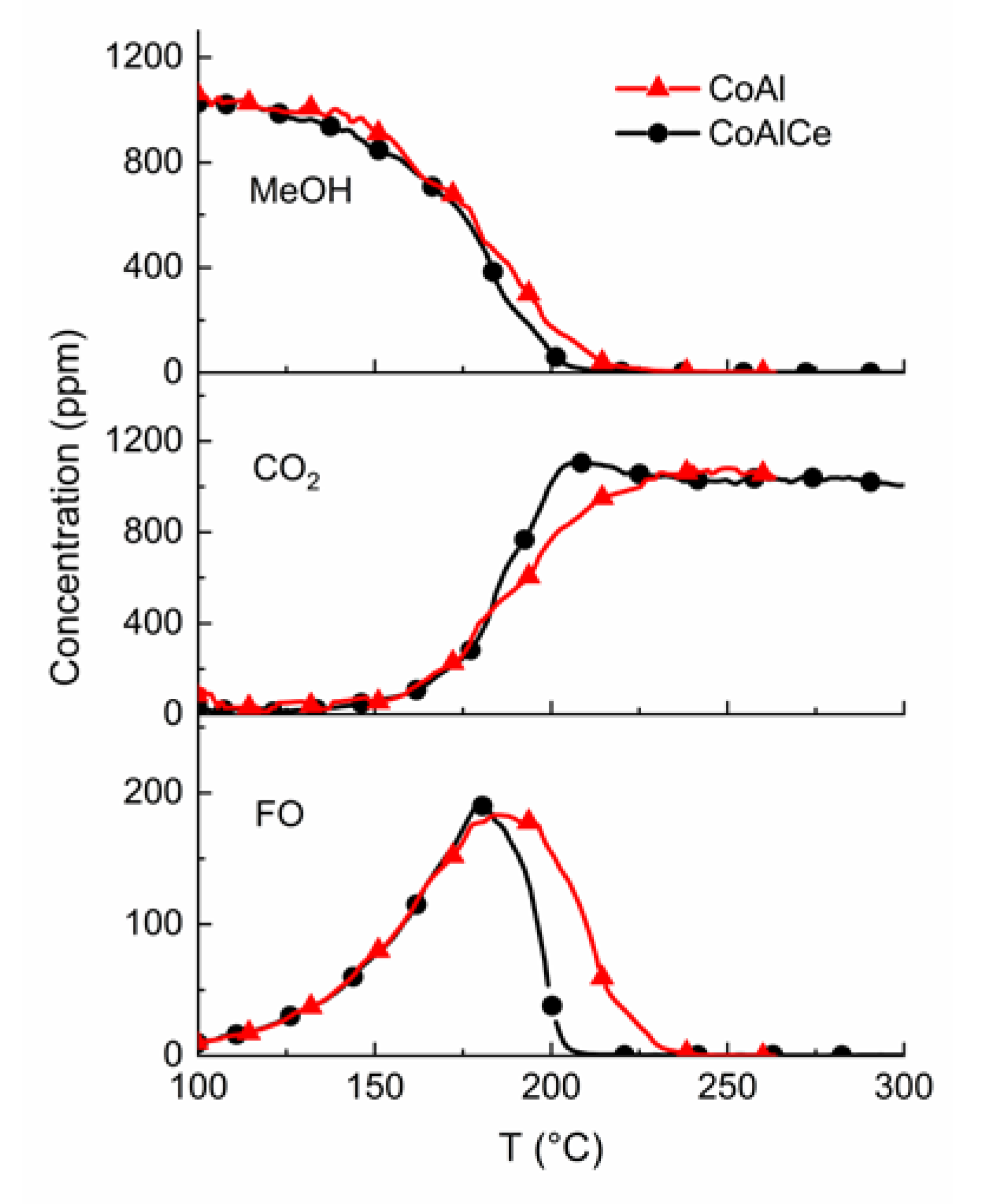
2.3. From Methanol to Formaldehyde
2.4. From Methanethiol to Formaldehyde
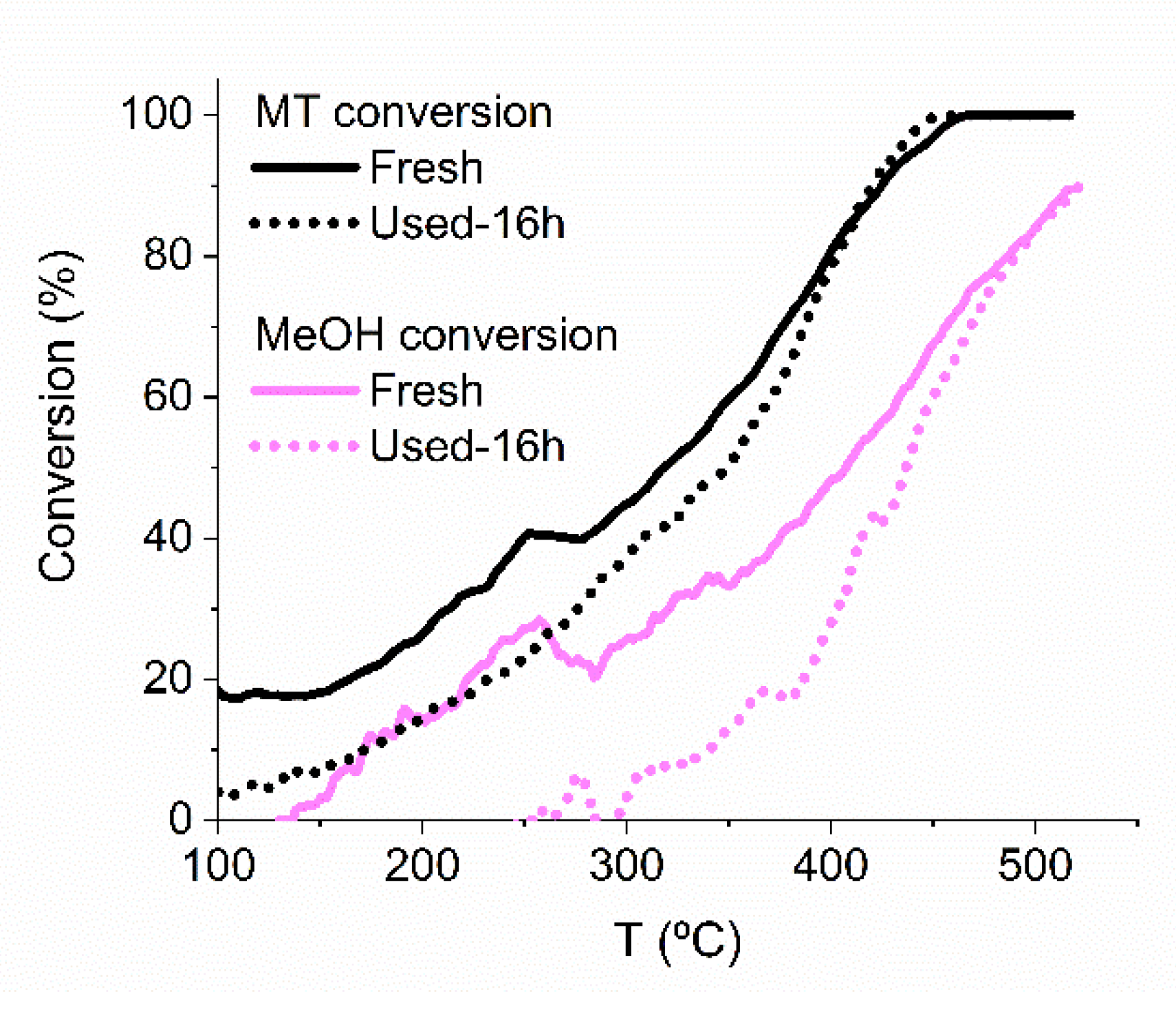
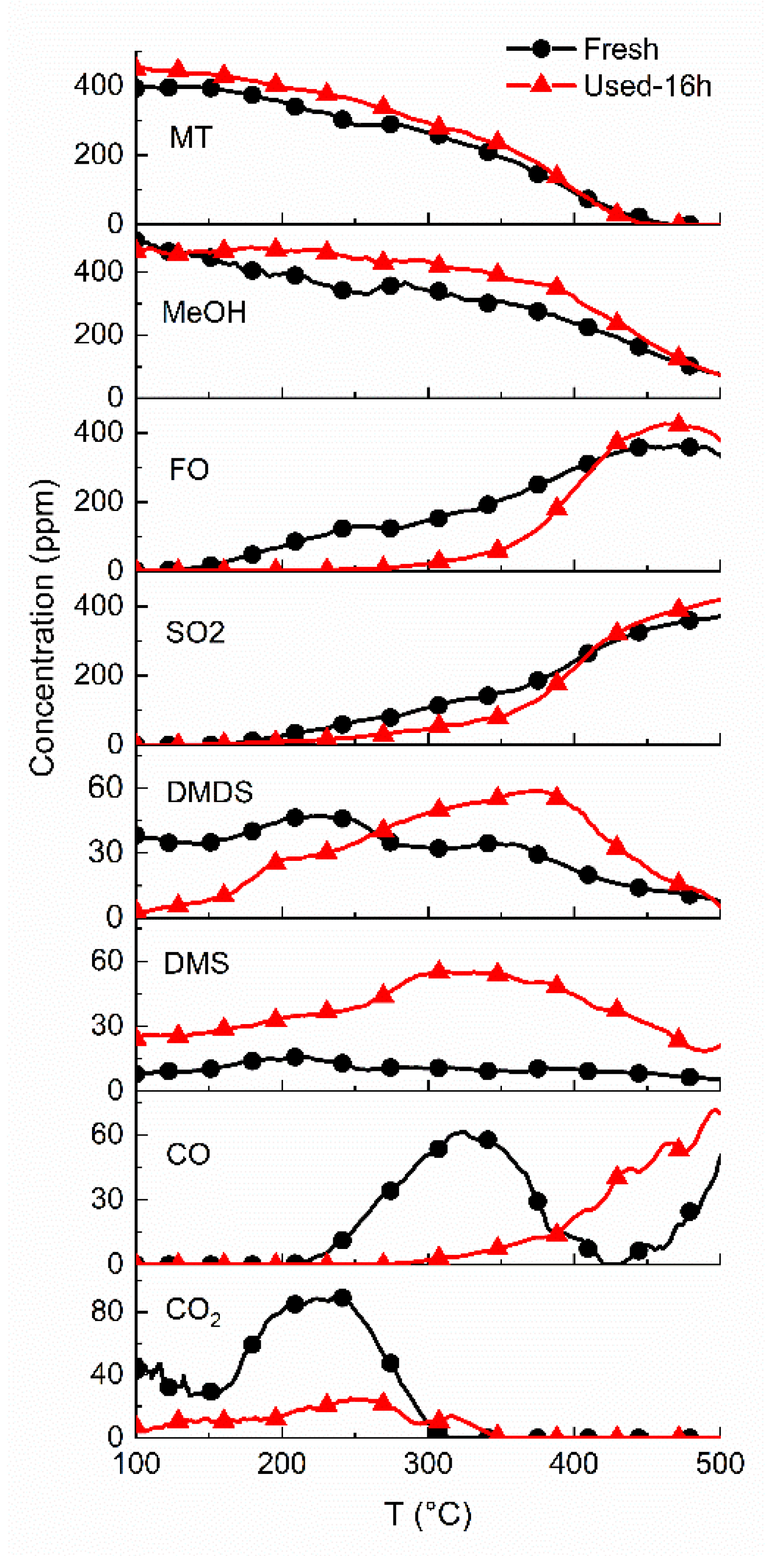
2.5. Stability Test
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Reaction and Stability Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burgess, T.L.; Gibson, A.G.; Furstein, S.J.; Wachs, I.E. Converting waste gases from pulp mills into value-added chemicals. Environ. Prog. 2002, 21, 137–141. [Google Scholar] [CrossRef]
- Reddy, B.M.; Ganesh, I.; Reddy, E.P. Study of dispersion and thermal stability of V2O5/TiO2-SiO2 catalysts by XPS and other techniques. J. Phys. Chem. B 1997, 101, 1769–1774. [Google Scholar] [CrossRef]
- Reddy, B.M.; Kumar, M.V.; Reddy, E.P.; Mehdi, S. Dispersion and thermal stability of vanadium oxide catalysts supported on titania-alumina binary oxide. Catal. Lett. 1996, 36, 187–193. [Google Scholar] [CrossRef]
- Yan, Z.; Shan, W.; Shi, X.; He, G.; Lian, Z.; Yu, Y.; Shan, Y.; Liu, J.; He, H. The way to enhance the thermal stability of V2O5-based catalysts for NH3-SCR. Catal. Today 2019, (in press). [Google Scholar] [CrossRef]
- Argyle, M.; Bartholomew, C. Heterogeneous catalyst deactivation and regeneration: A review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Moulijn, J.A.; Van Diepen, A.E.; Kapteijn, F. Catalyst deactivation: Is it predictable? What to do? Appl. Catal. A Gen. 2001, 212, 3–16. [Google Scholar] [CrossRef]
- Busca, G.; Daturi, M.; Finocchio, E.; Lorenzelli, V.; Ramis, G.; Willey, R.J. Transition metal mixed oxides as combustion catalysts: Preparation, characterization and activity mechanisms. Catal. Today 1997, 33, 239–249. [Google Scholar] [CrossRef]
- Trigueiro, F.; Ferreira, C.; Volta, J.; Gonzalez, W.; De Oliveria, P. Effect of niobium addition to Co/γ-Al2O3 catalyst on methane combustion. Catal. Today 2006, 118, 425–432. [Google Scholar] [CrossRef]
- Solsona, B.; Garcia, T.; Jones, C.; Taylor, S.; Carley, A.; Hutchings, G. Supported gold catalysts for the total oxidation of alkanes and carbon monoxide. Appl. Catal. A Gen. 2006, 312, 67–76. [Google Scholar] [CrossRef]
- Garcia, T.; Agouram, S.; Sánchez-Royo, J.F.; Murillo, R.; Mastral, A.M.; Aranda, A.; Vázquez, I.; Dejoz, A.; Solsona, B. Deep oxidation of volatile organic compounds using ordered cobalt oxides prepared by a nanocasting route. Appl. Catal. A Gen. 2010, 386, 16–27. [Google Scholar] [CrossRef]
- Solsona, B.; Aylón, E.; Murillo, R.; Mastral, A.M.; Monzonís, A.; Agouram, S.; Davies, T.E.; Taylor, S.H.; Garcia, T. Deep oxidation of pollutants using gold deposited on a high surface area cobalt oxide prepared by a nanocasting route. J. Hazard. Mater. 2011, 187, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, J.D.; Beyersmann, D. Cobalt and cobalt compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 467–497. ISBN 9783527306732. [Google Scholar]
- Papageorgiou, I.; Brown, C.; Schins, R.; Singh, S.; Newson, R.; Davis, S.; Fisher, J.; Ingham, E.; Case, C.P. The effect of nano- and micron-sized particles of cobalt-chromium alloy on human fibroblasts in vitro. Biomaterials 2007, 28, 2946–2958. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Dash, S.K.; Tripathy, S.; Das, B.; Mandal, D.; Pramanik, P.; Roy, S. Toxicity of cobalt oxide nanoparticles to normal cells; An in vitro and in vivo study. Chem. Biol. Interact. 2015, 226, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Liotta, L.F.; Wu, H.; Pantaleo, G.; Venezia, A.M. Co3O4 nanocrystals and Co3O4–MOx binary oxides for CO, CH4 and VOC oxidation at low temperatures: A review. Catal. Sci. Technol. 2013, 3, 3085. [Google Scholar] [CrossRef]
- Debecker, D.P.; Gaigneaux, E.M.; Busca, G. Exploring, tuning, and exploiting the basicity of hydrotalcites for applications in heterogeneous catalysis. Chem. A Eur. J. 2009, 15, 3920–3935. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef]
- Genty, E.; Cousin, R.; Capelle, S.; Gennequin, C.; Siffert, S. Catalytic oxidation of toluene and CO over nanocatalysts derived from hydrotalcite-like compounds (X62+Al23+): Effect of the bivalent cation. Eur. J. Inorg. Chem. 2012, 2012, 2802–2811. [Google Scholar] [CrossRef]
- Pérez, A.; Molina, R.; Moreno, S. Enhanced VOC oxidation over Ce/CoMgAl mixed oxides using a reconstruction method with EDTA precursors. Appl. Catal. A Gen. 2014, 477, 109–116. [Google Scholar] [CrossRef]
- Barrientos, J.; Montes, V.; Boutonnet, M.; Järås, S. Further insights into the effect of sulfur on the activity and selectivity of cobalt-based Fischer–Tropsch catalysts. Catal. Today 2016, 275, 119–126. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Wang, E.; Lin, C.; Shang, Y.; Ding, G.; Ma, X.; Qin, S.; Sun, Q. Effect of composite supports on the methanation activity of Co-Mo-based sulphur-resistant catalysts. J. Nat. Gas Chem. 2012, 21, 767–773. [Google Scholar] [CrossRef]
- Wang, B.; Yao, Y.; Jiang, M.; Li, Z.; Ma, X.; Qin, S.; Sun, Q. Effect of cobalt and its adding sequence on the catalytic performance of MoO3/Al2O3 toward sulfur-resistant methanation. J. Energy Chem. 2014, 23, 35–42. [Google Scholar] [CrossRef]
- Copperthwaite, R.G.; Gottschalk, F.M.; Sangiorgio, T.; Hutchings, G.J. Cobalt chromium oxide: A novel sulphur tolerant water-gas shift catalyst. Appl. Catal. 1990, 63, L11–L16. [Google Scholar] [CrossRef]
- Park, J.N.; Kim, J.H.; Lee, H.-I. A Study on the sulfur-resistant catalysts for water gas shift reaction. Bull. Korean Chem. Soc. 2000, 21, 1239–1244. [Google Scholar]
- Kloprogge, J.T.; Frost, R.L. Infrared emission spectroscopic study of the thermal transformation of Mg-, Ni- and Co-hydrotalcite catalysts. Appl. Catal. A Gen. 1999, 184, 61–71. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Mul, G.; Moulijn, J.A. In situ Fourier transform infrared and laser raman spectroscopic study of the thermal decomposition of Co-Al and Ni-Al hydrotalcites. Vib. Spectrosc. 2001, 27, 75–88. [Google Scholar] [CrossRef]
- Genty, E.; Brunet, J.; Pequeux, R.; Capelle, S.; Siffert, S.; Cousin, R. Effect of Ce substituted hydrotalcite-derived mixed oxides on total catalytic oxidation of air pollutant. Mater. Today Proc. 2016, 3, 277–281. [Google Scholar] [CrossRef]
- Mwenesongole, E. A Raman- and XRD Study of the Crystal Chemistry of Cobalt Blue. Master’s Thesis, University of Pretoria, Pretoria, South Africa, 2008. [Google Scholar]
- Huang, Y.; Yan, C.-F.; Guo, C.-Q.; Huang, S.-L. Enhanced photoreduction activity of carbon dioxide over Co3O4/CeO2 catalysts under visible light irradiation. Int. J. Photoenergy 2015, 2015, 1–11. [Google Scholar]
- Zheng, Y.; Liu, Y.; Zhou, H.; Huang, W.; Pu, Z. Complete combustion of methane over Co3O4 catalysts: Influence of pH values. J. Alloys Compd. 2018, 734, 112–120. [Google Scholar] [CrossRef]
- Farhadi, S.; Safabakhsh, J. Solid-state thermal decomposition of the [Co(NH3)5CO3]NO3·0.5H2O complex: A simple, rapid and low-temperature synthetic route to Co3O4 nanoparticles. J. Alloys Compd. 2012, 515, 180–185. [Google Scholar] [CrossRef]
- Pu, Z.; Zhou, H.; Zheng, Y.; Huang, W.; Li, X. Enhanced methane combustion over Co3O4 catalysts prepared by a facile precipitation method: Effect of aging time. Appl. Surf. Sci. 2017, 410, 14–21. [Google Scholar] [CrossRef]
- Pu, Z.; Liu, Y.; Zhou, H.; Huang, W.; Zheng, Y.; Li, X. Catalytic combustion of lean methane at low temperature over ZrO2-modified Co3O4 catalysts. Appl. Surf. Sci. 2017, 422, 85–93. [Google Scholar] [CrossRef]
- Gao, S.; Wang, P.; Yu, F.; Wang, H.; Wu, Z. Dual resistance to alkali metals and SO2: Vanadium and cerium supported on sulfated zirconia as an efficient catalyst for NH3-SCR. Catal. Sci. Technol. 2016, 6, 8148–8156. [Google Scholar] [CrossRef]
- Gómez, L.E.; Múnera, J.F.; Sollier, B.M.; Miró, E.E.; Boix, A.V. Raman in situ characterization of the species present in Co/CeO2 and Co/ZrO2 catalysts during the COPrOx reaction. Int. J. Hydrogen Energy 2016, 41, 4993–5002. [Google Scholar] [CrossRef]
- Reddy, B.M.; Sreekanth, P.M.; Lakshmanan, P.; Khan, A. Synthesis, characterization and activity study of SO42-/CexZr1-xO2 solid superacid catalyst. J. Mol. Catal. A Chem. 2006, 244, 1–7. [Google Scholar] [CrossRef]
- Shyu, J.Z.; Weber, W.H.; Gandhi, H.S. Surface characterization of alumina-supported ceria. J. Phys. Chem. 1988, 92, 4964–4970. [Google Scholar] [CrossRef]
- Laitinen, T.; Ojala, S.; Cousin, R.; Koivikko, N.; Poupin, C.; El Assal, Z.; Aho, A.; Keiski, R.L. Activity, selectivity, and stability of vanadium catalysts in formaldehyde production from emissionsof volatile organic compounds. J. Ind. Eng. Chem. 2020, 83, 375–386. [Google Scholar] [CrossRef]
- Ruan, H.D.; Frost, R.L.; Kloprogge, J.T. Comparison of raman spectra in characterizing gibbsite, bayerite, diaspore and boehmite. J. Raman Spectrosc. 2001, 32, 745–750. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Cheng, B.; Wang, K.K.; Ling, G.P.; Cai, J.; Song, C.L.; Han, G.R. Study of raman spectra for γ-Al2O3 models by using first-principles method. Solid State Commun. 2014, 178, 16–22. [Google Scholar] [CrossRef]
- Wolf, M.; Roberts, S.J.; Marquart, W.; Olivier, E.J.; Luchters, N.T.J.; Gibson, E.K.; Catlow, C.R.A.; Neethling, J.H.; Fischer, N.; Claeys, M. Synthesis, characterisation and water–gas shift activity of nano-particulate mixed-metal (Al, Ti) cobalt oxides. Dalt. Trans. 2019, 48, 13858–13868. [Google Scholar] [CrossRef]
- Choya, A.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Oxidation of lean methane over cobalt catalysts supported on ceria/alumina. Appl. Catal. A Gen. 2020, 591, 117381. [Google Scholar] [CrossRef]
- Bonnetot, B.; Rakic, V.; Yuzhakova, T.; Guimon, C.; Auroux, A. Preparation and characterization of Me2O3−CeO2 (Me = B, Al, Ga, In) mixed oxide catalysts. 2. Preparation by sol−gel method. Chem. Mater. 2008, 20, 1585–1596. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, X.; Tang, C.; Qi, L.; Liu, B.; Gao, F.; Sun, K.; Dong, L.; Chen, Y. Textural, structural, and morphological characterizations and catalytic activity of nanosized CeO2-MOx (M=Mg2+, Al3+, Si4+) mixed oxides for CO oxidation. J. Colloid Interface Sci. 2011, 354, 341–352. [Google Scholar] [CrossRef]
- Jongsomjit, B.; Panpranot, J.; Goodwin, J.G. Effect of zirconia-modified alumina on the properties of Co/γ-Al2O3 catalysts. J. Catal. 2003, 215, 66–77. [Google Scholar] [CrossRef]
- Taheri Najafabadi, A.; Khodadadi, A.A.; Parnian, M.J.; Mortazavi, Y. Atomic layer deposited Co/γ-Al2O3 catalyst with enhanced cobalt dispersion and Fischer-Tropsch synthesis activity and selectivity. Appl. Catal. A Gen. 2016, 511, 31–46. [Google Scholar] [CrossRef]
- Álvarez-Docio, C.M.; Reinosa, J.J.; del Campo, A.; Fernández, J.F. 2D particles forming a nanostructured shell: A step forward cool NIR reflectivity for CoAl2O4 pigments. Dye. Pigment. 2017, 137, 1–11. [Google Scholar] [CrossRef]
- Mo, S.; Zhang, Q.; Ren, Q.; Xiong, J.; Zhang, M.; Feng, Z.; Yan, D.; Fu, M.; Wu, J.; Chen, L.; et al. Leaf-like Co-ZIF-L derivatives embedded on Co2AlO4/Ni foam from hydrotalcites as monolithic catalysts for toluene abatement. J. Hazard. Mater. 2019, 364, 571–580. [Google Scholar] [CrossRef]
- Genty, E.; Brunet, J.; Poupin, C.; Casale, S.; Capelle, S.; Massiani, P.; Siffert, S.; Cousin, R. Co-Al mixed oxides prepared via LDH route using microwaves or ultrasound: Application for catalytic toluene total oxidation. Catalysts 2015, 5, 851–867. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Zhang, L.; Zheng, Y.; Zhang, S.; Liu, Q.; Gao, G.; Dong, D.; Wang, Y.; Xu, L.; Hu, X. Methanation of CO2 over nickel catalysts: Impacts of acidic/basic sites on formation of the reaction intermediates. Fuel 2020, 262, 116521. [Google Scholar] [CrossRef]
- Naresh, D.; Kumar, V.P.; Harisekhar, M.; Nagaraju, N.; Putrakumar, B.; Chary, K.V.R. Characterization and functionalities of Pd/hydrotalcite catalysts. Appl. Surf. Sci. 2014, 314, 199–207. [Google Scholar] [CrossRef]
- Arena, F.; Dario, R.; Parmaliana, A. A characterization study of the surface acidity of solid catalysts by temperature programmed methods. Appl. Catal. A Gen. 1998, 170, 127–137. [Google Scholar] [CrossRef]
- Koivikko, N.; Laitinen, T.; Ojala, S.; Pitkäaho, S.; Kucherov, A.; Keiski, R.L. Formaldehyde production from methanol and methyl mercaptan over titania and vanadia based catalysts. Appl. Catal. B Environ. 2011, 103, 72–78. [Google Scholar] [CrossRef]
- He, D.; Chen, D.; Hao, H.; Yu, J.; Liu, J.; Lu, J.; Wan, G.; He, S.; Li, K.; Luo, Y. Enhanced activity and stability of Sm-doped HZSM-5 zeolite catalysts for catalytic methyl mercaptan (CH3SH) decomposition. Chem. Eng. J. 2017, 317, 60–69. [Google Scholar] [CrossRef]
- Huguet, E.; Coq, B.; Durand, R.; Leroi, C.; Cadours, R.; Hulea, V. A highly efficient process for transforming methyl mercaptan into hydrocarbons and H2S on solid acid catalysts. Appl. Catal. B Environ. 2013, 134–135, 344–348. [Google Scholar] [CrossRef]
- Hulea, V.; Huguet, E.; Cammarano, C.; Lacarriere, A.; Durand, R.; Leroi, C.; Cadours, R.; Coq, B. Conversion of methyl mercaptan and methanol to hydrocarbons over solid acid catalysts–A comparative study. Appl. Catal. B Environ. 2014, 144, 547–553. [Google Scholar] [CrossRef]
- Dalai, A.K.; Tollefson, E.L.; Yang, A.; Sasaoka, E. Oxidation of methyl mercaptan over an activated carbon in a fixed-bed reactor. Ind. Eng. Chem. Res. 1997, 36, 4726–4733. [Google Scholar] [CrossRef]
- Koivikko, N.; Laitinen, T.; Mouammine, A.; Ojala, S.; Keiski, R. Catalytic activity studies of vanadia/silica–titania catalysts in SVOC partial oxidation to formaldehyde: Focus on the catalyst composition. Catalysts 2018, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Guo, Y.; Li, X.; Li, Z. Effects of cerium doping position on physicochemical properties and catalytic performance in methanol total oxidation. J. Rare Earths 2018, 36, 811–818. [Google Scholar] [CrossRef]
- Scirè, S.; Minicò, S.; Crisafulli, C.; Galvagno, S. Catalytic combustion of volatile organic compounds over group IB metal catalysts on Fe2O3. Catal. Commun. 2001, 2, 229–232. [Google Scholar] [CrossRef]
- Kaminski, P.; Ziolek, M. Mobility of gold, copper and cerium species in Au, Cu/Ce, Zr-oxides and its impact on total oxidation of methanol. Appl. Catal. B Environ. 2016, 187, 328–341. [Google Scholar] [CrossRef]
- Xia, Y.; Dai, H.; Jiang, H.; Zhang, L. Three-dimensional ordered mesoporous cobalt oxides: Highly active catalysts for the oxidation of toluene and methanol. Catal. Commun. 2010, 11, 1171–1175. [Google Scholar] [CrossRef]
- Ai, M. Catalytic activity for the oxidation of methanol and the acid-base properties of metal oxides. J. Catal. 1978, 54, 426–435. [Google Scholar] [CrossRef]
- Fu, Y.; Shen, J. Selective oxidation of methanol to dimethoxymethane under mild conditions over V2O5/TiO2 with enhanced surface acidity. Chem. Commun. 2007, 2, 2172–2174. [Google Scholar] [CrossRef]
- Tatibouët, J. Methanol oxidation as a catalytic surface probe. Appl. Catal. A Gen. 1997, 148, 213–252. [Google Scholar] [CrossRef]
- Gao, S.; Chen, X.; Wang, H.; Mo, J.; Wu, Z.; Liu, Y.; Weng, X. Ceria supported on sulfated zirconia as a superacid catalyst for selective catalytic reduction of NO with NH3. J. Colloid Interface Sci. 2013, 394, 515–521. [Google Scholar] [CrossRef]
- Bêche, E.; Charvin, P.; Perarnau, D.; Abanades, S.; Flamant, G. Ce 3d XPS investigation of cerium oxides and mixed cerium oxide (CexTiyOz). Surf. Interface Anal. 2008, 40, 264–267. [Google Scholar] [CrossRef]
- Väliheikki, A.; Kolli, T.; Huuhtanen, M.; Maunula, T.; Keiski, R.L. Activity enhancement of W–CeZr oxide catalysts by SO2 treatment in NH3-SCR. Top. Catal. 2015, 58, 1002–1011. [Google Scholar] [CrossRef]
- Moulder John, F.; Stickle William, F.; Sobol Peter, E.; Bomben Kenneth, D. Handbook of X-ray photoelectron spectroscopy: A reference book of standard spectra for identification and interpretation of XPS data. Perkin-Elmer Corp. 1992, 40, 221. [Google Scholar]
- Barr, T.L. An ESCA study of the termination of the passivation of elemental metals. J. Phys. Chem. 1978, 82, 1801–1810. [Google Scholar] [CrossRef]
- Zhao, H.; Bennici, S.; Cai, J.; Shen, J.; Auroux, A. Effect of vanadia loading on the acidic, redox and catalytic properties of V2O5-TiO2 and V2O5-TiO2/SO42- catalysts for partial oxidation of methanol. Catal. Today 2010, 152, 70–77. [Google Scholar] [CrossRef]
- Zhao, H.; Bennici, S.; Shen, J.; Auroux, A. The influence of the preparation method on the structural, acidic and redox properties of V2O5-TiO2/SO42- catalysts. Appl. Catal. A Gen. 2009, 356, 121–128. [Google Scholar] [CrossRef]
- Barakat, T.; Finne, G.; Franco, M.; Cousin, R.; Giraudon, J.M.; Lamonier, J.F.; Thomas, D.; Decroly, A.; Deweireld, G.; Siffert, S. Influence of shaping on Pd and Pt/TiO2 catalysts in total oxidation of VOCs. Adv. Mater. Res. 2011, 324, 162–165. [Google Scholar] [CrossRef]
- Yu, Y.; Takei, T.; Ohashi, H.; He, H.; Zhang, X.; Haruta, M. Pretreatments of Co3O4 at moderate temperature for CO oxidation at −80 °C. J. Catal. 2009, 267, 121–128. [Google Scholar] [CrossRef]
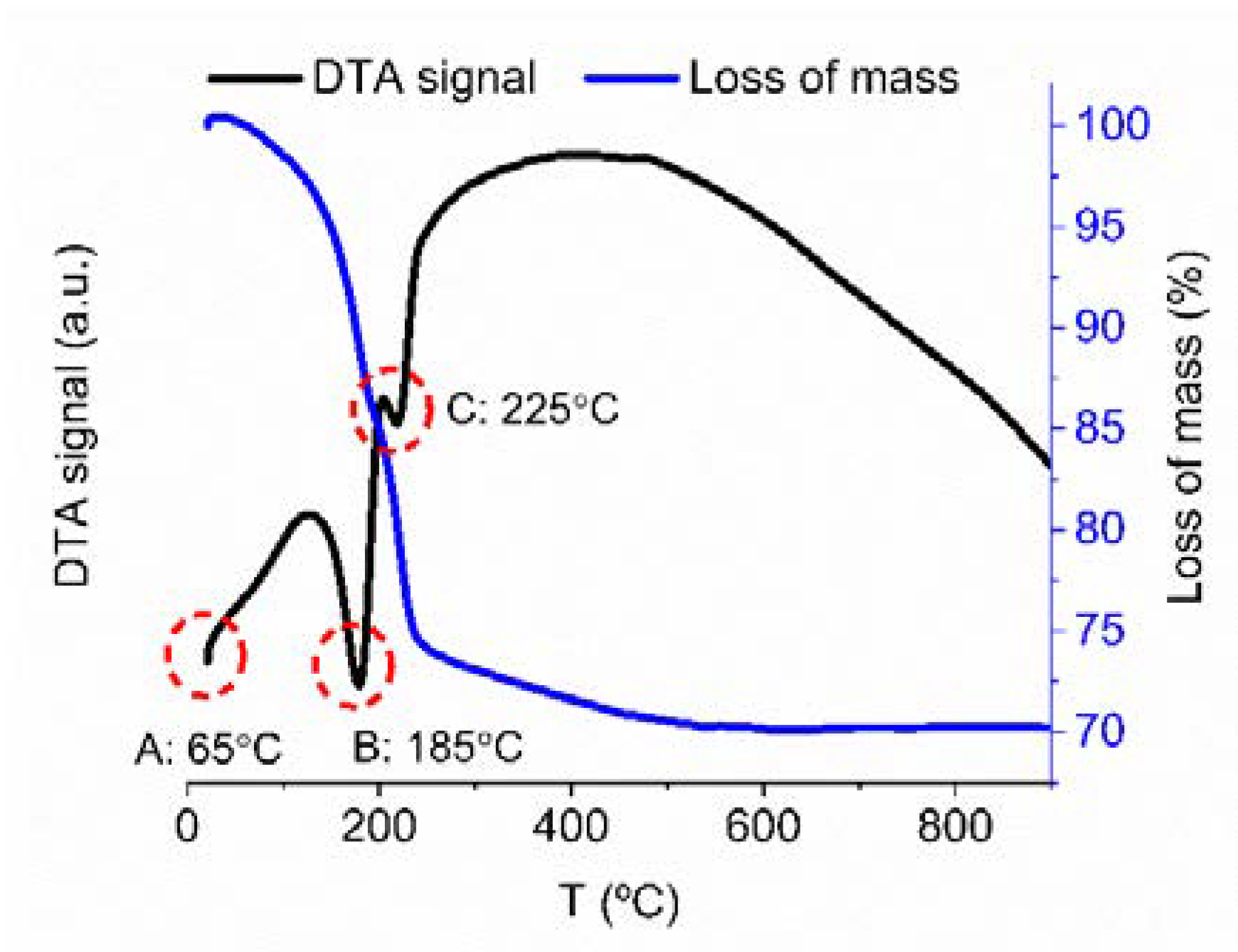
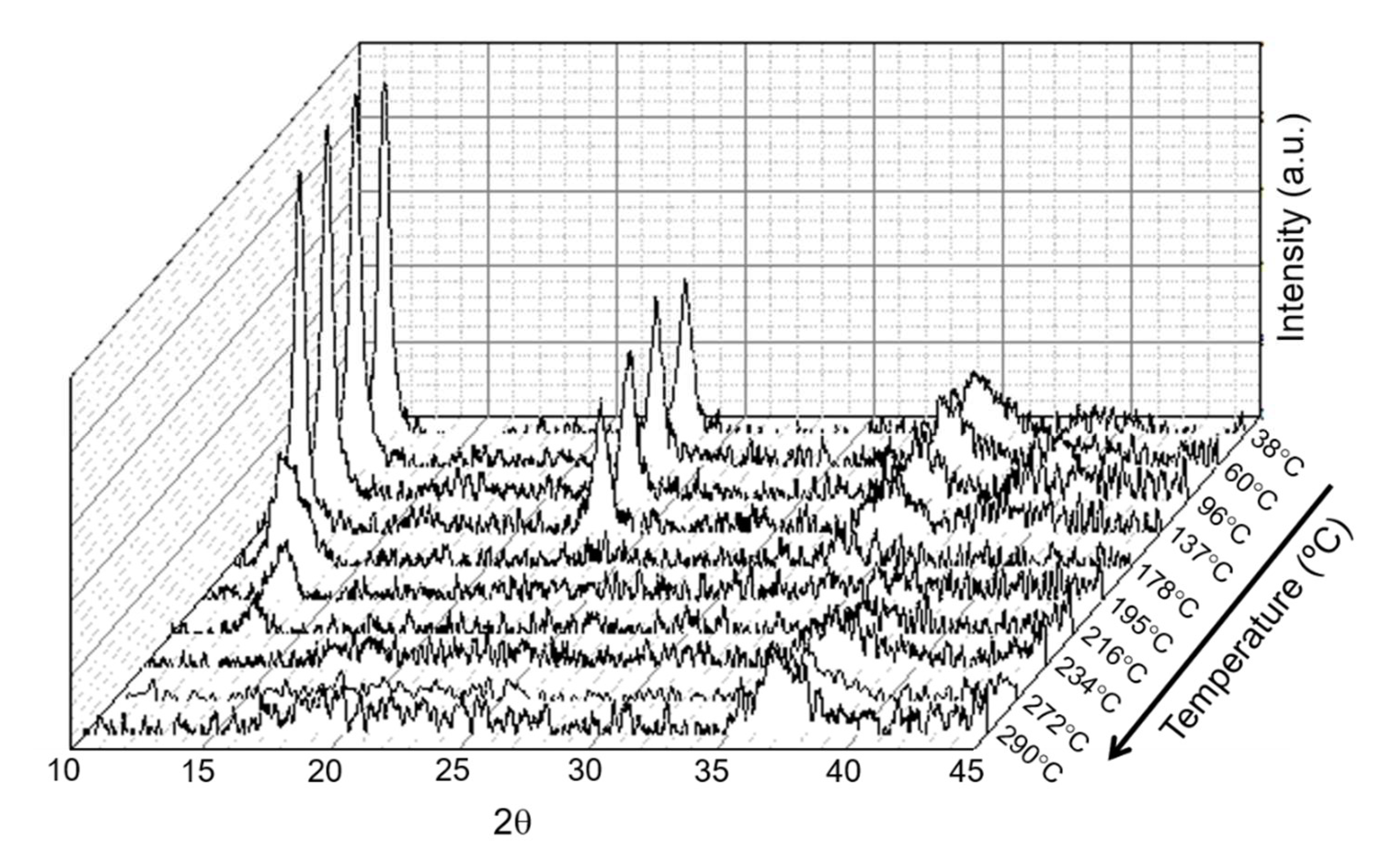

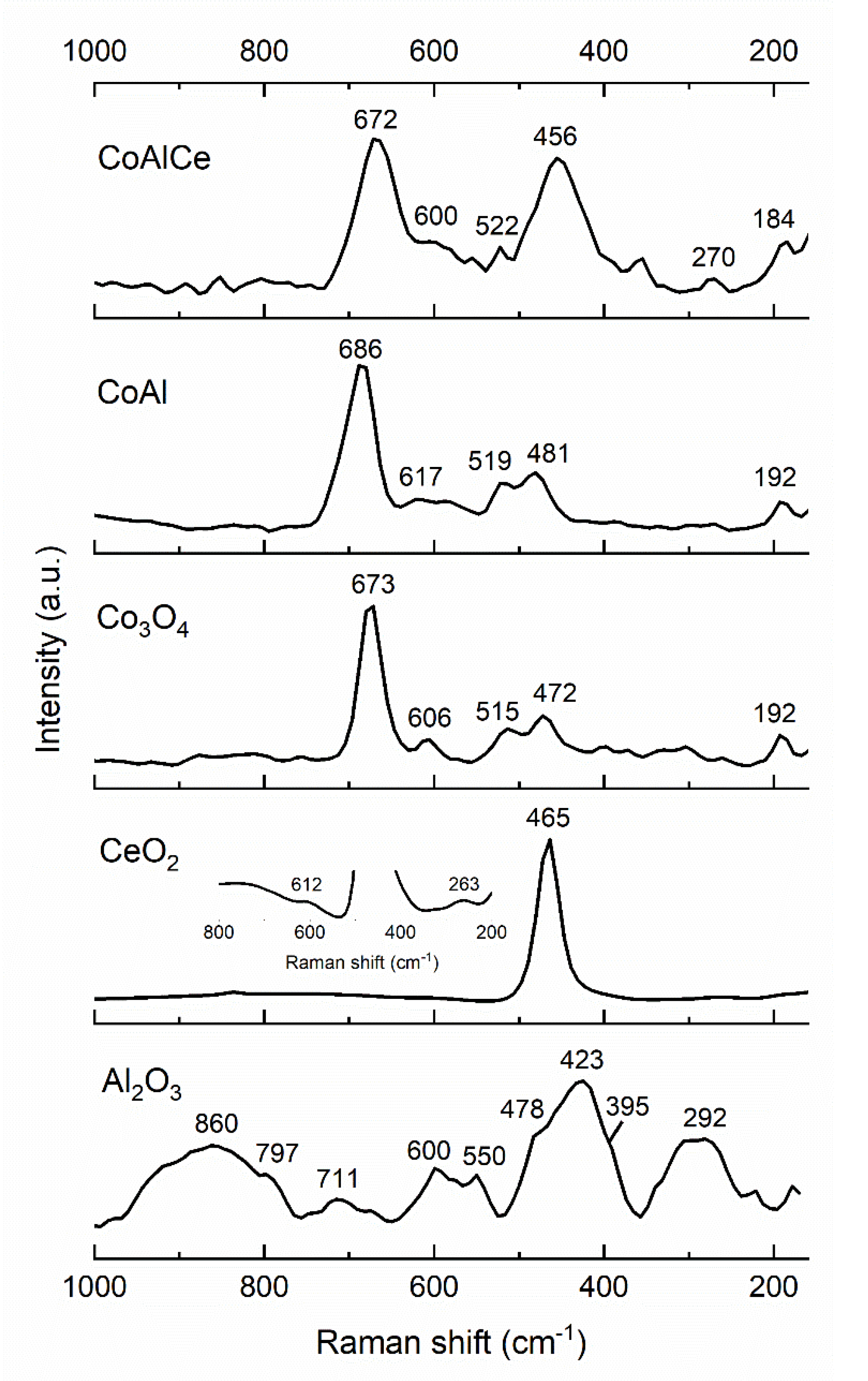
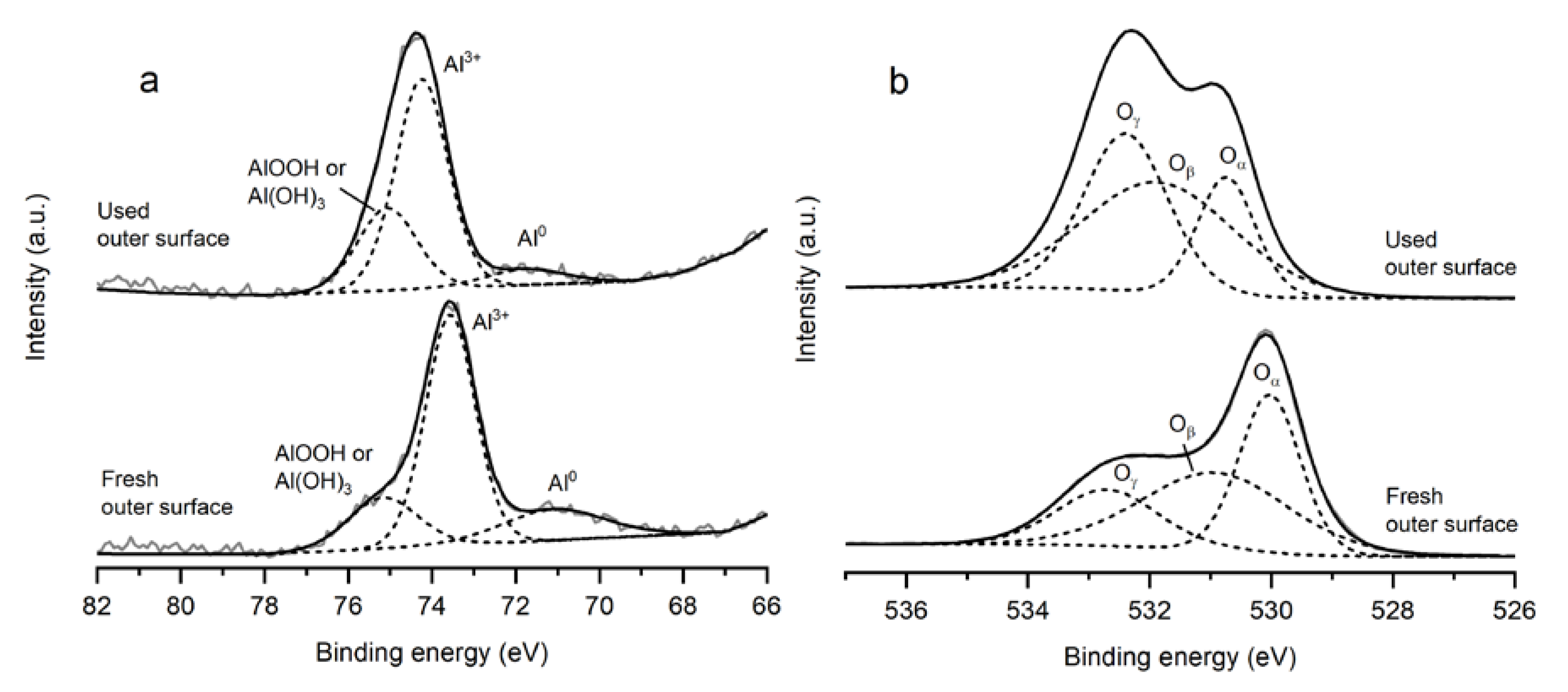
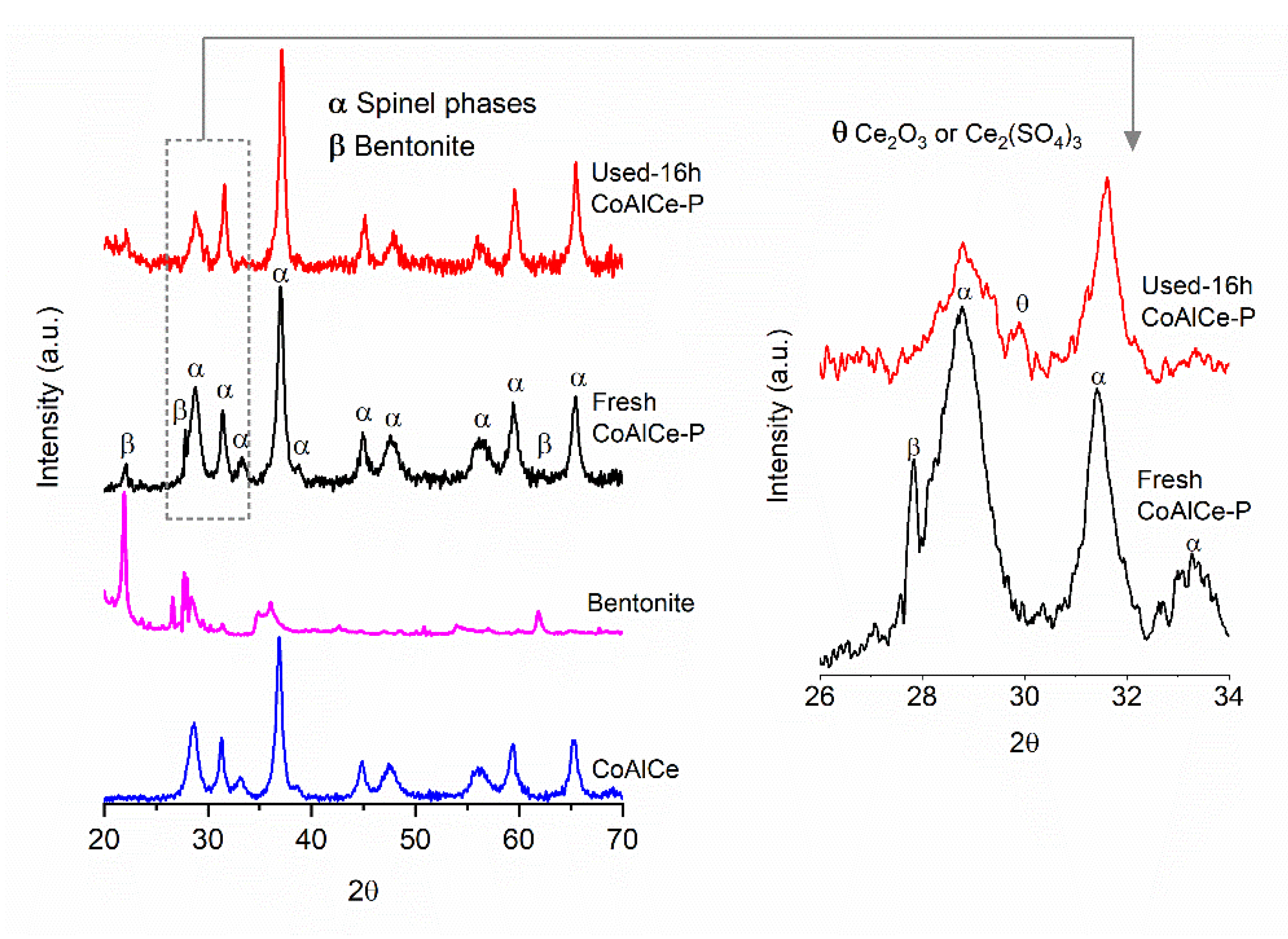
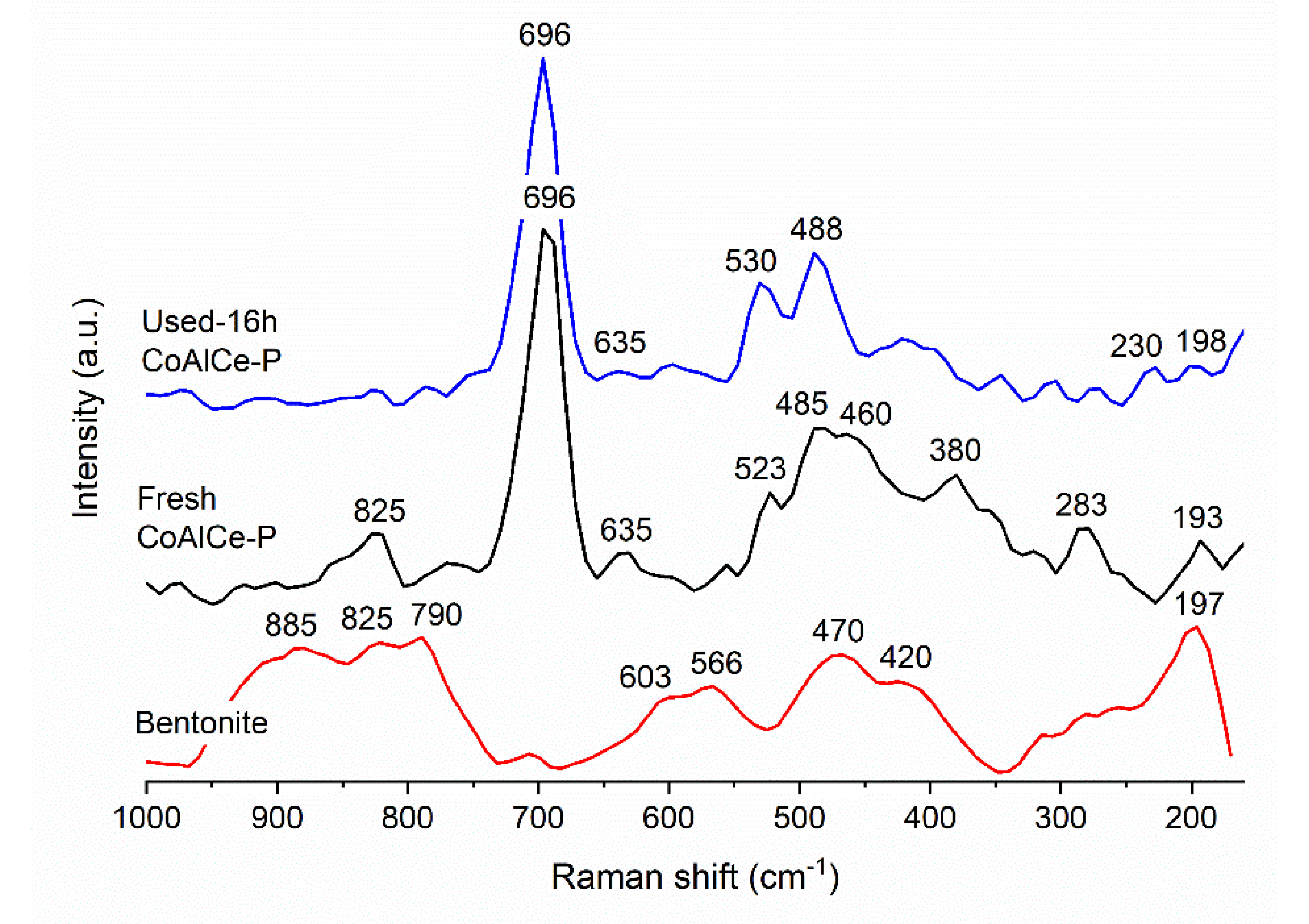
| Sample | Specific Surface Area (m2 g−1) | Total Pore Volume (cm3 g−1) | Average Pore Size (nm) |
|---|---|---|---|
| Co3O4 | 25 | 0.1 | 14 |
| Al2O3 | 130 | 0.2 | 7 |
| CeO2 | 125 | 0.2 | 6 |
| CoAl | 125 | 0.2 | 8 |
| CoAlCe | 110 | 0.1 | 4 |
| Sample | Stoichiometry | ||
|---|---|---|---|
| Co | Al | Ce | |
| CoAl | 6 | 2.1 | - |
| CoAlCe | 6 | 1.1 | 0.8 |
| Sample | H2 Consumption | O2 Consumption | NH3 Consumption | CO2 Consumption | ||||
|---|---|---|---|---|---|---|---|---|
| μmol g−1 | μmol m−2 | μmol g−1 | μmol m−2 | μmol g−1 | μmol m−2 | μmol g−1 | μmol m−2 | |
| CoAl | 2400 | 19.2 | 204 | 1.6 | 151 | 1.2 | 132 | 1.1 |
| CoAlCe | 2016 | 18.3 | 202 | 1.8 | 182 | 1.7 | 184 | 1.7 |
| CoAlCe-P | 1556 | 18.3 | 155 | 1.8 | 130 | 1.5 | 102 | 1.2 |
| Sample | TFO,max (°C) | YFO (%) | XMT (%) | XMeOH (%) |
|---|---|---|---|---|
| CoAl | 400 | 55 | 100 | 89 |
| CoAlCe | 370 | 57 | 100 | 84 |
| Co3O4 | 395 | 83 | 100 | 81 |
| Al2O3 | 540 | 35 | 100 | 100 |
| CeO2 | 395 | 83 | 100 | 84 |
| QS 1 | 590 | 47 | 100 | 32 |
| Thermal 2 | 550 | 34 | 100 | 0 |
| Sample | Reactant | T50 (°C) 1 | T90 (°C) 1 | TFO, max (°C) 2 | YFO (%) |
|---|---|---|---|---|---|
| CoAl | MeOH | 178 | 207 | 182 | 16 |
| CoAlCe | MeOH | 178 | 197 | 178 | 17 |
| CoAl | MeOH+MT | 248 | 417 | 400 | 55 |
| CoAlCe | MeOH+MT | 255 | 407 | 370 | 57 |
| Sample | Reactant | T50 (°C) 1 | T90 (°C) 1 | TFO, max (°C) 2 | YFO (%) |
|---|---|---|---|---|---|
| CoAl | MT | 222 | 356 | 390 | 52 |
| CoAlCe | MT | 208 | 323 | 370 | 60 |
| CoAl | MeOH+MT | 189 | 320 | 400 | 55 |
| CoAlCe | MeOH+MT | 195 | 311 | 370 | 57 |
| CoAlCe-P | Amount of Sulfur (w-%) | Specific Surface Area (m2 g−1) | Total Pore Volume (cm3 g−1) | Average Pore Size (nm) |
|---|---|---|---|---|
| Fresh | - | 85 | 0.33 | 15.4 |
| 16 h | 0.1–0.9 1 2.9–5.8 2 | 55 | 0.26 | 18.6 |
| Thermal-16h 3 | - | 82 | 0.33 | 15.8 |
| CoAlCe-P | Ce3+/Ce4+ | Co3+/Co2+ | Oβ/Ototal | S (w-%) |
|---|---|---|---|---|
| Fresh inner surface | 0.27 | 1.11 | 33.2 | - |
| Fresh outer surface | 0.28 | 1.53 | 42.7 | - |
| 16 h inner surface | 0.92 | 3.05 | 37.9 | 5.0 |
| 16 h outer surface | 0.89 | 3.28 | 45.6 | 5.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laitinen, T.; Ojala, S.; Genty, E.; Brunet, J.; De Weireld, G.; Poupin, C.; Siffert, S.; Cousin, R.; Keiski, R.L. On the Activity and Selectivity of CoAl and CoAlCe Mixed Oxides in Formaldehyde Production from Pulp Mill Emissions. Catalysts 2020, 10, 424. https://doi.org/10.3390/catal10040424
Laitinen T, Ojala S, Genty E, Brunet J, De Weireld G, Poupin C, Siffert S, Cousin R, Keiski RL. On the Activity and Selectivity of CoAl and CoAlCe Mixed Oxides in Formaldehyde Production from Pulp Mill Emissions. Catalysts. 2020; 10(4):424. https://doi.org/10.3390/catal10040424
Chicago/Turabian StyleLaitinen, Tiina, Satu Ojala, Eric Genty, Julien Brunet, Guy De Weireld, Christophe Poupin, Stéphane Siffert, Renaud Cousin, and Riitta L. Keiski. 2020. "On the Activity and Selectivity of CoAl and CoAlCe Mixed Oxides in Formaldehyde Production from Pulp Mill Emissions" Catalysts 10, no. 4: 424. https://doi.org/10.3390/catal10040424