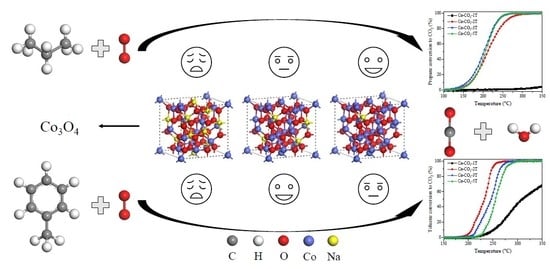
The Influence of Residual Sodium on the Catalytic Oxidation of Propane and Toluene over Co3O4 Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Decomposition of the Catalyst Precursors
2.2. Characterization of the Catalysts
2.3. Catalytic Activity
2.4. Stability Test
2.5. Discussion
3. Experimental
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Activity Test
3.4. Kinetic Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Alifanti, M.; Florea, M.; Pârvulescu, V.I. Ceria-based oxides as supports for LaCoO3 perovskite; catalysts for total oxidation of VOC. Appl. Catal. B Environ. 2007, 70, 400–405. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Liotta, L.F.; Wu, H.; Pantaleo, G.; Venezia, A.M. Co3O4 nanocrystals and Co3O4–MOx binary oxides for CO, CH4 and VOC oxidation at low temperatures: A review. Catal. Sci. Technol. 2013, 3, 3085–3102. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Shangguan, W. Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Hua, W.; Guo, Y.; Lu, G.; Gil, S.; Giroir-Fendler, A. Catalytic oxidation of vinyl chloride emissions over Co-Ce composite oxide catalysts. Chem. Eng. J. 2017, 315, 392–402. [Google Scholar] [CrossRef]
- Hua, W.; Zhang, C.; Guo, Y.; Chai, G.; Wang, C.; Guo, Y.; Wang, L.; Wang, Y.; Zhan, W. An efficient SnyMn1-yOx composite oxide catalyst for catalytic oxidation of vinyl chloride emissions. Appl. Catal. B Environ. 2019, 255, 117748–117757. [Google Scholar] [CrossRef]
- Weng, X.; Sun, P.; Long, Y.; Meng, Q.; Wu, Z. Catalytic oxidation of chlorobenzene over MnxCe1-xO2/HZSM-5 catalysts: A study with practical implications. Environ. Sci. Technol. 2017, 51, 8057–8066. [Google Scholar] [CrossRef]
- Gluhoi, A.C.; Bogdanchikova, N.; Nieuwenhuys, B.E. Total oxidation of propene and propane over gold-copper oxide on alumina catalysts: Comparison with Pt/Al2O3. Catal. Today 2006, 113, 178–181. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Wang, L.; Zhang, C.; He, H. Catalytic oxidation of formaldehyde over manganese oxides with different crystal structures. Catal. Sci. Technol. 2015, 5, 2305–2313. [Google Scholar] [CrossRef]
- Wu, J.C.S.; Chang, T.Y. VOC deep oxidation over Pt catalysts using hydrophobic supports. Catal. Today 1998, 44, 111–118. [Google Scholar]
- Hu, Z.; Wang, Z.; Guo, Y.; Wang, L.; Guo, Y.; Zhang, J.; Zhan, W. Total oxidation of propane over a Ru/CeO2 catalyst at low temperature. Environ. Sci. Technol. 2018, 52, 9531–9541. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Q.; Lu, E.; Wang, Z.; Gong, X.; Yu, Z.; Guo, Y.; Wang, L.; Guo, Y.; Zhan, W.; et al. Taming the stability of Pd active phases through a compartmentalizing strategy toward nanostructured catalyst supports. Nat. Commun. 2019, 10, 1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.C.; Shim, W.G. Properties and performance of Pd based catalysts for catalytic oxidation of volatile organic compounds. Appl. Catal. B Environ. 2009, 92, 429–436. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Xu, W.; Xu, Z.; Chen, J.; Jia, H.; Chen, J. Hydrolysis driving redox reaction to synthesize Mn-Fe binary oxides as highly active catalysts for the removal of toluene. Chem. Eng. J. 2017, 330, 281–293. [Google Scholar] [CrossRef]
- Lu, H.F.; Zhou, Y.; Han, W.F.; Huang, H.F.; Chen, Y.F. Promoting effect of ZrO2 carrier on activity and thermal stability of CeO2-based oxides catalysts for toluene combustion. Appl. Catal. A Gen. 2013, 464, 101–108. [Google Scholar] [CrossRef]
- Cai, T.; Yuan, J.; Zhang, L.; Yang, L.; Tong, Q.; Ge, M.; Xiao, B.; Zhang, X.; Zhao, K.; He, D. Ni–Co–O solid solution dispersed nanocrystalline Co3O4 as a highly active catalyst for low-temperature propane combustion. Catal. Sci. Technol. 2018, 8, 5416–5427. [Google Scholar] [CrossRef]
- Ye, Z.; Giraudon, J.M.; Nuns, N.; Simon, P.; De Geyter, N.; Morent, R.; Lamonier, J.F. Influence of the preparation method on the activity of copper-manganese oxides for toluene total oxidation. Appl. Catal. B Environ. 2018, 223, 154–166. [Google Scholar] [CrossRef]
- Delimaris, D.; Ioannides, T. VOC oxidation over CuO–CeO2 catalysts prepared by a combustion method. Appl. Catal. B Environ. 2009, 89, 295–302. [Google Scholar] [CrossRef]
- Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Deganello, G. Co3O4/CeO2 and Co3O4/CeO2–ZrO2 composite catalysts for methane combustion: Correlation between morphology reduction properties and catalytic activity. Catal. Commun. 2005, 6, 329–336. [Google Scholar] [CrossRef]
- Pu, Z.; Liu, Y.; Zhou, H.; Huang, W.; Zheng, Y.; Li, X. Catalytic combustion of lean methane at low temperature over ZrO2-modified Co3O4 catalysts. Appl. Surf. Sci. 2017, 422, 85–93. [Google Scholar] [CrossRef]
- Zhang, W.; Díez-Ramírez, J.; Anguita, P.; Descorme, C.; Valverde, J.L.; Giroir-Fendler, A. Nanocrystalline Co3O4 catalysts for toluene and propane oxidation: Effect of the precipitation agent. Appl. Catal. B Environ. 2020, 273, 118894. [Google Scholar] [CrossRef]
- Salek, G.; Alphonse, P.; Dufour, P.; Guillemet-Fritsch, S.; Tenailleau, C. Low-temperature carbon monoxide and propane total oxidation by nanocrystalline cobalt oxides. Appl. Catal. B Environ. 2014, 147, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Pantaleo, G.; Di Carlo, G.; Guo, S.; Marci, G.; Concepción, P.; Venezia, A.M.; Liotta, L.F. Co3O4 particles grown over nanocrystalline CeO2: Influence of precipitation agents and calcination temperature on the catalytic activity for methane oxidation. Catal. Sci. Technol. 2015, 5, 1888–1901. [Google Scholar] [CrossRef] [Green Version]
- Bai, G.; Dai, H.; Deng, J.; Liu, Y.; Wang, F.; Zhao, Z.; Qiu, W.; Au, C.T. Porous Co3O4 nanowires and nanorods: Highly active catalysts for the combustion of toluene. Appl. Catal. A Gen. 2013, 450, 42–49. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.C.; Chen, M.; Gao, Y.; He, H.Y.; Fan, K.N. Dry citrate-precursor synthesized nanocrystalline cobalt oxide as highly active catalyst for total oxidation of propane. J. Catal. 2009, 263, 104–113. [Google Scholar] [CrossRef]
- Pu, Z.; Zhou, H.; Zheng, Y.; Huang, W.; Li, X. Enhanced methane combustion over Co3O4 catalysts prepared by a facile precipitation method: Effect of aging time. Appl. Surf. Sci. 2017, 410, 14–21. [Google Scholar] [CrossRef]
- Jun, K.W.; Shen, W.J.; Rama Rao, K.S.; Lee, K.W. Residual sodium effect on the catalytic activity of Cu/ZnO/Al2O3 in methanol synthesis from hydrogenation. Appl. Catal. A Gen. 1998, 174, 231–238. [Google Scholar] [CrossRef]
- Yu, Y.; Zhan, W.; Guo, Y.; Lu, G.; Adjimi, S.; Guo, Y. Gas-phase hydrogenation of maleic anhydride to γ-butyrolactone over Cu-CeO2-Al2O3 catalyst at atmospheric pressure: Effect of the residual sodium and water in the catalyst precursor. J. Mol. Catal. A Chem. 2014, 395, 392–397. [Google Scholar] [CrossRef]
- Choya, A.; de Rivas, B.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Effect of residual Na+ on the combustion of methane over Co3O4 bulk catalysts prepared by precipitation. Catalysts 2018, 8, 427. [Google Scholar] [CrossRef] [Green Version]
- Chromčáková, Ž.; Obalová, L.; Kovanda, F.; Legut, D.; Titov, A.; Ritz, M.; Fridrichová, D.; Michalik, S.; Kuśtrowski, P.; Jirátová, K. Effect of precursor synthesis on catalytic activity of Co3O4 in N2O decomposition. Catal. Today 2015, 257, 18–25. [Google Scholar] [CrossRef]
- Haneda, M.; Kintaichi, Y.; Bion, N.; Hamada, H. Alkali metal-doped cobalt oxide catalysts for NO decomposition. Appl. Catal. B Environ. 2003, 46, 473–482. [Google Scholar] [CrossRef]
- Xu, R.; Zeng, H.C. Dimensional control of cobalt-hydroxide-carbonate nanorods and their thermal conversion to one-dimensional arrays of Co3O4 nanoparticles. J. Phys. Chem. B 2003, 107, 12643–12649. [Google Scholar] [CrossRef]
- Yan, Q.; Li, X.; Zhao, Q.; Chen, G. Shape-controlled fabrication of the porous Co3O4 nanoflower clusters for efficient catalytic oxidation of gaseous toluene. J. Hazard. Mater. 2012, 209, 385–391. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, G.; Zhang, Z.; Guo, Y.; Guo, Y.; Wang, Y. Highly active and stable Co3O4/ZSM-5 catalyst for propane oxidation: Effect of the preparation method. ACS Catal. 2013, 3, 1154–1164. [Google Scholar] [CrossRef]
- Sulaiman, M.; Dzulkarnain, N.A.; Rahman, A.A.; Mohamed, N.S. Sol–gel synthesis and characterization of LiNO3–Al2O3 composite solid electrolyte. Solid State Sci. 2012, 14, 127–132. [Google Scholar] [CrossRef]
- Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Deganello, G. Catalytic performance of Co3O4/CeO2 and Co3O4/CeO2–ZrO2 composite oxides for methane combustion: Influence of catalyst pretreatment temperature and oxygen concentration in the reaction mixture. Appl. Catal. B Environ. 2007, 70, 314–322. [Google Scholar] [CrossRef]
- Hu, L.; Sun, K.; Peng, Q.; Xu, B.; Li, Y. Surface active sites on Co3O4 nanobelt and nanocube model catalysts for CO oxidation. Nano Res. 2010, 3, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Imamura, S.; Fukuda, K.; Nishida, T.; Inui, T. Effect of Sm on the catalytic activity of Co3O4 in the oxidation of toluene. J. Catal. 1985, 93, 186–191. [Google Scholar] [CrossRef]
- Ren, Q.; Mo, S.; Peng, R.; Feng, Z.; Zhang, M.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Controllable synthesis of 3D hierarchical Co3O4 nanocatalysts with various morphologies for the catalytic oxidation of toluene. J. Mater. Chem. A 2018, 6, 498–509. [Google Scholar] [CrossRef]
- Hu, Z.; Qiu, S.; You, Y.; Guo, Y.; Guo, Y.; Wang, L.; Zhan, W.; Lu, G. Hydrothermal synthesis of NiCeOx nanosheets and its application to the total oxidation of propane. Appl. Catal. B Environ. 2018, 225, 110–120. [Google Scholar] [CrossRef]











| Catalyst | Na Content a (wt.%) | d b (nm) | a b (Å) | SSA c (m2 g−1) | Average Pore Size c (nm) | Vpore c (cm3 g−1) |
|---|---|---|---|---|---|---|
| Co-CO3-1T | 2.40 | 19.8 | 8.091 | 28 | 12.9 | 0.085 |
| Co-CO3-2T | 0.33 | 30.8 | 8.088 | 27 | 11.8 | 0.081 |
| Co-CO3-3T | 0.14 | 31.3 | 8.088 | 25 | 10.3 | 0.064 |
| Co-CO3-5T | <0.1 | 33.0 | 8.088 | 22 | 8.8 | 0.049 |
| Catalyst | T50 (°C) | T90 (°C) | r × 108 (mol g−1 s−1) | TOF × 109 (mol m−2 s−1) | Ea (kJ mol−1) | lnA |
|---|---|---|---|---|---|---|
| Co-CO3-1T | / | / | 0.3 | 0.1 | / | / |
| Co-CO3-2T | 211 | 258 | 6.6 | 2.4 | 80 | 4.8 |
| Co-CO3-3T | 201 | 236 | 9.9 | 3.9 | 76 | 4.1 |
| Co-CO3-5T | 206 | 238 | 6.9 | 3.1 | 92 | 8.2 |
| Catalyst | T50 (°C) | T90 (°C) | r × 108 (mol g−1 s−1) | TOF × 109 (mol m−2 s−1) | Ea (kJ mol−1) | lnA |
|---|---|---|---|---|---|---|
| Co-CO3-1T | 309 | / | 0.3 | 0.1 | 151 | 18.0 |
| Co-CO3-2T | 232 | 248 | 10.0 | 3.7 | 160 | 22.8 |
| Co-CO3-3T | 246 | 264 | 3.0 | 1.2 | 189 | 29.1 |
| Co-CO3-5T | 255 | 276 | 0.5 | 0.3 | 198 | 29.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, G.; Zhang, W.; Guo, Y.; Valverde, J.L.; Giroir-Fendler, A. The Influence of Residual Sodium on the Catalytic Oxidation of Propane and Toluene over Co3O4 Catalysts. Catalysts 2020, 10, 867. https://doi.org/10.3390/catal10080867
Chai G, Zhang W, Guo Y, Valverde JL, Giroir-Fendler A. The Influence of Residual Sodium on the Catalytic Oxidation of Propane and Toluene over Co3O4 Catalysts. Catalysts. 2020; 10(8):867. https://doi.org/10.3390/catal10080867
Chicago/Turabian StyleChai, Guangtao, Weidong Zhang, Yanglong Guo, Jose Luis Valverde, and Anne Giroir-Fendler. 2020. "The Influence of Residual Sodium on the Catalytic Oxidation of Propane and Toluene over Co3O4 Catalysts" Catalysts 10, no. 8: 867. https://doi.org/10.3390/catal10080867