Pd Nanoparticles and Mixture of CO2/CO/O2 Applied in the Carbonylation of Aniline
Abstract
:1. Introduction


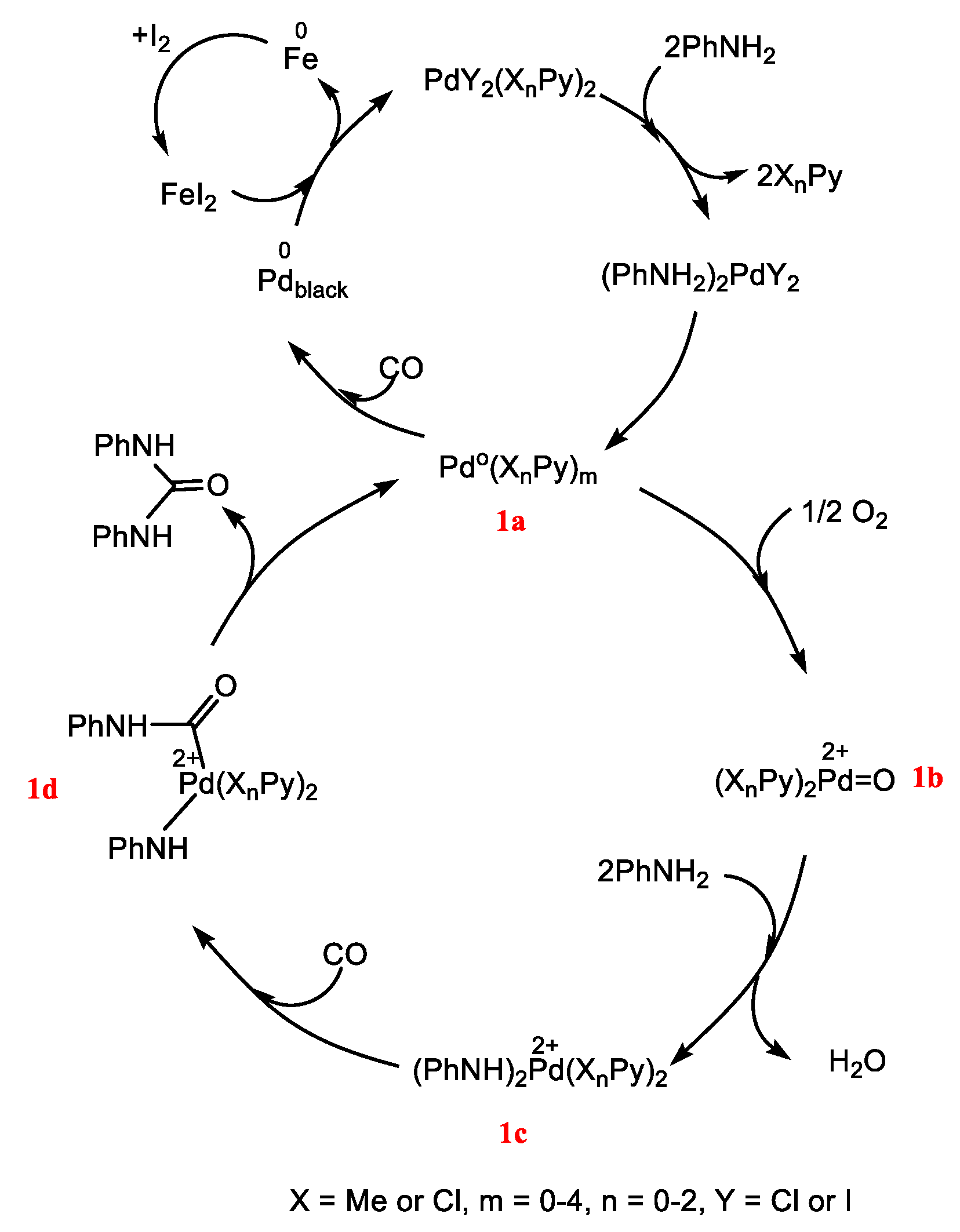
2. Results and Discussion
2.1. Optimization of Reaction Conditions of AN Carbonylation with CO2/CO/O2 Mixture
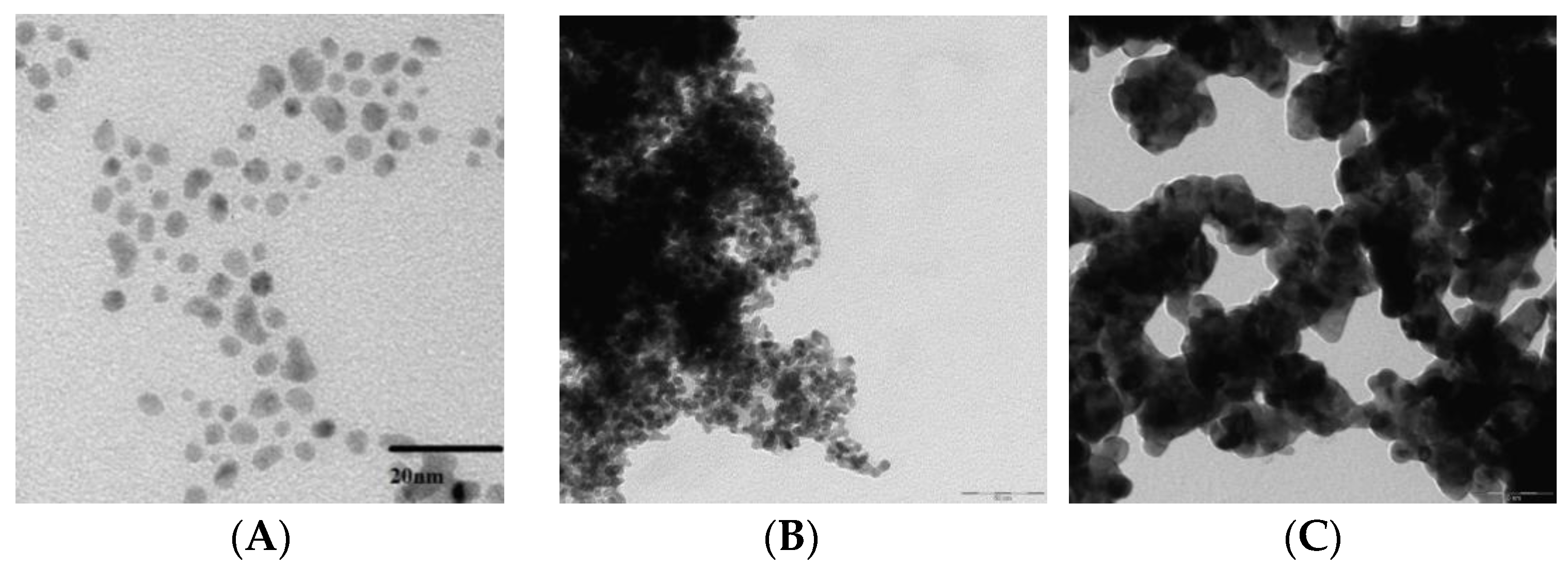
2.2. Synthesis of PdNPs
2.3. Catalytic Activity of NPs Compared with Other Pd Species
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Palladium Nanoparticles
3.3. Techniques
3.4. Carbonylation of Aniline by CO/CO2/O2 to N,N’-Diphenylurea
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ferretti, F.; Barraco, E.; Gatti, C.; Ramadan, D.R.; Ragaini, F. Palladium/iodide catalyzed oxidative carbonylation of aniline to diphenylurea: Effect of ppm amounts of iron salts. J. Catal. 2019, 369, 257–266. [Google Scholar] [CrossRef]
- Wu, X.-F.; Neumann, H.; Beller, M. Palladium-Catalyzed Oxidative Carbonylation Reactions. ChemSusChem 2013, 6, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Didgikar, M.R.; Roy, D.; Gupte, S.P.; Joshi, S.S.; Chaudhari, R.V. Immobilized Palladium Nanoparticles Catalyzed Oxidative Carbonylation of Amines. Ind. Eng. Chem. Res. 2010, 49, 1027–1032. [Google Scholar] [CrossRef]
- Guan, Z.H.; Lei, H.; Chen, M.; Ren, Z.H.; Bai, Y.; Wang, Y.Y. Palladium-Catalyzed Carbonylation of Amines: Switchable Approaches to Carbamates and N,N’-Disubstituted Ureas. Adv. Synth. Catal. 2012, 354, 489–496. [Google Scholar] [CrossRef]
- Gabriele, B.; Salerno, G.; Mancuso, R.; Costa, M. Efficient Synthesis of Ureas by Direct Palladium-Catalyzed Oxidative Carbonylation of Amines. J. Org. Chem. 2004, 69, 4741–4750. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Oliferenko, A.; Lomaka, A.; Karelson, M. Six-Membered cyclic ureas as HIV-1 protease inhibitors: A QSAR study based on CODESSA PRO approach. Bioorg. Med. Chem. Lett. 2002, 12, 3453–3457. [Google Scholar] [CrossRef]
- Patel, M.; Rodgers, J.D.; McHugh, R.J., Jr.; Johnson, B.L.; Cordova, B.C.; Klabe, R.M.; Bacheler, L.T.; Erickson-Viitanen, S. Ko SS Unsymmetrical cyclic ureas as HIV-1 protease inhibitors: Novel biaryl indazoles as P2/P2’ substituents. Bioorg. Med. Chem. Lett. 1999, 9, 3217–3220. [Google Scholar] [CrossRef]
- Inaloo, I.D.; Majnooni, S.A. Fe3O4@SiO2/Schiff Base/Pd Complex as an Efficient Heterogeneous and Recyclable Nanocatalyst for One-Pot Domino Synthesis of Carbamates and Unsymmetrical Ureas. Eur. J. Org. Chem. 2019, 37, 6359–6368. [Google Scholar] [CrossRef]
- Klaus, S.; Lehenmeier, M.W.; Anderson, C.E.; Rieger, B. Recent advances in CO2/epoxide copolymerization—New strategies and cooperative mechanisms. Coord. Chem. Rev. 2011, 255, 1460–1479. [Google Scholar] [CrossRef]
- Mulla, S.A.R.; Rode, C.V.; Kelkar, A.A.; Gupte, S.P. Activity of homogeneous transition metal catalysts for oxidative carbonylation of aniline to N,N’diphenyl urea. J. Mol. Catal. A Chem. 1997, 122, 103–109. [Google Scholar] [CrossRef]
- Bigi, F.; Maggi, R.; Sartori, G. Selected syntheses of ureas throuth phosgene substitutes. Green Chem. 2000, 2, 140–148. [Google Scholar] [CrossRef]
- Zahrtmann, N.; Claver, C.; Godard, C.; Riisager, A.; Garcia-Suarez, E. Selective Oxidative Carbonylation of Aniline to Diphenylurea with Ionic Liquids. J. ChemCatChem 2018, 10, 2450–2457. [Google Scholar] [CrossRef]
- Mancuso, R.; Raut, D.S.; Della Ca’, N.; Fini, F.; Carfagna, C.; Gabriele, B. Catalytic Oxidative Carbonylation of Amino Moieties to Ureas, Oxamides, 2-Oxazolidinones, and Benzoxazolones. ChemSusChem 2015, 13, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- Krogul, A.; Litwinienko, G. Application of Pd(II) Complexes with Pyridines as Catalysts for the Reduction of Aromatic Nitro Compounds by CO/H2O. Org. Process. Res. Dev. 2015, 19, 2017–2021. [Google Scholar] [CrossRef]
- Krogul, A.; Litwinienko, G. One pot synthesis of ureas and carbamates via oxidative carbonylation of aniline-type substrates by CO/O2 mixture catalyzed by Pd-complexes. J. Mol. Catal. A Chem. 2015, 407, 204–211. [Google Scholar] [CrossRef]
- Krogul, A.; Skupinska, J.; Litwinienko, G. Tuning of the catalytic properties of PdCl2(XnPy)2 complexes by variation of the basicity of aromatic ligands. J. Mol. Catal. A Chem. 2014, 385, 141–148. [Google Scholar] [CrossRef]
- Krogul, A.; Skupinska, J.; Litwinienko, G. Catalytic activity of PdCl2 complexes with pyridines in nitrobenzene carbonylation. J. Mol. Catal. A Chem. 2011, 337, 9–16. [Google Scholar] [CrossRef]
- Bartish, C.M.; Drissel, G.M. Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed.; Wiley-Interscience: New York, NY, USA, 1978; Volume 4, p. 774. [Google Scholar]
- Cheng, J.; Zhou, F. Revised Explosibility Diagram to Judge Best Practice of Controlling an Explosive Gas-Mixture. Fire Technol. 2015, 51, 293–308. [Google Scholar] [CrossRef]
- Chen, Y.; Hone, C.A.; Gutmann, B.; Kappe, C.O. Continuous Flow Synthesis of Carbonylated Heterocycles via Pd-Catalyzed Oxidative Carbonylation Using CO and O2 at Elevated Temperatures and Pressures. Org. Process Res. Dev. 2017, 21, 1080–1087. [Google Scholar] [CrossRef]
- Liu, A.H.; Li, Y.N.; He, L.N. Organic synthesis using carbon dioxide as phosgene-free carbonyl reagent. Pure Appl. Chem. 2012, 84, 581–602. [Google Scholar] [CrossRef]
- Darensbourg, D.J. Making Plastics from Carbon Dioxide: Salen Metal Complexes as Catalysts for the Production of Polycarbonates from Epoxides and CO2. Chem. Rev. 2007, 107, 2388–2410. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, P.; Matt, D.; Nobel, D. Reactions of Carbon Dioxide with Carbon-Carbon Bond Formation Catalyzed by Transition-Metal Complexes. Chem. Rev. 1988, 88, 747–764. [Google Scholar] [CrossRef]
- Sakakura, T.; Choi, J.C.; Yasuda, H. Transformation of Carbon Dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, A.J.; Picchioni, F.; Pescarmona, P.P. CO2-fixation into cyclic and polymeric carbonates: Principles and applications. Green Chem. 2019, 21, 406–448. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y. Carbon dioxide bio-fixation and wastewater treatment via algae photochemical synthesis for biofuels production. RSC Adv. 2014, 4, 49672–49722. [Google Scholar] [CrossRef]
- Leclaire, J.; Heldebrant, D.J. A call to (green) arms: A rallying cry for green chemistry and engineering for CO2 capture, utilisation and storage. Green Chem. 2018, 20, 5058–5081. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, L.; Jackstell, R.; Beller, B. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015, 6, 5933. [Google Scholar] [CrossRef]
- Wu, C.; Wang, J.; Chang, P.; Cheng, H.; Yu, Y.; Wu, Z.; Donga, D.; Zhao, F. Polyureas from diamines and carbon dioxide: Synthesis, structures and properties. Phys. Chem. Chem. Phys. 2012, 14, 464–468. [Google Scholar] [CrossRef]
- Franz, M.; Stalling, T.; Steinert, H.; Martens, J. First catalyst-free CO2 trapping of N-acyliminium ions under ambient conditions: Sustainable multicomponent synthesis of thia- and oxazolidinyl carbamates. Org. Biomol. Chem. 2018, 16, 8292–8304. [Google Scholar] [CrossRef]
- Krawczyk, T.; Jasiak, K.; Kokolus, A.; Baj, S. Polymer- and Carbon Nanotube-Supported Heterogeneous Catalysts for the Synthesis of Carbamates from Halides, Amines, and CO2. Catal. Lett. 2016, 146, 1163–1168. [Google Scholar] [CrossRef] [Green Version]
- Nomura, R.; Hasegawa, Y.; Ishimoto, M.; Toyasaki, T.; Matauda, H. Carbonylation of Amines by Carbon Dioxide in the Presence of an Organoantimony Catalyst. J. Org. Chem. 1992, 57, 7339–7342. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Aresta, M.; Narracci, M. Carbon Dioxide Capture by Amines: Increasing the Efficiency by Amine Structure Modification. Fuel Chem. Div. Prep. 2002, 47, 53. [Google Scholar]
- Abla, M.; Choi, J.C.; Sakakura, T. Halogen-free process for the conversion of carbon dioxide to urethanes by homogeneous catalysis. Chem. Commun. 2001, 2238–2239. [Google Scholar] [CrossRef] [PubMed]
- Heyn, R.H.; Jacobs, I.; Carr, R.H. Synthesis of Aromatic Carbamates from CO2: Implications for the Polyurethane Industry. Adv. Inorg. Chem. 2014, 66, 83–115. [Google Scholar]
- Choi, J.C.; Yuan, H.Y.; Fukaya, N.; Onozawa, S.; Zhang, Q.; Choi, S.J.; Yasuda, H. Halogen-Free Synthesis of Carbamates from CO2 and Amines Using Titanium Alkoxides. Chem. Asian J. 2017, 12, 1297–1300. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, H.Y.; Fukaya, N.; Choi, J.C. Alkali Metal Salt as Catalyst for Direct Synthesis of Carbamate from Carbon Dioxide. ACS Sustain. Chem. Eng. 2018, 6, 6675–6681. [Google Scholar] [CrossRef]
- Ren, Y.; Rousseaux, S.A.L. Metal-Free Synthesis of Unsymmetrical Ureas and Carbamates from CO2 and Amines via Isocyanate Intermediates. J. Org. Chem. 2018, 83, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Inaloo, I.D.; Majnoonib, S. Carbon dioxide utilization in the efficient synthesis of carbamates by deep eutectic solvents (DES) as green and attractive solvent/catalyst Systems. New J. Chem. 2019, 43, 11275–11281. [Google Scholar] [CrossRef]
- Lam, R.H.; McQueen, C.M.A.; Pernik, I.; McBurney, R.T.; Hill, A.F.; Messerle, B.A. Selective formylation or methylation of amines using carbon dioxide catalysed by a rhodium perimidine-based NHC complex. Green Chem. 2019, 21, 538–549. [Google Scholar] [CrossRef]
- Riemer, D.; Hirapara, P.; Das, S. Chemoselective Synthesis of Carbamates Using CO2 as Carbon Source. ChemSusChem 2016, 9, 1916–1920. [Google Scholar] [CrossRef]
- Wang, P.; Fei, Y.; Deng, Y. Transformation of CO2 into polyureas with 3-amino-1,2,4-triazole potassium as a solid base catalyst. New J. Chem. 2018, 42, 1202–1207. [Google Scholar] [CrossRef]
- Honda, M.; Sonehara, S.; Yasuda, H.; Nakagawaa, Y.; Tomishige, K. Heterogeneous CeO2 catalyst for the one-pot synthesis of organic carbamates from amines, CO2 and alcohols. Green Chem. 2011, 13, 3406–3413. [Google Scholar] [CrossRef]
- Ca’, N.D.; Bottarelli, P.; Dibenedetto, A.; Aresta, M.; Gabriele, B.; Salerno, G.; Costa, M. Palladium-catalyzed synthesis of symmetrical urea derivatives by oxidative carbonylation of primary amines in carbon dioxide medium. J. Catal. 2011, 282, 120–127. [Google Scholar] [CrossRef]
- Ma, L.; Xiao, Y.; Deng, J.; Wang, Q. Effect of CO2 on explosion limits of flammable gases in goafs. Min. Sci. Technol. 2010, 20, 193–197. [Google Scholar] [CrossRef]
- Leitner, W. Supercritical Carbon Dioxide as a Green Reaction Medium for Catalysis. Acc. Chem. Res. 2002, 35, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, B. Gas Expanded Liquids for Sustainable Catalysis. Encycl. Sustain. Sci. Technol. 2012, 3933. [Google Scholar]
- Flanagan, K.A.; Sullivan, J.A.; Mueller-Bunz, H. Preparation and Characterization of 4-Dimethylaminopyridine-Stabilized Palladium Nanoparticles. Langmuir 2007, 23, 12508–12520. [Google Scholar] [CrossRef] [Green Version]
- Shaughnessy, K.H.; DeVasher, R.B. Palladium-Catalyzed Cross-Coupling in Aqueous Media: Recent Progress and Current Applications. Curr. Org. Chem. 2005, 9, 585–604. [Google Scholar] [CrossRef]
- Vasylyev, M.V.; Maayan, G.; Hovav, Y.; Haimov, A.; Neumann, R. Palladium Nanoparticles Stabilized by Alkylated Polyethyleneimine as Aqueous Biphasic Catalysts for the Chemoselective Stereocontrolled Hydrogenation of Alkenes. Org. Lett. 2006, 8, 5445–5448. [Google Scholar] [CrossRef]
- Rucareanu, S.; Gandubert, V.J.; Lennox, R.B. 4-(N,N-Dimethylamino)pyridine-Protected Au Nanoparticles: Versatile Precursors for Water- and Organic-Soluble Gold Nanoparticles. Chem. Mater. 2006, 18, 4674–4680. [Google Scholar] [CrossRef]
- Kaim, A.; Szydłowska, J.; Piotrowski, P.; Megiel, E. One-pot synthesis of gold nanoparticles densely coated with nitroxide spins. Polyhedron 2012, 46, 119–123. [Google Scholar] [CrossRef]
- Oh, S.K.; Niu, Y.; Crooks, R.M. Size-Selective Catalytic Activity of Pd Nanoparticles Encapsulated within End-Group Functionalized Dendrimers. Langmuir 2005, 21, 10209–10213. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Crooks, R.M. Homogeneous Hydrogenation Catalysis with Monodisperse, Dendrimer-Encapsulated Pd and Pt Nanoparticles. Angew. Chem. Int. Ed. 1999, 38, 364–366. [Google Scholar] [CrossRef]
- Krogul-Sobczak, A.; Kasperska, P.; Litwinienko, G. N-heterocyclic monodentate ligands as stabilizing agents for catalytically active Pd-nanoparticles. Catal. Commun. 2018, 104, 86–90. [Google Scholar] [CrossRef]
- Krogul-Sobczak, A.; Cedrowski, J.; Kasperska, P.; Litwinienko, G. Reduction of Nitrobenzene to Aniline by CO/H2O in the Presence of Palladium Nanoparticles. Catalysts 2019, 9, 404. [Google Scholar] [CrossRef] [Green Version]
- Ragaini, F.; Cenini, S. Mechanistic studies of palladium-catalysed carbonylation reactions of nitro compounds to isocyanates, carbamates and ureas. J. Mol. Catal. A Chem. 1996, 109, 1–25. [Google Scholar] [CrossRef]
- Stahl, S.S. Palladium Oxidase Catalysis: Selective Oxidation of Organic Chemicals by Direct Dioxygen-Coupled Turnover. Angew. Chem. Int. Ed. 2004, 43, 3400–3420. [Google Scholar] [CrossRef]
- Fukuoka, S.; Chono, M.; Kohno, M. Isocyanate without phosgene. Chemtech 1984, 14, 670–676. [Google Scholar]
- Gupte, S.P.; Chaudhari, R.V. Oxidative carbonylation of aniline over PdC catalyst: Effect of promoters, solvents, and reaction conditions. J. Catal. 1988, 114, 246–258. [Google Scholar] [CrossRef]
- Pri-Bar, I.; Schwartz, J. I2-Promoted Palladium-Catalyzed Carbonylation of Amines. J. Org. Chem. 1995, 60, 8124–8125. [Google Scholar] [CrossRef]
- Shi, F.; Deng, Y.; SiMa, T.; Yang, H. A novel ZrO2-SO42− supported palladium catalyst for syntheses of disubstituted ureas from amines by oxidative carbonylation. Tetrahedron Lett. 2001, 42, 2161–2163. [Google Scholar] [CrossRef]
- McCusker, J.E.; Qian, F.; McElwee-White, L. Catalytic oxidative carbonylation of aliphatic secondary amines to tetrasubstituted ureas. J. Mol. Catal. A Chem. 2000, 159, 11–17. [Google Scholar] [CrossRef]
- Maitlis, P.M.; Haynes, A.; James, B.R.; Catellani, M.; Chiusoli, G.P. Iodide effects in transition metal catalyzed reactions. Dalton Trans. 2004, 3409–3419. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, B.; Mancuso, R.; Veltri, L.; Della Ca’, N. Polemic against conclusions drawn in “Palladium/iodide catalyzed oxidative carbonylation of aniline to diphenylurea: Effect of ppm amounts of iron salts”. J. Catal. 2019, 380, 387–390. [Google Scholar] [CrossRef]
- Benesovsky, F. Gmelin Handbuch der Anorganischen Chemie; Springer: New York, NY, USA, 1979; Volume 59, p. 337. [Google Scholar]
- Kwiatkowski, A.; Jędrzejewska, B.; Józefowicz, M.; Grela, I.; Ośmiałowski, B. The trans/cis photoisomerization in hydrogen bonded complexes with stability controlled by substituent effects: 3-(6-aminopyridin-3-yl) acrylate case study. RSC Adv. 2018, 8, 23698–23710. [Google Scholar] [CrossRef] [Green Version]

| Entry | CO2 in CO/CO2 b (%) | I2 (mmoL) | Fe (mmoL) | CAN (%) | SDPU c (%) | TOFDPU d |
|---|---|---|---|---|---|---|
| 1 e | 100 | 0.12 | 2.68 | 12 | 0 | 0 |
| 2 e | 88 | 37 | 57 | 202 | ||
| 3 e | 65 | 83 | 83 | 666 | ||
| 4 e | 56 | 84 | 87 | 704 | ||
| 5 e | 0 | 68 | 93 | 608 | ||
| 6 | 56 | 0 | 2.68 | 10 | 0 | 0 |
| 7 | 0 | 0 | 10 | 0 | 0 | |
| 8 | 0.01 | 46 | 96 | 434 | ||
| 9 | 0.04 | 71 | 96 | 657 | ||
| 10 | 0.12 | 73 | 95 | 669 | ||
| 11 | 0.39 | 69 | 94 | 617 | ||
| 12 | 0.04 | 0.25 | 75 | 96 | 694 | |
| 13 | 0.5 | 78 | 98 | 733 | ||
| 14 | 1.2 | 74 | 96 | 685 | ||
| 15 | 2.68 | 72 | 95 | 660 | ||
| 16 f | 2.68 | 72 | 96 | 667 |
| Entry | T (°C) | CAN (%) | SDPU b (%) | TOFDPU c |
|---|---|---|---|---|
| 1 | 80 | 17 | 98 | 161 |
| 2 | 100 | 71 | 98 | 671 |
| 3 | 120 | 86 | 95 | 791 |
| 4 | 140 | 80 | 71 (20) d | 550 (154) e |
| Entry | Catalyst | CAN (%) | SDPU b (%) | TOFDPU c |
|---|---|---|---|---|
| 1 | PdNPs/4MePy | 92 | 88 | 781 |
| 2 d | PdNPs/4MePy | 27 | 89 | 926 |
| 3 | PdNM/4MePy e | 80 | 84 | 646 |
| 4 | Pdblack | 60 | 89 | 515 |
| 5 | Pdblack f | 72 | 89 | 617 |
| 6 | PdCl2(4MePy)2 | 83 | 87 | 656 |
| 7 d | PdCl2(2,4Cl2Py)2 | 16 | 88 | 540 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madej, D.; Konopko, A.; Piotrowski, P.; Krogul-Sobczak, A. Pd Nanoparticles and Mixture of CO2/CO/O2 Applied in the Carbonylation of Aniline. Catalysts 2020, 10, 877. https://doi.org/10.3390/catal10080877
Madej D, Konopko A, Piotrowski P, Krogul-Sobczak A. Pd Nanoparticles and Mixture of CO2/CO/O2 Applied in the Carbonylation of Aniline. Catalysts. 2020; 10(8):877. https://doi.org/10.3390/catal10080877
Chicago/Turabian StyleMadej, Dominik, Adrian Konopko, Piotr Piotrowski, and Agnieszka Krogul-Sobczak. 2020. "Pd Nanoparticles and Mixture of CO2/CO/O2 Applied in the Carbonylation of Aniline" Catalysts 10, no. 8: 877. https://doi.org/10.3390/catal10080877






