Carbon Nanotube Modified by (O, N, P) Atoms as Effective Catalysts for Electroreduction of Oxygen in Alkaline Media
Abstract
:1. Introduction
2. Results and Discussion
2.1. X-ray Photoelectron Spectrum (XPS) Studies
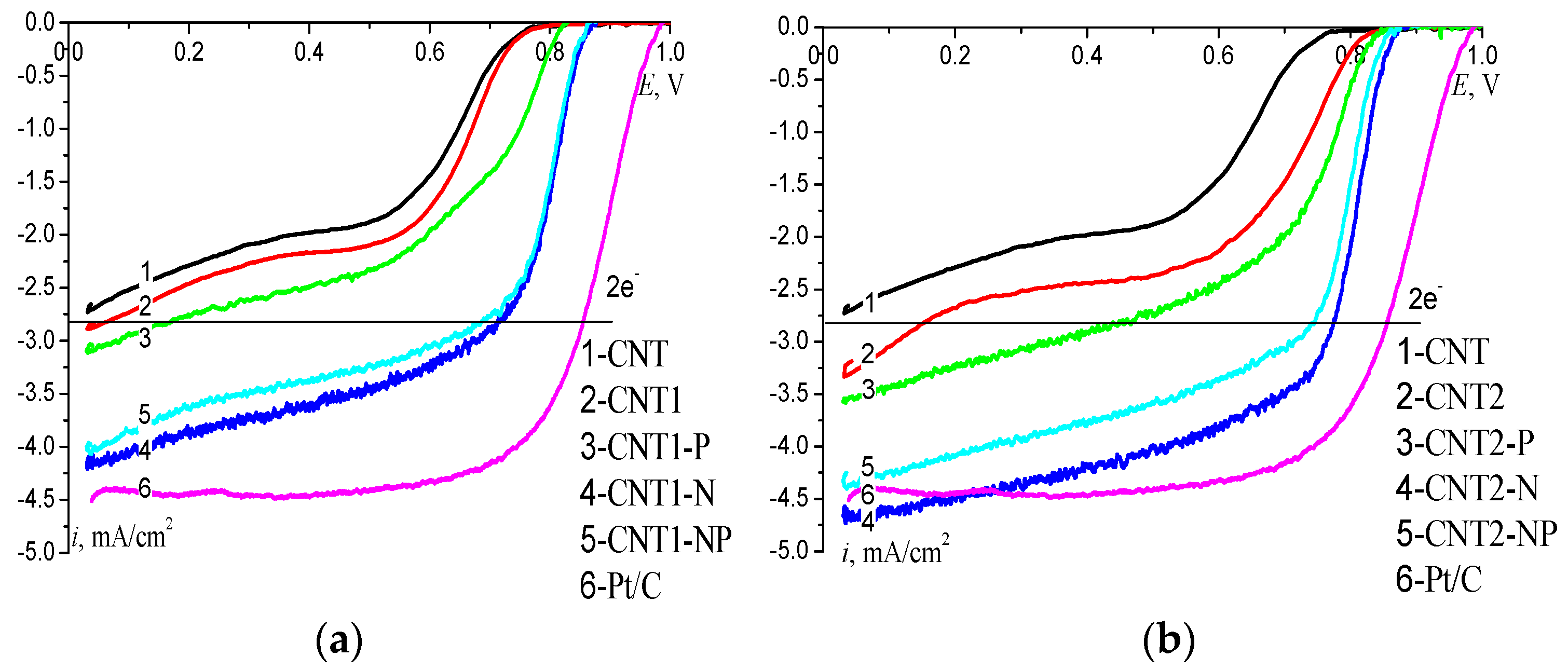
2.2. Electrochemical Studies
2.3. Accelerated Corrosion Testing
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Electrochemical Methods
3.3. CNT Modification Methods
3.4. Structural Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dumitrescu, I.; Unwin, P.R.; Macpherson, J.V. Electrochemistry at carbon nanotubes: Perspective and issues. Chem. Commun. 2009, 45, 6886–6901. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Li, X.; Liu, J.; Geng, D.; Yang, J.; Li, R.; Sun, X. Nitrogen-doped carbon nanotubes as cathode for lithium–Air batteries. Electrochem. Commun. 2011, 13, 668–672. [Google Scholar] [CrossRef]
- Antolini, E. Carbon supports for low-temperature fuel cell catalysts. Appl. Catalysis B Environm. 2009, 88, 1–24. [Google Scholar] [CrossRef]
- Melchionna, M.; Marchesan, S.; Prato, M.; Fornasiero, P. Carbon nanotubes and catalysis: The many facets of a successful marriage. Catal. Sci. Technol. 2015, 5, 3859–3875. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Song, W.; Li, M.; Wu, Z. Oxygen Reduction Reaction Mechanisms on Heteroatom-Doped Single Walled Carbon Nanotube Catalysts: Insights from a Theoretical Study. J. Electrochem. Soc. 2019, 166, 670–678. [Google Scholar] [CrossRef]
- Sang, Y.; Fu, A.; Li, H.; Zhang, J.; Li, Z.; Li, H.; Zhao, X.S.; Guo, P. Experimental and theoretical studies on the effect of functional groups on carbon nanotubes to its oxygen reduction reaction activity. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 476–484. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, C.; Dou, S.; Liu, D.; Wang, S. Oxidized carbon nanotubes as an efficient metal free electrocatalyst for the oxygen reduction reaction. RSC Adv. 2015, 5, 41901–41904. [Google Scholar] [CrossRef]
- Dumitrua, A.; Mamlouk, M.; Scott, K. Effect of different chemical modification of carbon nanotubes for the oxygen reduction reaction in alkaline media. Electrochim. Acta 2014, 35, 428–438. [Google Scholar] [CrossRef]
- Othman, S.H.; Ritter, U.; McCarthy, E.K.; Fernandes, D.; Kelarakis, A.; Tsierkezos, N.G. Synthesis and electrochemical characterization of nitrogen-doped and nitrogen–phosphorus-doped multi-walled carbon nanotubes. Ionics 2017, 2025–2035. [Google Scholar] [CrossRef]
- Wei, Q.; Tong, X.; Zhang, G.; Qiao, J.; Gong, Q.; Sun, S. Nitrogen-Doped Carbon Nanotube and Graphene Materials for Oxygen Reduction Reactions. Catalysts 2015, 5, 1574–1602. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Silva, E.; Lopez-Urias, F.; Munoz-Sandoval, E.; Sumpter, B.G.; Terrones, H.; Charlier, J.-C.; Meunier, V.; Terrones, M. Electronic Transport and Mechanical Properties of Phosphorus and Phosphorus-Nitrogen-Doped Carbon Nanotube. ACS Nano 2009, 3, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dai, L. Carbon nanomaterials as metal-free catalysts in next generation fuel cells. Nano Energy 2012, 1, 514–517. [Google Scholar] [CrossRef]
- Cruz-Silva, E.; Cullen, D.A.; Gu, L.; Romo-Herrera, J.M.; Muñoz-Sandoval, E.; López-Urías, F.; Sumpter, B.G.; Meunier, V.; Charlier, J.-C.; Smith, D.J.; et al. Heterodoped Nanotubes: Theory, Synthesis, and Characterization of Phosphorus Nitrogen Doped Multiwalled Carbon Nanotubes. ACS Nano 2008, 2, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Shi, L.; Xu, J.; Yan, X.; Zhao, T.S. Role of phosphorus in nitrogen, phosphorus dual-doped ordered mesoporous carbon electrocatalyst for oxygen reduction reaction in alkaline media. Intern. J. Hydrog. Energy 2018, 43, 1470–1478. [Google Scholar] [CrossRef]
- Kanninen, P.; Eriksson, B.; Davodi, F.; Melandsø, M.E.; Sorsa, O.; Kallio, T.; Lindstrom, R.W. Carbon corrosion properties and performance of multi-walled carbon nanotube support with and without nitrogen-functionalization in fuel cell electrodes. Electrochim. Acta 2020, 3321, 135384. [Google Scholar] [CrossRef]
- Zhong, R.S.; Qin, Y.H.; Niu, D.F.; Tian, J.W.; Zhang, X.S.; Zhou, X.G.; Sun, S.G.; Yuan, W.K. Effect of carbon nanofiber surface functional groups on oxygen reduction in alkaline solution. J. Power Sources 2013, 225, 192–199. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Dias de Tuesta, J.L.; Quintanilla, A.; Casas, J.A.; Rodrigues, J.J. P-, B- and N-doped carbon black for the catalytic wet peroxide oxidation of phenol: Activity, stability and kinetic studies. Catalysis Commun. 2017, 102, 131–135. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Aplications; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Zhao, D.; Tang, Z.; Xu, W.; Wu, Z.; Ma, L.i.-J.; Cui, Z.; Yang, C.; Li, L. N, S-codoped CNTs supported Co4S3 nanoparticles prepared by using CdS nanorods as sulfur sources and hard templates: An efficient catalyst for reversible oxygen electrocatalysis. J. Colloid Interface Sci. 2020, 560, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Bogdanovskaya, V.A.; Kol’tsova, E.M.; Tarasevich, M.R.; Radina, M.V.; Zhutaeva, G.V.; Kuzov, A.V.; Gavrilova, N.N. Highly active and stable catalysts based on nanotubes and modified platinum for fuel cells. Russ. J. Electrochem. 2016, 52, 723–734. [Google Scholar] [CrossRef]




| Nos. | Material | Q, C/g (as SEAS) | SBET, m2/g | E1/2, V | ikin, A/cm2 (E, V) * | ilim, A/cm2 at 0.4 V (n) ** | Element/Overall Content, at. % |
|---|---|---|---|---|---|---|---|
| 157 rad/s | |||||||
| 1 | CNT | 23 | 320 | 0.64 | 0.1 (0.750) | 2 (1.4) | О/0.46 |
| 2 | CNT 1 | 37.5 | 297 | 0.66 | 0.13 (0.750) | 2.2 (1.7) | O/2.18 |
| 3 | CNT 1–Р | 25 | - | 0.72 | 0.6 (0.777) | 2.5 (1.8) | О/11.1 N/0.70 Р/0.40 |
| 4 | CNT 1–N | 47.5 | 269 | 0.79 | 1.02 (0.813) | 3.62 (2.6) | O/10.08 N/1.15 |
| 5 | CNT 1–NP | 48 | 172.2 | 0.79 | 1.02 (0.819) | 3.36 (2.5) | О/2.59 N/1.49 Р/0.82 |
| 6 | CNT 2 | 78 | - | 0.71 | 0.23 (0.800) | 2.42 (1.8) | О/15.4 N/1.2 |
| 7 | CNT 2–P | 37.5 | - | 0.74 | 0.49 (0.805) | 2.9 (2) | О/10.8 N/1.0 Р/0.2 |
| 8 | CNT 2–N | 61 | - | 0.79 | 0.92 (0.815) | 4.2 (3) | O/12.84 N/1.98 |
| 9 | CNT 2–NP | 54 | 216.6 | 0.78 | 1.19 (0.823) | 3.75 (2.7) | O/10.8 N/1.55 P/0.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanovskaya, V.; Vernigor, I.; Radina, M.; Andreev, V.; Korchagin, O.; Novikov, V. Carbon Nanotube Modified by (O, N, P) Atoms as Effective Catalysts for Electroreduction of Oxygen in Alkaline Media. Catalysts 2020, 10, 892. https://doi.org/10.3390/catal10080892
Bogdanovskaya V, Vernigor I, Radina M, Andreev V, Korchagin O, Novikov V. Carbon Nanotube Modified by (O, N, P) Atoms as Effective Catalysts for Electroreduction of Oxygen in Alkaline Media. Catalysts. 2020; 10(8):892. https://doi.org/10.3390/catal10080892
Chicago/Turabian StyleBogdanovskaya, Vera, Inna Vernigor, Marina Radina, Vladimir Andreev, Oleg Korchagin, and Vasilii Novikov. 2020. "Carbon Nanotube Modified by (O, N, P) Atoms as Effective Catalysts for Electroreduction of Oxygen in Alkaline Media" Catalysts 10, no. 8: 892. https://doi.org/10.3390/catal10080892






