Light Cycle Oil Source for Hydrogen Production through Autothermal Reforming using Ruthenium doped Perovskite Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. LCO Feedstock Characterization
2.2. Synthesis of Perovskite Catalysts
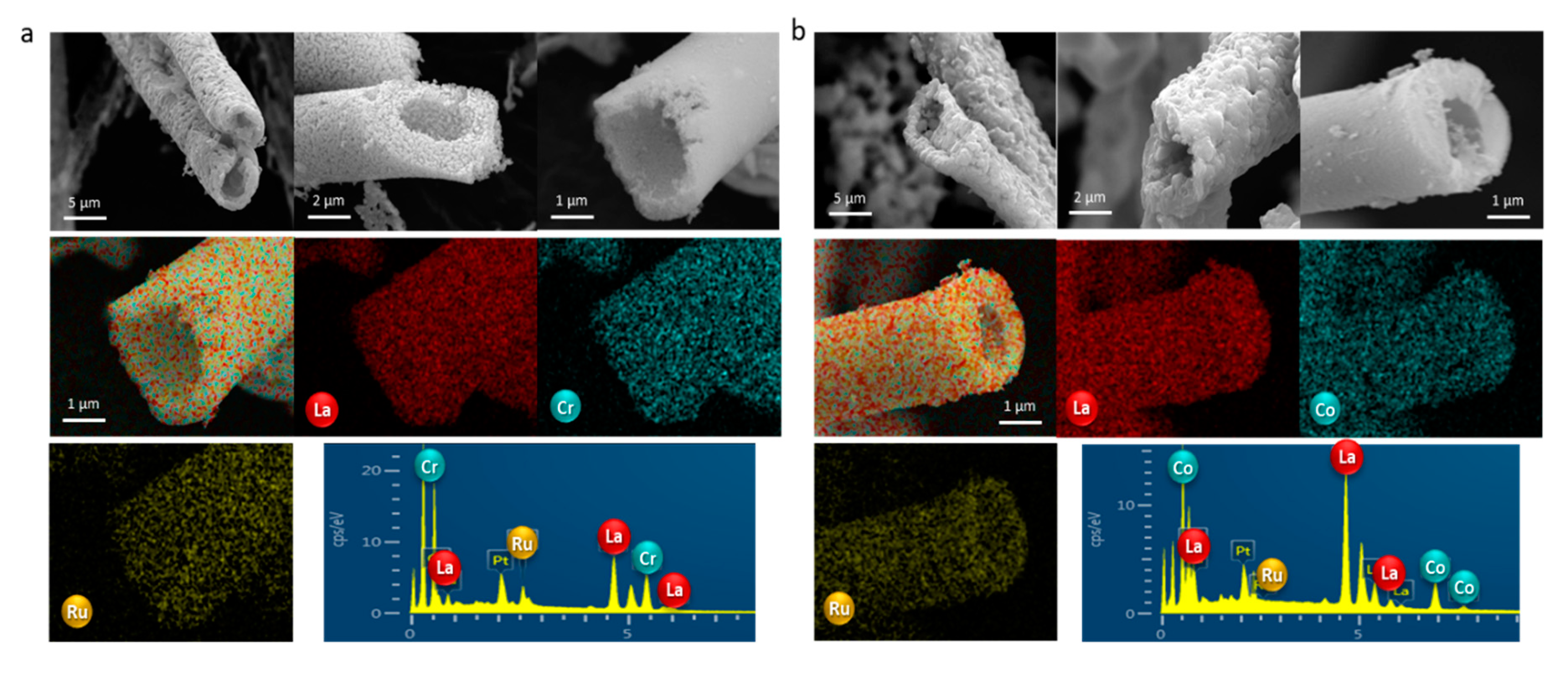
2.2.1. SEM-EDX (Scanning Electron Microscopy-Energy-Dispersive X-ray)
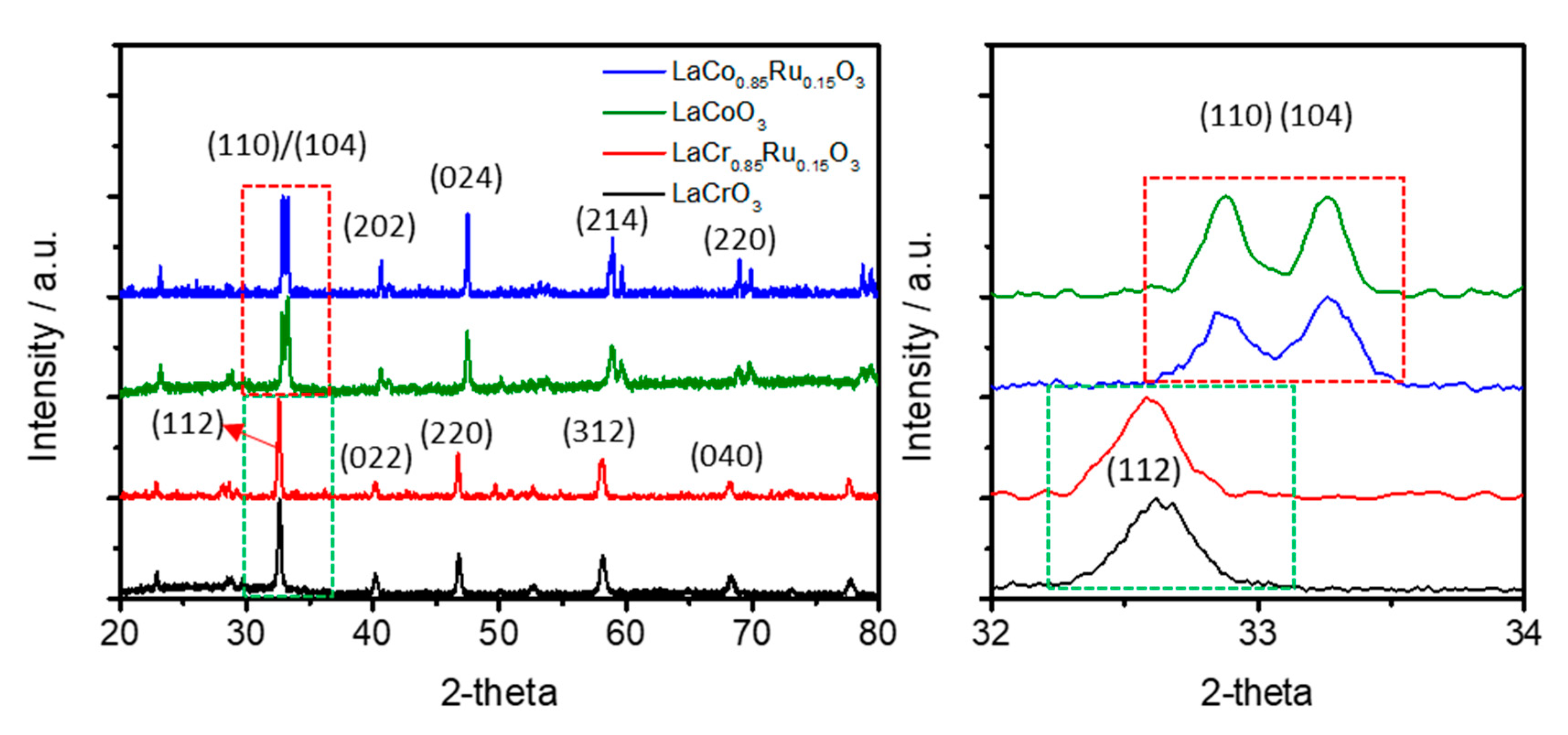
2.2.2. XRD (X-Ray Diffraction)
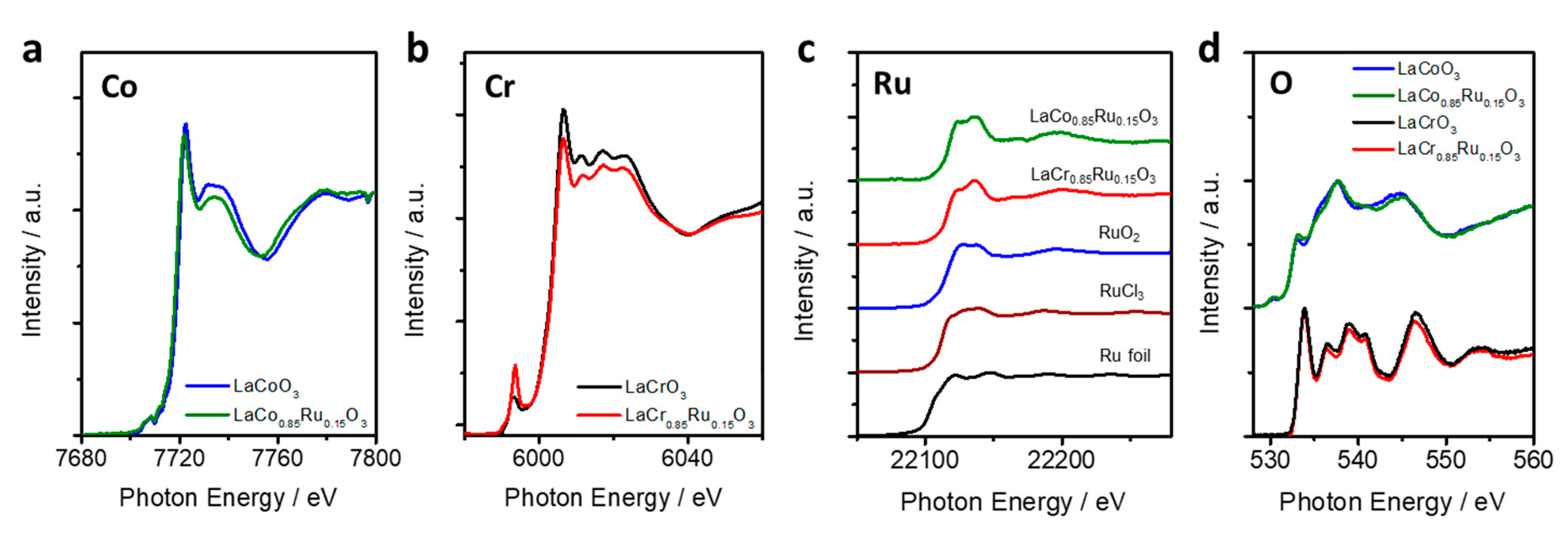
2.2.3. XANES (X-Ray Absorption Near Edge Structure)
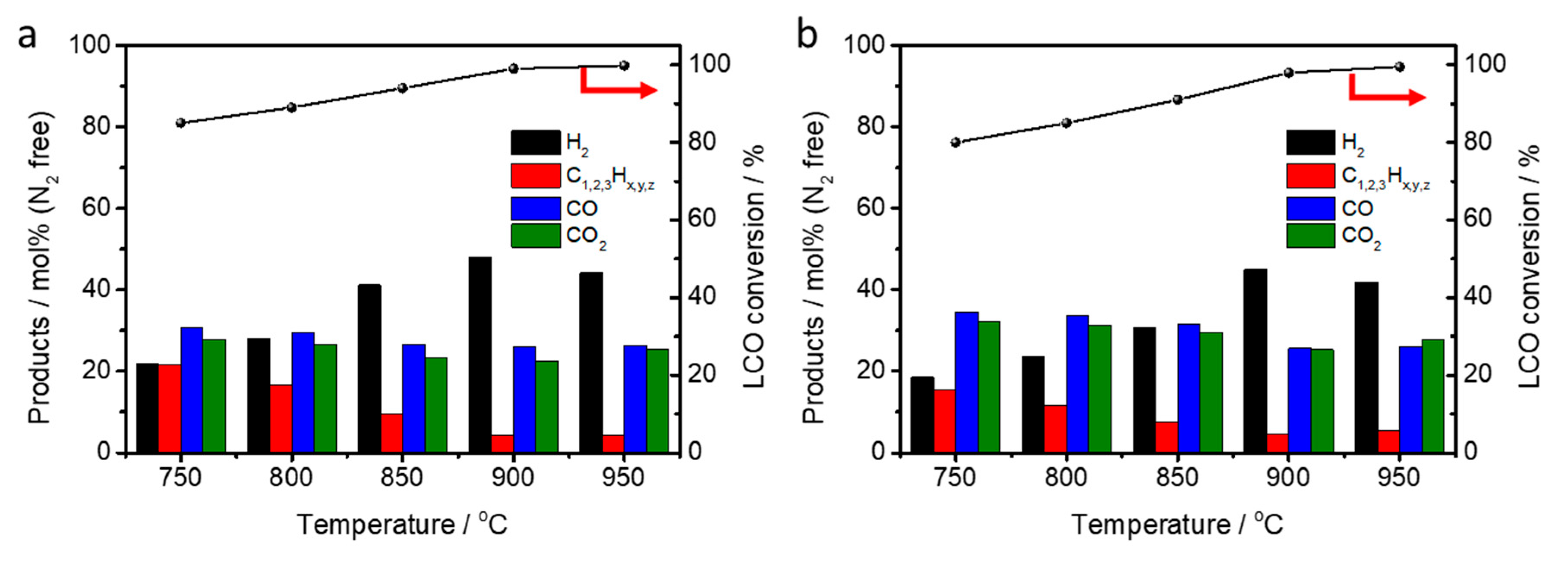
2.3. LCO-ATR by Various Reaction Temperature
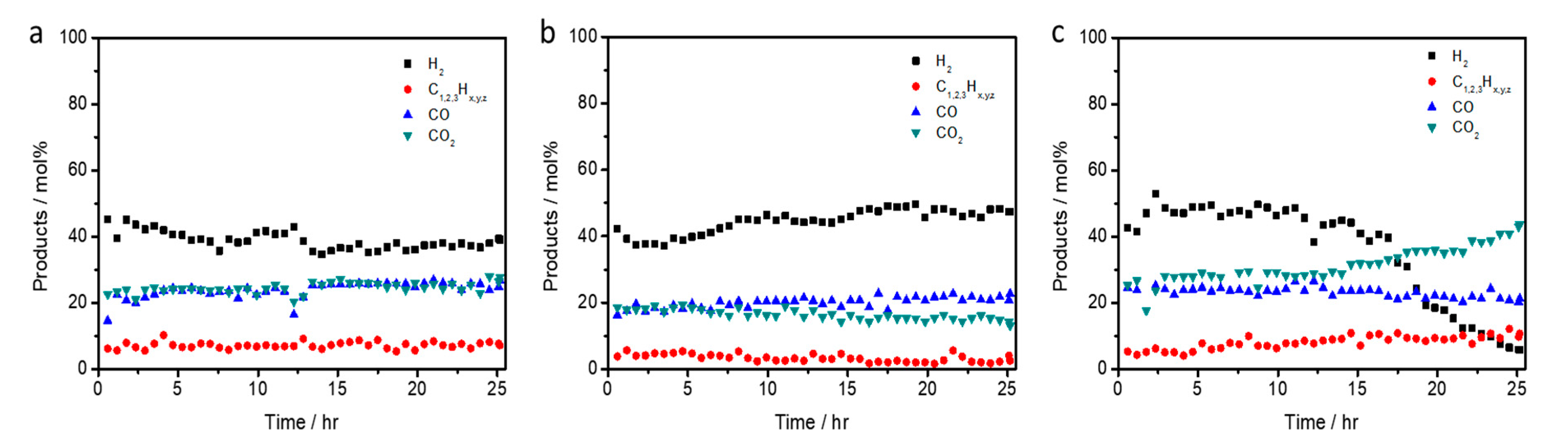
2.4. Long-Term Test of LCO-ATR Reactions
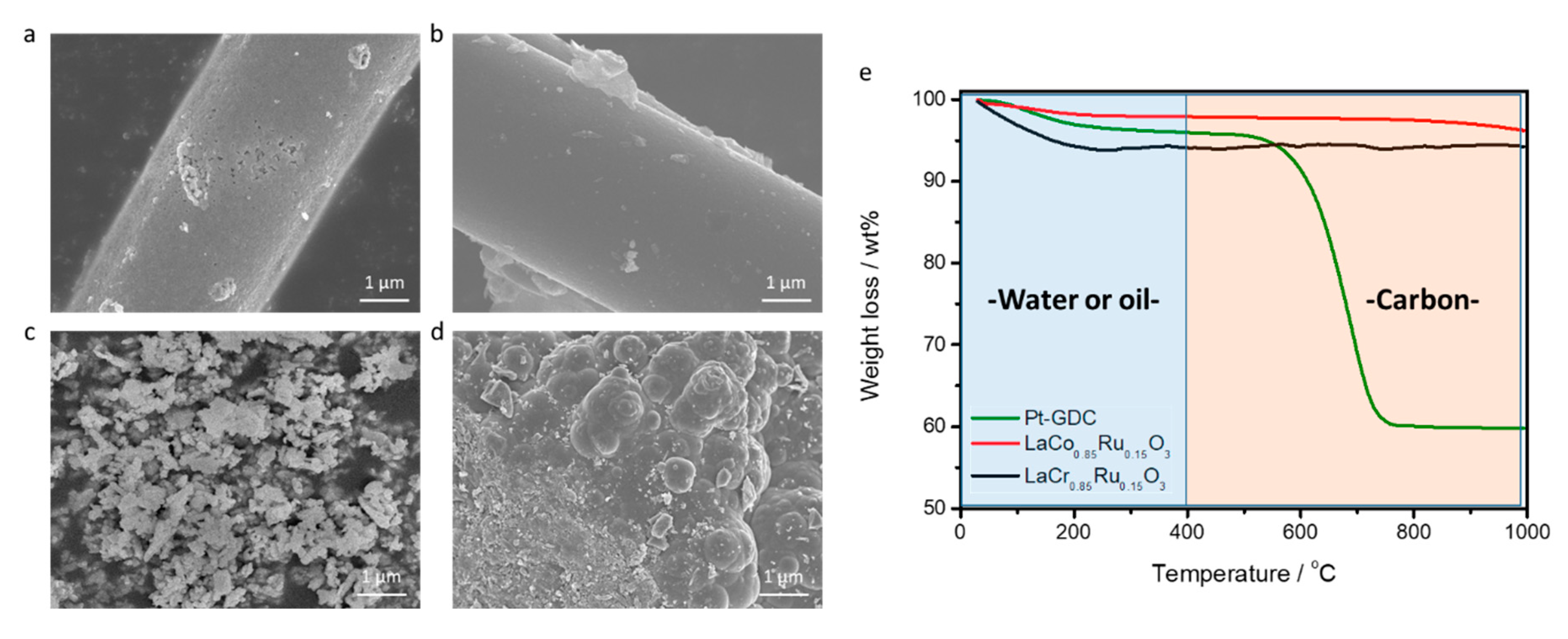
2.5. Post Characterization of the Used Perovskite Catalysts
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Damo, U.M.; Ferrari, M.L.; Turan, A.; Massardo, A.F. Solid oxide fuel cell hybrid system: A detailed review of an environmentally clean and efficient source of energy. Energy 2019, 168, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Cai, F.; Ibrahim, J.J.; Fu, Y.; Kong, W.; Zhang, J.; Sun, Y. Low-temperature hydrogen production from methanol steam reforming on Zn-modified Pt/MoC catalysts. Appl. Catal. B Environ. 2020, 264, 118500. [Google Scholar] [CrossRef]
- United Nations Framework Convention on Climate Change (UNFCCC). Adoption of the Paris Agreement, Report No. FCCC/CP/2015/L.9/Rev.1, 2015. Available online: http://unfccc.int/resource/docs/2015/cop21/eng/l09r01.pdf (accessed on 13 August 2020).
- Rogelj, J.; Den elzen, M.; Höhne, N.; Fransen, T.; Fekete, H.; Winkler, H.; Schaeffer, R.; Sha, F.; Riahi, K.; Meinshausen, M. Paris Agreement climate proposals need a boost to keep warming well below 2 °C. Nature 2016, 534, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Jang, W.; Shim, J.-O.; Kim, H.-M.; Yoo, S.-Y.; Roh, H.-S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Zhai, P.; Pörtner, H.O.; Roberts, D. (Eds.) Summary for Policymakers, in Global warming of 15 °C, An IPCC Special Report on the impacts of global warming of 15 °C above pre-industrial levels and related global greenhouse gas emission pathways. In The Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2018; Volume 32. [Google Scholar]
- IEA. World Energy Outlook; IEA: Paris, France, 2018. [Google Scholar]
- Antonini, C.; Treyer, K.; Streb, A.; Spek, M.; Bauer, C.; Mazzotti, M. Hydrogen production from natural gas and biomethane with carbon capture and storage–A techno-environmental analysis. Sustain. Energy Fuels 2020, 4, 2967–2986. [Google Scholar] [CrossRef] [Green Version]
- International Energy Agency (IEA). Energy Technology Perspectives 2017; International Energy Agency (IEA): Paris, France, 2017. [Google Scholar]
- Lee, Y.-L.; Mnoyan, A.; Na, H.-S.; Ahn, S.-Y.; Kim, K.-J.; Shim, J.-O.; Lee, K.; Roh, H.-S. Comparison of the effects of the catalyst preparation method and CeO2 morphology on the catalytic activity of Pt/CeO2 catalysts for the water–gas shift reaction. Catal. Sci. Technol. 2020. accepted. [Google Scholar] [CrossRef]
- Du, H.; Kong, R.M.; Guo, X.; Qu, F.; Li, J. Recent progress in transition metal phosphides with enhanced electrocatalysis for hydrogen evolution. Nanoscale 2018, 10, 21617–21624. [Google Scholar] [CrossRef]
- Valente, A.; Iribarren, D.; Dufour, J. Harmonised life-cycle global warming impact of renewable hydrogen. J. Clean. Prod. 2017, 149, 762–772. [Google Scholar] [CrossRef]
- Parkinson, B.; Tabatabaei, M.; Upham, D.C.; Ballinger, B.; Greig, C.; Smart, S.; McFarland, E. Hydrogen production using methane: Techno-economics of decarbonizing fuels and chemicals. Int. J. Hydrogen Energy 2018, 43, 2540–2555. [Google Scholar] [CrossRef]
- Sun, P.; Young, B.; Elgowainy, A.; Lu, Z.; Wang, M.; Morelli, B.; Hawkins, T. Criteria Air Pollutants and Greenhouse Gas Emissions from Hydrogen Production in U.S. Steam Methane Reforming Facilities. Environ. Sci. Technol. 2019, 53, 7103–7113. [Google Scholar] [CrossRef] [PubMed]
- Navarro, R.M.; Álvarez-Galván, M.C.; Mota, M.; Villoria de la Mano, J.A.; Al-Zahrani, S.M.; Fierro, J.L.G. Catalysts for Hydrogen Production from Heavy Hydrocarbons. ChemCatChem 2011, 3, 440–457. [Google Scholar] [CrossRef]
- Jeon, Y.; Lee, C.; Rhee, J.; Lee, G.; Myung, J.; Park, M.; Park, J.-I.; Einaga, H.; Shul, Y.-G. Autothermal reforming of heavy-hydrocarbon fuels by morphology controlled perovskite catalysts using carbon templates. Fuel 2017, 187, 446–456. [Google Scholar] [CrossRef]
- Jeon, Y.; Kwon, O.; Lee, C.; Lee, G.; Myung, J.; Park, S.; Irvine, J.T.S.; Shul, Y. Positional influence of Ru on Perovskite structured catalysts for efficient H2 production process by heavy-hydrocarbon source. Appl. Catal. A Gen. 2019, 582, 117111. [Google Scholar] [CrossRef]
- Nahar, G.; Dupont, V. Recent Advances in Hydrogen Production Via Autothermal Reforming Process (ATR): A Review of Patents and Research Articles. Recent Patents Chem. Eng. 2013, 6, 8–42. [Google Scholar] [CrossRef]
- Reese, M.A.; Turn, S.Q.; Cui, H. Kinetic modeling of high pressure autothermal reforming. J. Power Sources 2010, 195, 553–558. [Google Scholar] [CrossRef]
- Jeon, Y.; Park, D.-H.; Park, J.-I.; Yoon, S.-H.; Mochida, I.; Choy, J.-H.; Shul, Y.-G. Hollow Fibers Networked with Perovskite Nanoparticles for H2 Production from Heavy Oil. Sci. Rep. 2013, 3, 2902. [Google Scholar] [CrossRef] [PubMed]
- Cortright, R.D.; Davda, R.R.; Dumesic, J.A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature 2002, 418, 964–967. [Google Scholar] [CrossRef]
- Pasadakis, N.; Karonis, D.; Mintza, A. Detailed compositional study of the Light Cycle Oil (LCO) solvent extraction products. Fuel Process. Tech. 2011, 92, 1568–1573. [Google Scholar] [CrossRef]
- Laredo, G.C.; Vega Merino, P.M.; Hernández, P.S. Light Cycle Oil Upgrading to High Quality Fuels and Petrochemicals: A Review. Ind. Eng. Chem. Res. 2018, 57, 7315–7321. [Google Scholar] [CrossRef]
- Thakkar, V.P.; Abdo, S.F.; Gembicki, V.A.; Mc Gehee, J.F. LCO Upgrading: A Novel Approach for Greater Added Value and Improved Returns; UOP Report AM-05-53; UOP LLC: Des Plaines, IL, USA, 2005. [Google Scholar]
- Kaila, R.K.; Gutierrez, A.; Krause, Q.I. Autothermal reforming of simulated and commercial diesel: The performance of zirconia-supported RhPt catalyst in the presence of sulfur. Appl. Catal. B Environ. 2008, 84, 324–331. [Google Scholar] [CrossRef]
- Alvarez-Galvan, M.C.; Navarro, R.M.; Rosa, F.; Briceno, Y.; Gordillo Alvarez, F.; Fierro, J.L.G. Performance of La,Ce-modified alumina-supported Pt and Ni catalysts for the oxidative reforming of diesel hydrocarbons. Int. J. Hydrogen Energy 2008, 33, 652–663. [Google Scholar] [CrossRef]
- Karatzas, X.; Jansson, K.; González, A.; Dawody, J.; Svensson, A.; Pettersson, L. Zone-coated Rh-based monolithic catalyst for autothermal reforming of diesel. Appl. Catal. B Environ. 2011, 101, 226–238. [Google Scholar] [CrossRef]
- Pena, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2017. [Google Scholar] [CrossRef]
- Mota, N.; Alvarez-Galván, M.C.; Navarro, R.M.; Al-Zahrani, S.M.; Goguet, A.; Daly, H.; Zhang, W.; Trunschke, A.; Schlögl, R.; Fierro, J.L.G. Insights on the role of Ru substitution in the properties of LaCoO3-based oxides as catalysts precursors for the oxidative reforming of diesel fuel. Appl. Catal. B Environ. 2012, 113, 271–282. [Google Scholar] [CrossRef]
- Qia, A.; Wang, S.; Fu, G.; Ni, C.; Wu, D. La–Ce–Ni–O monolithic perovskite catalysts potential for gasoline autothermal reforming system. Appl. Catal. A Gen. 2005, 281, 233–246. [Google Scholar] [CrossRef]
- Voorhoeve, R.J.H.; Johnson, D.W.; Remeika, J.P.; Gallagher, P.K. Perovskite oxides: Materials science in catalysis. Science 1977, 4, 827–833. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal-air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef]
- Mota, N.; Navarro, R.M.; Alvarez-Galvana, M.C.; Al-Zahrani, S.M.; Fierro, J.L.G. Hydrogen production by reforming of diesel fuel over catalysts derived from LaCo1−xRuxO3 perovskites: Effect of the partial substitution of Co by Ru (x = 0.01–0.1). J. Power Sources 2011, 196, 9087–9095. [Google Scholar] [CrossRef]
- Mota, N.; Alvarez-Galvan, M.C.; Villoria, J.A.; Rosa, F.; Fierro, J.L.G.; Navarro, R.M. Reforming of Diesel Fuel for Hydrogen Production over Catalysts Derived from LaCo1−xMxO3 (M = Ru, Fe). Top Catal. 2009, 52, 1995–2000. [Google Scholar] [CrossRef]
- Fino, D.; Russo, N.; Cauda, E.; Saracco, G.; Specchia, V. La–Li–Cr perovskite catalysts for diesel particulate combustion. Catal. Today 2006, 114, 31–39. [Google Scholar] [CrossRef]
- Sunarso, J.; Torriero, A.A.J.; Zhou, W.; Howlett, P.C.; Forsyth, M. Oxygen Reduction Reaction Activity of La-Based Perovskite Oxides in Alkaline Medium: A Thin-Film Rotating Ring-Disk Electrode Study. J. Phys. Chem. C 2012, 116, 5827–5834. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Simböck, J.; Ghiasi, M.; Schönebaum, S.; Simon, U.; de Groot, F.M.; Palkovits, R. Electronic parameters in cobalt-based perovskite-type oxides as descriptors for chemocatalytic reactions. Nat. Comm. 2020, 11, 652. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Lin, L.; Wang, X. A perovskite oxide LaCoO3 cocatalyst for efficient photocatalytic reduction of CO2 with visible light. Chem. Commun. 2018, 54, 2272–2275. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, S.; Xi, S.; Ren, X.; Zhou, Y.; Zhang, G.; Yang, H.; Du, Y.; Xu, Z.J. Tailoring the Co 3d-O 2p Covalency in LaCoO3 by Fe Substitution To Promote Oxygen Evolution Reaction. Chem. Mater. 2017, 29, 10534–10541. [Google Scholar] [CrossRef]
- Song, S.; Zhou, J.; Su, X.; Wang, Y.; Li, J.; Zhang, L.; Xiao, G.; Guan, C.; Liu, R.; Chen, S.; et al. Operando X-ray spectroscopic tracking of self-reconstruction for anchored nanoparticles as high-performance electrocatalysts towards oxygen evolution. Energy Environ. Sci. 2018, 11, 2945–2953. [Google Scholar] [CrossRef]
- Getty, K.; Delgado-Jaime, M.U.; Kennepohl, P. Assignment of pre-edge features in the Ru K-edge X-ray absorption spectra of organometallic ruthenium complexes. Inorg. Chim. Acta 2008, 361, 1059–1065. [Google Scholar] [CrossRef] [Green Version]
- Masuda, Y.; Hosokawa, S.; Inoue, M. Combustion activities of the Ru catalysts supported on hexagonal YbFeO3. J. Ceram. Soc. Jpn. 2011, 119, 850. [Google Scholar] [CrossRef] [Green Version]
- Arcon, I.; Bencan, A.; Kodre, A.; Kosec, M. X-ray absorption spectroscopy analysis of Ru in La2RuO5. X-ray Spectrom. 2007, 36, 301–304. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, L.; Zhou, J.; Jiang, Z.; Shen, B. Chemoselectivity-induced multiple interfaces in MWCNT/Fe3O4@ZnO heterotrimers for whole X-band microwave absorption. Nanoscale 2014, 6, 12298–12302. [Google Scholar] [CrossRef]
- Hong, W.T.; Stoerzinger, K.A.; Moritz, B.; Devereaux, T.P.; Yang, W.; Shao-Horn, Y. Probing LaMO3 Metal and Oxygen Partial Density of States Using X-ray Emission, Absorption, and Photoelectron Spectroscopy. J. Phys. Chem. C 2015, 119, 2063–2072. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Chen, C.; Zhang, Y.; Peng, L.; Ma, S.; Yang, T.; Guo, H.; Zhang, Z.; Su, D.S.; Zhang, J. In Situ oxidation of carbon-encapsulated cobalt nanocapsules creates highly active cobalt oxide catalysts for hydrocarbon combustion. Nat. Comm. 2015, 6, 7181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, C.; Chen, Y.; Engelhard, M.H.; Song, C. Comparative study on the sulfur tolerance and carbon resistance of supported noble metal catalysts in steam reforming of liquid hydrocarbon fuel. ACS Catal. 2012, 2, 1127–1137. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, C.; Li, Y.; Song, C.; Bolin, T.B. Sulfur poisoning mechanism of steam reformingcatalysts: An X-ray absorption near edge structure (XANES) spectroscopic stud. Phys. Chem. Chem. Phys. 2010, 12, 5707–5711. [Google Scholar] [CrossRef] [PubMed]
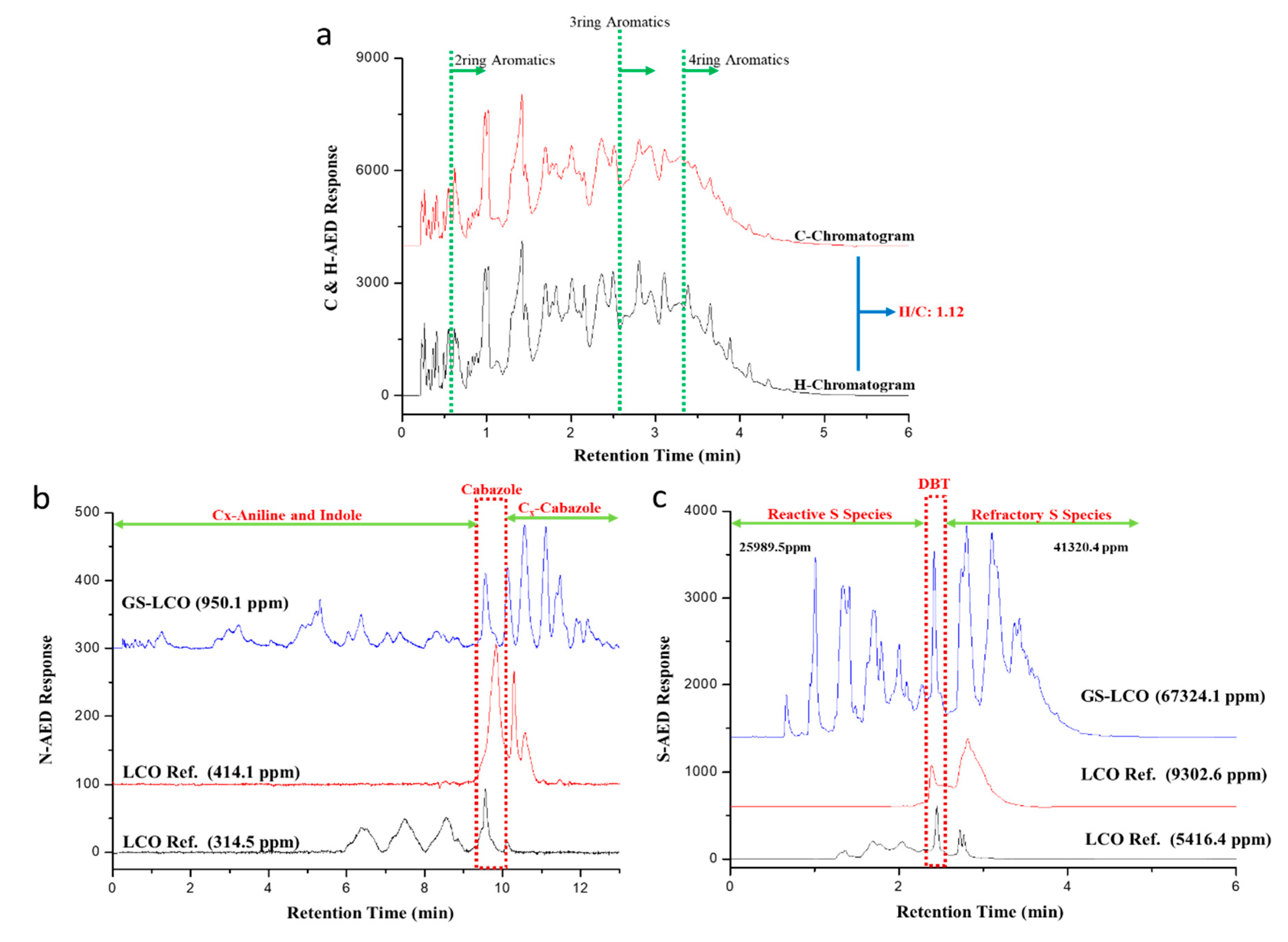
| Species | Ring | SA/USA 1 | Compositions/(%) | % |
|---|---|---|---|---|
| HC | 57.92 | |||
| 0 | - | Eicosane (2.38), Octadecane (2.50), Nonadecane (0.92), etc. | 8.94 | |
| 1 | SA | 1,7-Dimethyl-4-(1-methylethyl)cyclodecane (0.165), etc. | 0.17 | |
| 1 | USA | Benzene (1.48), Octane (0.21), Cyclopropane (0.11), etc. | 2.06 | |
| 2 | SA | Cyclopropane, 1,1’-methylenebis- (0.07), etc. | 0.01 | |
| 2 | USA | Naphthalene (5.39), 1,1’-Biphenyl (1.12), 1H-Indene (0.55), etc. | 10.65 | |
| 3 | SA | Tricyclo-octane, 8-methylene (0.01), etc. | 0.02 | |
| 3 | USA | Phenanthrene (17.57), Anthracene (6.87), Fluorene (1.47), etc. | 31.40 | |
| 4 | USA | Pyrene (2.06), Benz(a)anthracene (1.24), etc. | 4.68 | |
| S | 30.75 | |||
| 0 | - | 1-Methyl-prop-2-enyl Dithiopropanoate (0.07,  ), etc. ), etc. | 0.01 | |
| 1 | - | - | 0.00 | |
| 2 | - | 1-Propene-2-thiol (10.08,  ), Benzo[b]thiophene (0.119, ), Benzo[b]thiophene (0.119,  ), etc. ), etc. | 10.78 | |
| 3 | - | Dibenzothiophene (8.94, 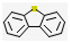 ), 2,7-Dimethyldibenzothiophene (9.63, ), 2,7-Dimethyldibenzothiophene (9.63,  ), etc. ), etc. | 19.90 | |
| 4 | - | Phenaleno [1,9-bc]thiophene (0.04,  ), etc. ), etc. | 0.07 | |
| N | 1.35 | |||
| 0 | - | Butanenitrile (0.002), etc. | 0.003 | |
| 1 | - | 3-Methyl-1,2-diazirine (0.017,  ), etc. ), etc. | 0.04 | |
| 2 | - | Pyridine (0.02, 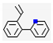 ), etc. ), etc. | 0.07 | |
| 3 | - | Carbazole (0.637, 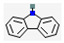 ), 3,3-Diphenyl-5-methyl-3H-pyrazole (0.281, ), 3,3-Diphenyl-5-methyl-3H-pyrazole (0.281, 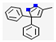 ), etc. ), etc. | 1.23 | |
| 4 | - | Benzo(a)phenazine (0.006, 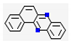 ), etc. ), etc. | 0.01 | |
| Total | 90.02 |
| Catalysts | Elemental Compositions 1 | BET Analysis 2 | ||||
|---|---|---|---|---|---|---|
| La | Cr vs. Co | Ru | SBET (m2/g) | Total Pore Volume (p/p0 = 0.990) (mL/g) | Average Pore Diameter (nm) | |
| LaCo0.85Ru0.15O3 | 1.00 | 0.86 | 0.14 | 15.5 | 0.047 | 12.6 |
| LaCr0.85Ru0.15O3 | 1.00 | 0.84 | 0.16 | 14.3 | 0.051 | 15.9 |
| Peak 1 | d-Value | Cell Parameters | Particles Size 2 | |||
|---|---|---|---|---|---|---|
| Pp | d (Å) | a (Å) | b (Å) | c (Å) | Ps | |
| LaCoO3 | 33.15 | 2.783 | 5.468 | 5.453 | 13.17 | 38.2 |
| LaCo0.85Ru0.15O3 | 32.72 | 2.794 | 5.514 | 5.511 | 13.73 | 21.3 |
| LaCrO3 | 32.63 | 2.741 | 5.493 | 5.477 | 7.745 | 27.9 |
| LaCr0.85Ru0.15O3 | 31.82 | 2.761 | 5.515 | 5.512 | 7.793 | 18.3 |
| Catalysts | Weight Loss 1 (wt %) | Composition 2 (%) | |||
|---|---|---|---|---|---|
| Carbon | Hydrogen | Nitrogen | Sulfur | ||
| Pt-GDC | 31.87 | 94.87 | 0.51 | 0.43 | 4.19 |
| LaCo0.85Ru0.15O3 | 2.56 | 95.79 | 0.82 | 0.26 | 3.01 |
| LaCr0.85Ru0.15O3 | 1.12 | 96.74 | 0.82 | 0.15 | 2.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, Y.; Jung, H.-K.; Park, C.-I.; Shul, Y.; Park, J.-i. Light Cycle Oil Source for Hydrogen Production through Autothermal Reforming using Ruthenium doped Perovskite Catalysts. Catalysts 2020, 10, 1039. https://doi.org/10.3390/catal10091039
Jeon Y, Jung H-K, Park C-I, Shul Y, Park J-i. Light Cycle Oil Source for Hydrogen Production through Autothermal Reforming using Ruthenium doped Perovskite Catalysts. Catalysts. 2020; 10(9):1039. https://doi.org/10.3390/catal10091039
Chicago/Turabian StyleJeon, Yukwon, Hoi-Kyoeng Jung, Cho-I Park, Yonggun Shul, and Joo-il Park. 2020. "Light Cycle Oil Source for Hydrogen Production through Autothermal Reforming using Ruthenium doped Perovskite Catalysts" Catalysts 10, no. 9: 1039. https://doi.org/10.3390/catal10091039