Synthesis of 14-Substituted-14H-Dibenzo[a,j]Xanthene Derivatives in Presence of Effective Synergetic Catalytic System Bleaching Earth Clay and PEG-600
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Reaction Conditions and Scope of the Reaction
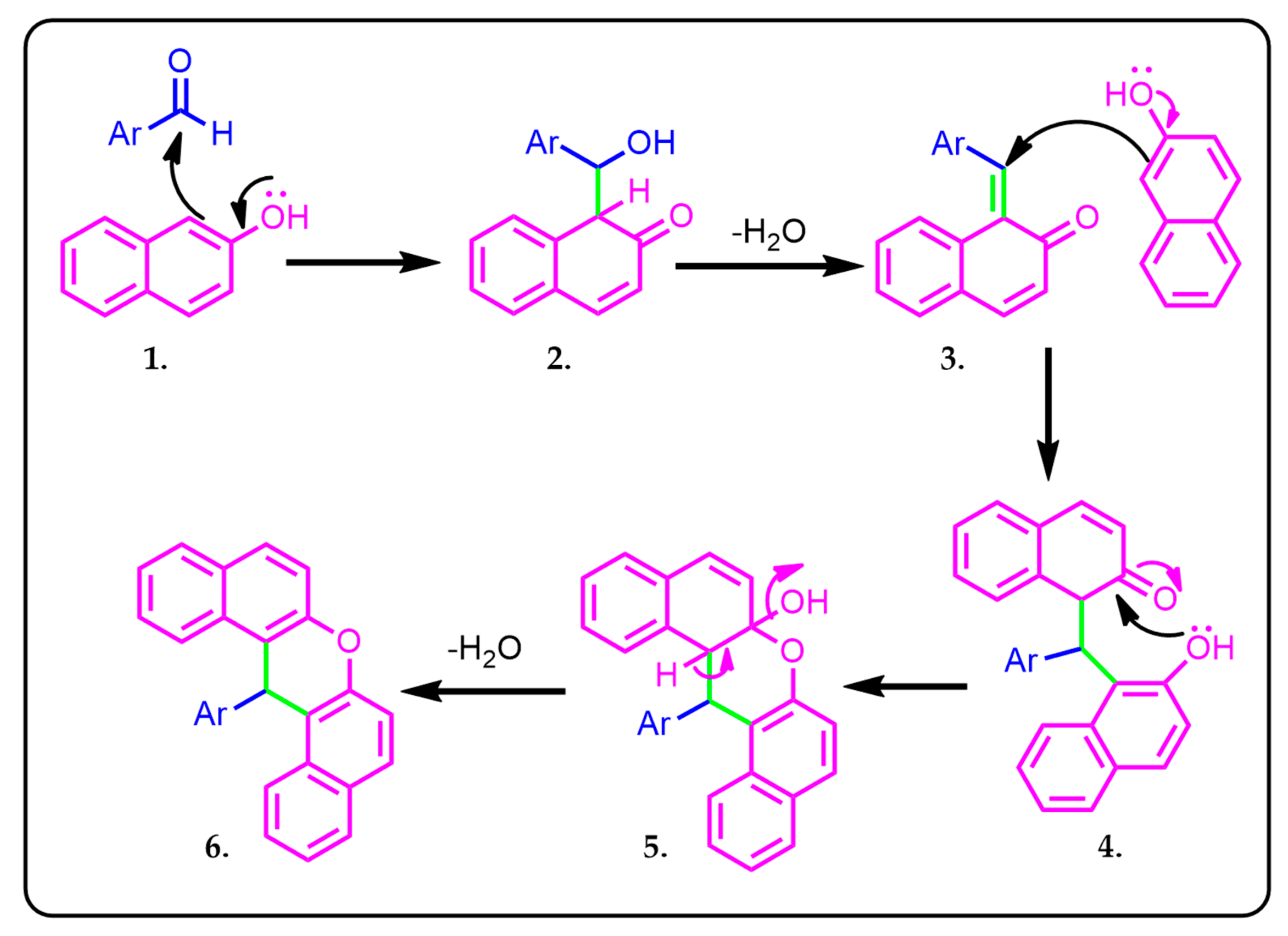
2.2. Reaction Mechanism
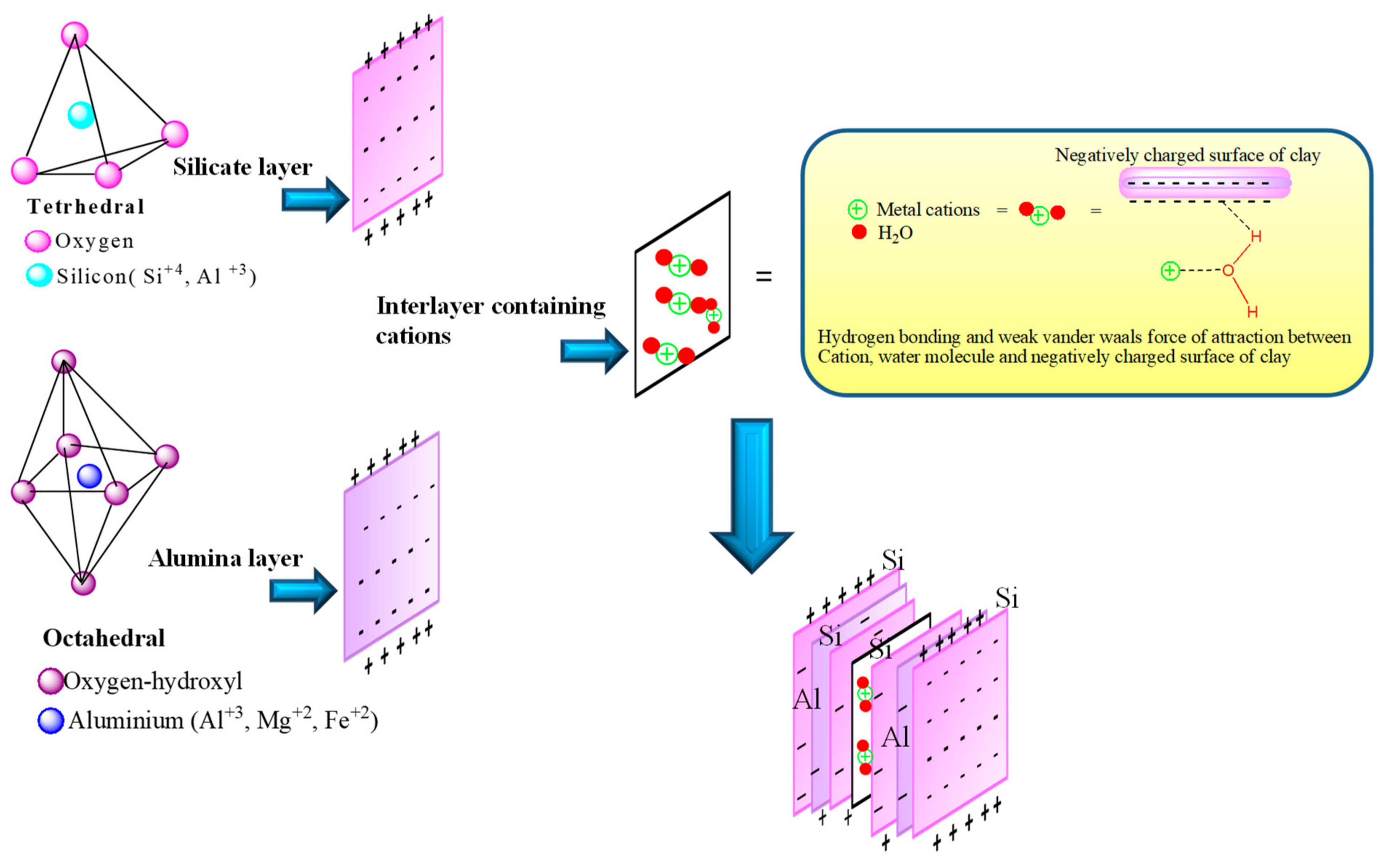
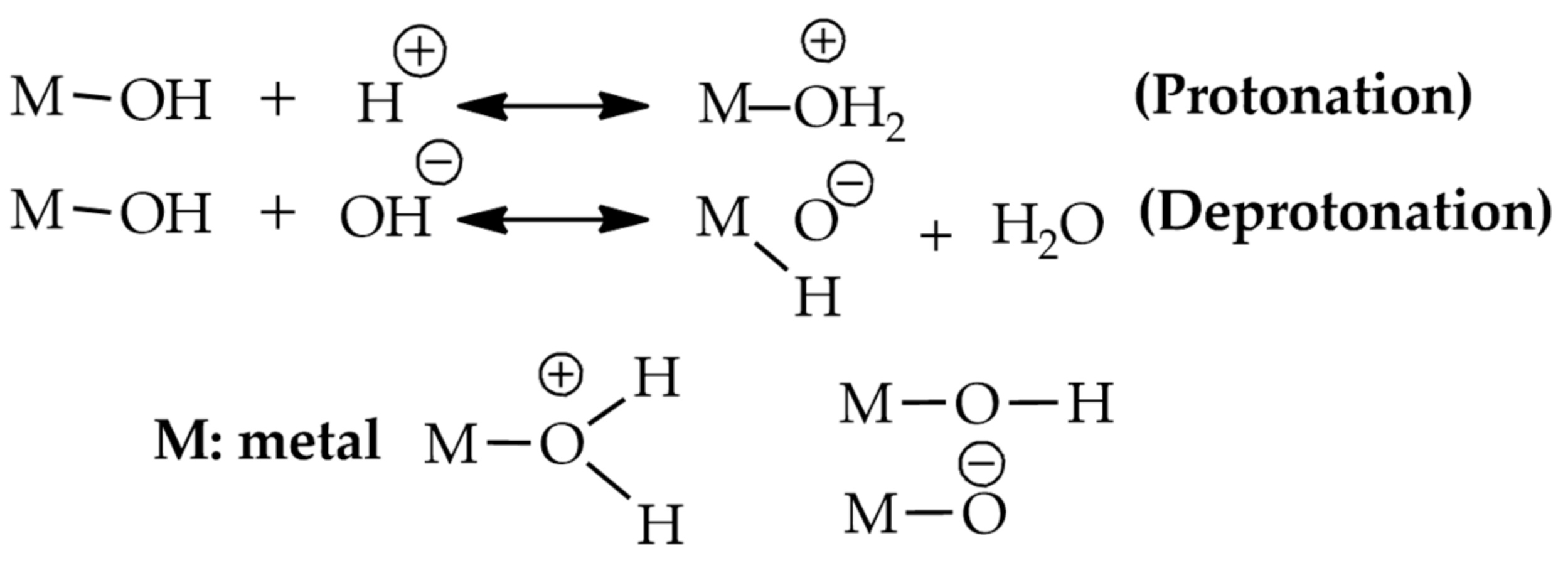
2.2.1. Structure of Clay
2.2.2. Polyethylene Glycol
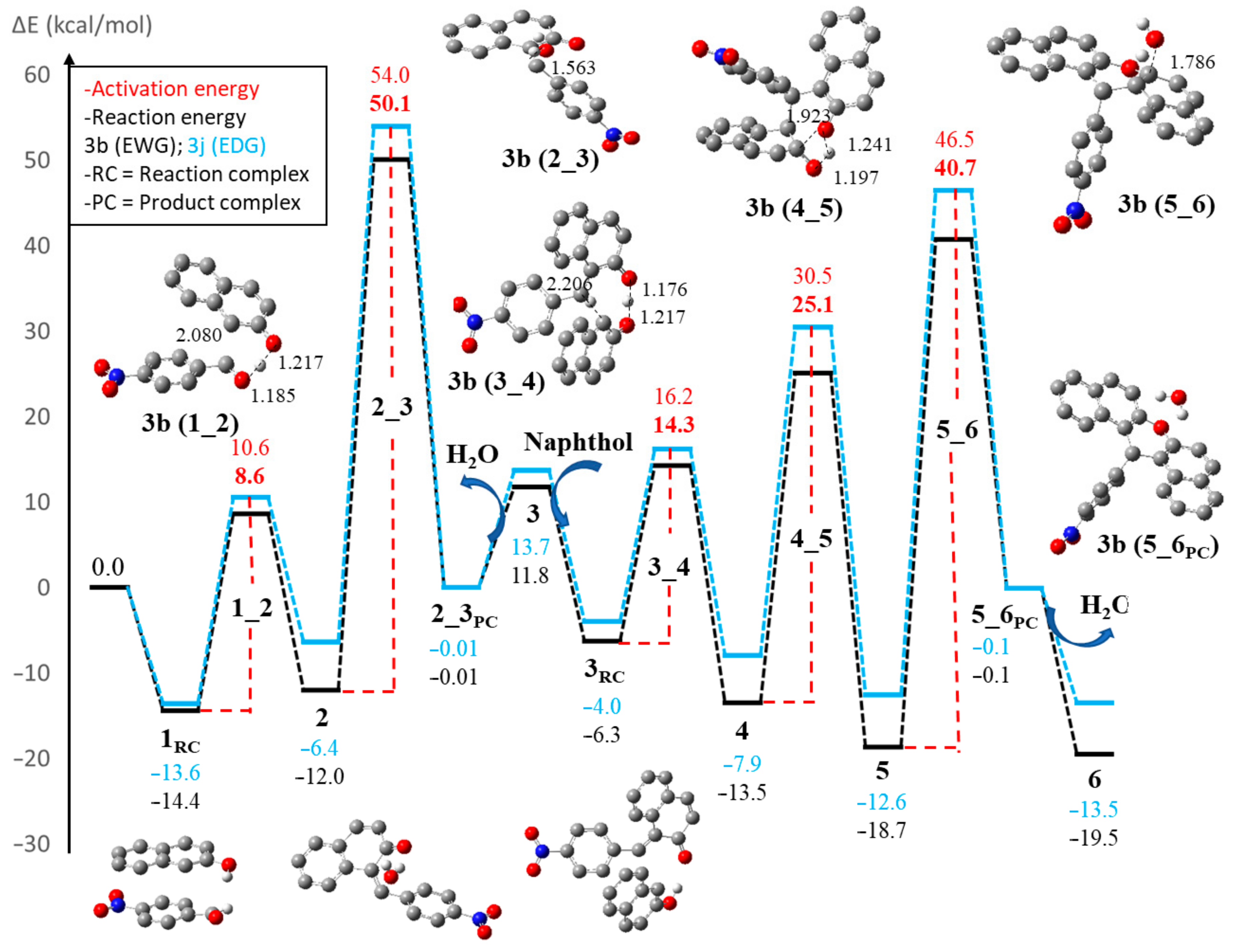
2.2.3. DFT Studies on the Mechanistic Details of the Regioselective Reactions
3. Experimental
3.1. Typical Procedure for the Synthesis of 14-(4-Chlorophenyl)-14H-Dibenzo[a,j]Xanthene (3a)
3.2. Spectral Data of Representative Compounds
3.2.1. 14-(4-Chlorophenyl)-14H-Dibenzo[a,j]Xanthene (3a)
3.2.2. 14-(4-Nitrophenyl)-14H-Dibenzo[a,j]Xanthene (3b)
3.2.3. 14-(3-Nitrophenyl)-14H-Dibenzo[a,j]Xanthene(3c)
3.2.4. 14-(3-Flurophenyl)-14H-Dibenzo[a,j]Xanthene (3d)
3.2.5. 14-(2,4-Dichlorophenyl)-14H-Dibenzo[a,j]Xanthene (3e)
3.2.6. 14-Phenyl-14H-Dibenzo[a,j]Xanthene (3f)
3.2.7. 14-(4-Bromophenyl)-14H-Dibenzo[a,j]Xanthene (3g)
3.2.8. 14-(4-Methoxyphenyl)-14H-Dibenzo[a,j]Xanthene (3h)
3.2.9. 14-(4-Methylphenyl)-14H-Dibenzo[a,j]Xanthene (3i)
3.2.10. 14-(4-Dimethylamino-phenyl)-14H-Dibenzo[a,j]Xanthene (3j)
3.2.11. 14-(4-Methylsulfanylphenyl)-14H-Dibenzo[a,j]Xanthene (3k)
3.2.12. 14-(4-t-Butylphenyl)-14H-Dibenzo[a,j]Xanthene (3l)
3.2.13. 14-Ethyl-14H-Dibenzo[a,j]Xanthene (3m)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poupelin, J.; Saint-Ruf, G.; Foussard-blanpin, O.; Narcisse, G.; Uchida-Ernouf, G.; Lacroix, R. Synthesis and anti-inflammatory properties of bis(2-hydroxy,1-naphthyl) methane derivatives. Chem. Inf.-Dienst 1978, 9, 50. [Google Scholar]
- Ion, R.M.; Planner, A.; Wiktorowicz, K.; Frackowiak, D. The incorporation of various porphyrins into blood cells measured via flow cytometry, absorption and emission spectroscopy. Acta Biochim. Pol. 1998, 45, 833–845. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; King, T.A.; Ko, D.-K.; Cha, B.H.; Lee, J. Performance and photostability of xanthene and pyrromethene laser dyes in sol-gel phases. J. Phys. D: Appl. Phys. 2002, 35, 1473–1476. [Google Scholar] [CrossRef]
- Callan, J.F.; de Silva, A.P.; Magri, D.C. Luminescent sensors and switches in the early 21st century. Tetrahedron 2005, 61, 8551–8588. [Google Scholar] [CrossRef]
- Knight, D.W.; Little, P.B. The first efficient method for the intramolecular trapping of benzynes by phenols: A new approach to xanthenes. J. Chem. Soc., Perkin Trans. 1 2001, 1, 1771–1777. [Google Scholar] [CrossRef]
- Jha, A.; Beal, J. Convenient synthesis of 12H-benzo[a]xanthenes from 2-tetralone. Tetrahedron Lett. 2004, 45, 8999–9001. [Google Scholar] [CrossRef]
- Bekaert, A.; Andrieux, J.; Plat, M. New total synthesis of bikaverin. Tetrahedron Lett. 1992, 33, 2805–2806. [Google Scholar] [CrossRef]
- Papini, P.; Cimmarusti, R. The action of formamide and formanilide on naphthols and on barbituric acid. Gazz. Chim. Ital. 1947, 77, 142. [Google Scholar]
- Sen, R.N.; Sarkar, N.N. The condensation of primary alcohols with resorcinol and other hydroxyl aromatic compounds. J. Am. Chem. Soc. 1925, 47, 1079–1091. [Google Scholar] [CrossRef]
- Koki, O.; Taketoshi, K. An Improved Synthesis of Dibenzoxanthene. Bull. Chem. Soc. Jpn. 1976, 49, 1167–1168. [Google Scholar]
- Azizi, N.; Abbasi, F.; Abdoli-Senejani, M. Natural Acidic Ionic Liquid Immobilized on Magnetic Silica: Preparation and Catalytic Performance in Chemoselective Synthesis of Dicoumarols and Substituted Xanthene Derivatives. ChemSelect 2018, 3, 3797–3802. [Google Scholar] [CrossRef]
- Azizi, N.; Shirdel, F. Task specific dicationic acidic ionic liquids catalyzed efficient and rapid synthesis of benzoxanthenones derivatives. J. Mol. Liq. 2016, 222, 783–787. [Google Scholar] [CrossRef]
- Xing, L.H.Y.; Xing, X.; Hui, Y. MCM-41@Schiff Base-Mn(OAc)2 Catalyzed Synthesis of Xanthene Derivatives in Water. Chin. J. Org. Chem. 2018, 38, 912–918. [Google Scholar] [CrossRef] [Green Version]
- Ko, S.; Yao, C.-F. Heterogeneous catalyst: Amberlyst-15 catalyzes the synthesis of 14-substituted-14H-dibenzo[a,j]xanthenes under solvent-free conditions. Tetrahedron Lett. 2006, 47, 8827–8829. [Google Scholar] [CrossRef]
- Saini, A.; Kumar, S.; Sandhu, J.S. A New LiBr-Catalyzed, Facile and Efficient Method for the Synthesis of 14-Alkyl or Aryl-14H-dibenzo[a,j]xanthenes and Tetrahydrobenzo[b]pyrans under Solvent-Free Conventional and Microwave Heating. Synlett 2006, 2006, 1928–1932. [Google Scholar] [CrossRef]
- Rajitha, B.; Sunil Kumar, B.; Thirupathi Reddy, Y.; Narsimha Reddy, P.; Sreenivasulu, N. Sulfamic acid: A novel and efficient catalyst for the synthesis of aryl-14H-dibenzo[a,j]xanthenes under conventional heating and microwave irradiation. Tetrahedron Lett. 2005, 46, 8691–8693. [Google Scholar] [CrossRef]
- Khosropour, A.R.; Khodaei, M.M.; Moghannian, H. A Facile, Simple and Convenient Method for the Synthesis of 14-Alkyl or Aryl-14-H-dibenzo[a,j]xanthenes Catalyzed by pTSA in Solution and Solvent-Free Conditions. ChemInform 2005, 36, 955–958. [Google Scholar] [CrossRef]
- Pasha, M.A.; Jayashankara, V.P. Molecular iodine catalyzed synthesis of aryl-14H-dibenzo[a,j]xanthenes under solvent-free condition. Bioorg. Med. Chem. Lett. 2007, 17, 621–623. [Google Scholar] [CrossRef] [Green Version]
- Eshghi, H.; Bakavoli, M.; Moradi, H. Fe(HSO4)3: An efficient, heterogeneous and reusable catalyst for the synthesis of 14-aryl- or alkyl-14H-dibenzo[a,j]xanthenes. Chin. Chem. Lett. 2008, 19, 1423–1426. [Google Scholar] [CrossRef]
- Mirjalili, B.B.F.; Bamoniri, A.; Akbari, A. BF3·SiO2: An efficient alternative for the synthesis of 14-aryl or alkyl-14H-dibenzo[a,j]xanthenes. Tetrahedron Lett. 2008, 49, 6454–6456. [Google Scholar] [CrossRef]
- Kokare, N.D.; Sangshetti, J.N.; Shinde, D.B. Oxalic acid as a catalyst for efficient synthesis of bis-(indolyl)methanes, and 14-aryl-14H-dibenzo[a,j]xanthenes in water. Chin. Chem. Lett. 2008, 19, 1186–1189. [Google Scholar] [CrossRef]
- Madhav, J.V.; Kuarm, B.S.; Rajitha, B. Dipyridine cobalt chloride: A novel and efficient catalyst for the synthesis of 14-aryl 14H-dibenzo[a.j]xanthenes under solvent-free conditions. ARKIVOC 2008, 2008, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Bigdeli, M.A.; Heravi, M.M.; Hossein Mahdavinia, G. Wet cyanuric chloride catalyzed simple and efficient synthesis of 14-aryl or alkyl-14-H-dibenzo[a,j]xanthenes. Catal. Commun. 2007, 8, 1595–1598. [Google Scholar] [CrossRef]
- Bigdeli, M.A.; Heravi, M.M.; Mahdavinia, G.H. Silica supported perchloric acid (HClO4-SiO2): A mild, reusable and highly efficient heterogeneous catalyst for the synthesis of 14-aryl or alkyl-14-H-dibenzo[a,j]xanthenes. J. Mol. Catal. A Chem. 2007, 275, 25–29. [Google Scholar] [CrossRef]
- Shaterian, H.R.; Ghashang, M.; Mir, N. Aluminium hydrogensulfate as an efficient and heterogeneous catalyst for preparation of aryl 14H-dibenzo[a,j]xanthene derivatives under thermal and solvent-free conditions. ARKIVOC 2007, 2007, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.S.; Kumar, B.S.; Rajitha, B.; Reddy, P.N.; Sreenivasulu, N.; Reddy, Y.T. A novel one pot synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes catalyzed by SelectfluorTM under solvent free conditions. ARKIVOC 2006, 2006, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, K.-z.; Zahra, K.; Hadi, A.R. Structural investigation and preparation of 14-alkyl-14H-dibenzo [a,j] xanthenes revised. J. Korean Chem. Soc. 2002, 46, 541–544. [Google Scholar]
- Sarma, R.J.; Baruah, J.B. One step synthesis of dibenzoxanthenes. Dyes Pigm. 2005, 64, 91–92. [Google Scholar] [CrossRef]
- Shaterian, H.R.; Ghashang, M.; Hassankhani, A. One-pot synthesis of aryl 14H-dibenzo[a,j]xanthene leuco-dye derivatives. Dyes Pigm. 2008, 76, 564–568. [Google Scholar] [CrossRef]
- Balogh, M.; Laszlo, P. Organic Chemistry Using Clays, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1993; p. 184. [Google Scholar]
- Acharya, A.; Kamble, R.; Patil, S.; Hese, S.; Yemul, O.; Patil, S.; Halale, S.; Dawane, B. “Green synthesis” of benzothiazepine library of indeno analogues and their in vitro antimicrobial activity. Chem. Pap. 2014, 68, 719–724. [Google Scholar] [CrossRef]
- Kamble, R.D.; Jawadwar, G.V.; Patil, S.D.; Shrikant, V.H.; Hese, V.; Acharya, A.P.; Dawane, B.; Pekamwar, S. Bleaching earth clay (pH 12.5): A novel and reusabl e catalyst for rapid synthesis of 7-Hydroxy 4-Styryl coumarin deri vatives and their antihelmintic activity. Org. Commun. 2013, 6, 95–101. [Google Scholar]
- Kamble, R.D.; Dawane, B.S.; Yemul, O.S.; Kale, A.B.; Patil, S.D. Bleaching earth clay (pH 12.5): A green catalyst for rapid synthesis of pyranopyrazole derivatives via a tandem three-component reaction. Res. Chem. Intermed. 2013, 39, 3859–3866. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Keim, W. Ionic Liquids—New “Solutions” for Transition Metal Catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Sheldon, R. Catalytic reactions in ionic liquids. Chem. Commun. 2001, 2001, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Namboodiri, V.V.; Varma, R.S. Microwave-accelerated Suzuki cross-coupling reaction in polyethylene glycol (PEG). Green Chem. 2001, 3, 146–148. [Google Scholar] [CrossRef]
- Parfitt, R.L.; Greenland, D.J. The Adsorption of Poly(Ethylene Glycols) on Clay Minerals. Clay Miner. 1970, 8, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Theng, B.K.G. Clay-Polymer Interactions: Summary and Perspectives. Clays Clay Miner. 1982, 30, 1–10. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision A. 03; Gaussian: Wallingford, CT, USA, 2016. [Google Scholar]
- Hussain, M.A.; Mahadevi, A.S.; Sastry, G.N. Estimating the binding ability of onium ions with CO2 and π systems: A computational investigation. Phy. Chem. Chem. Phy. 2015, 17, 1763–1775. [Google Scholar] [CrossRef] [PubMed]
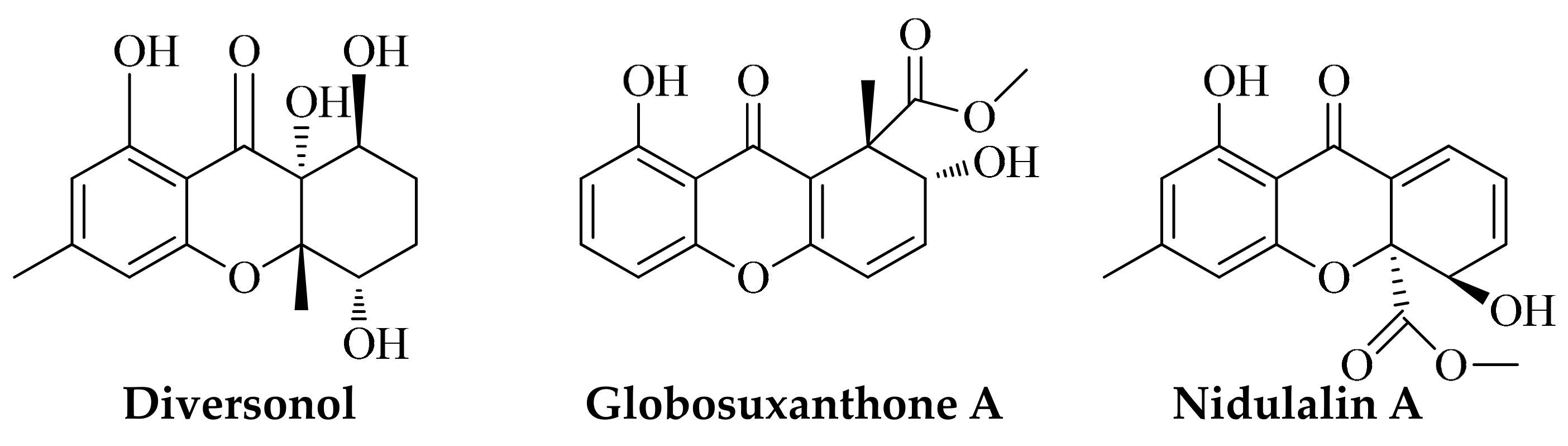




| Entry | Solvent/Catalyst | Temp (°C) | Time (min) | Yield (%) b |
|---|---|---|---|---|
| 1 | Neat | 90 | 300 | No reaction |
| 2 | Et3N | RT/90 | 300 | No reaction/10 |
| 3 | Piperidine | RT/90 | 300 | No reaction/15 |
| 4 | Morpholine | RT/90 | 300 | 15 |
| 5 | Bleaching earth clay | RT/90 | 100 | 40 |
| 6 | PEG-200 | 90 | 100 | 25 |
| 7 | PEG-400 | 90 | 100 | 30 |
| 8 | PEG-600 | 90 | 100 | 40 |
| 9 | PEG-200/bleaching earth clay | 90 | 100 | 70 |
| 10 | PEG-400/bleaching earth clay | 90 | 60 | 75 |
| 11 | PEG-600/bleaching earth clay | RT | 45 | 70 |
| 12 | PEG-600/bleaching earth clay | 50/70/90/100 | 45 | 70/80/95/95 |
| 13 | Water/bleaching earth clay | 90 | 45 | 20 |
| 14 | DCM/bleaching earth clay | 90 | 45 | 30 |
| 15 | Me0H/bleaching earth clay | 90 | 45 | 40 |
| 16 | EtOH/bleaching earth clay | 90 | 45 | 40 |
| 17 | Acetonitrile/bleaching earth clay | 90 | 45 | 40 |
| Entry | Catalyst (wt.%) | Temp (°C) | Time (min) | Yield (%) |
|---|---|---|---|---|
| 1 | 5 | RT | 100 | 40 |
| 2 | 5 | 80 | 100 | 60 |
| 3 | 5 | 90 | 80 | 70 |
| 4 | 7 | 90 | 80 | 70 |
| 5 | 10 | 80 | 60 | 90 |
| 6 | 10 | 90 | 45 | 95 |
| 7 | 12 | 90 | 45 | 95 |
| Entry | Aldehyde | Product | Time (min) | Yield (%) | |
|---|---|---|---|---|---|
| 1 | 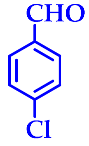 |  | 3a | 45 | 95 |
| 2 | 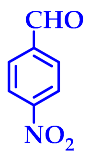 | 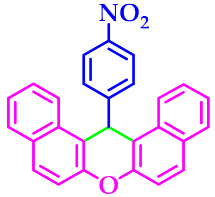 | 3b | 50 | 96 |
| 3 | 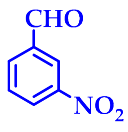 | 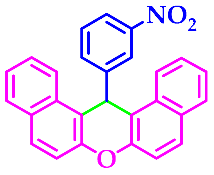 | 3c | 50 | 94 |
| 4 | 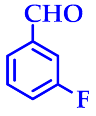 | 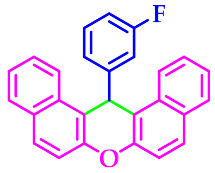 | 3d | 50 | 95 |
| 5 | 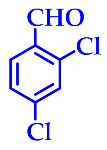 | 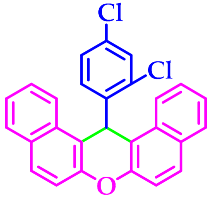 | 3e | 40 | 96 |
| 6 | 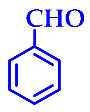 | 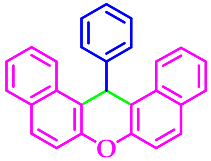 | 3f | 43 | 95 |
| 7 |  | 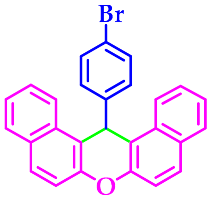 | 3g | 50 | 96 |
| 8 | 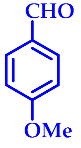 | 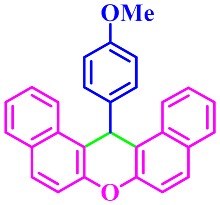 | 3h | 65 | 90 |
| 9 | 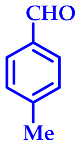 | 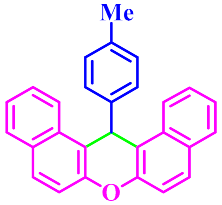 | 3i | 60 | 91 |
| 10 |  | 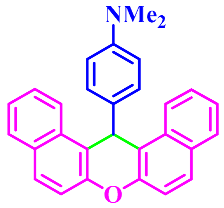 | 3j | 70 | 89 |
| 11 |  | 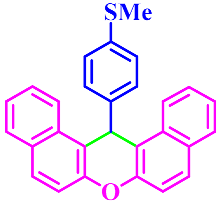 | 3k | 60 | 91 |
| 12 |  |  | 3l | 70 | 89 |
| 13 |  | 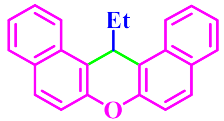 | 3m | 80 | 87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atkore, S.T.; Bondle, G.M.; Raithak, P.V.; Kamble, V.T.; Varala, R.; Kuniyil, M.; Hatshan, M.R.; Shaik, B.; Adil, S.F.; Hussain, M.A. Synthesis of 14-Substituted-14H-Dibenzo[a,j]Xanthene Derivatives in Presence of Effective Synergetic Catalytic System Bleaching Earth Clay and PEG-600. Catalysts 2021, 11, 1294. https://doi.org/10.3390/catal11111294
Atkore ST, Bondle GM, Raithak PV, Kamble VT, Varala R, Kuniyil M, Hatshan MR, Shaik B, Adil SF, Hussain MA. Synthesis of 14-Substituted-14H-Dibenzo[a,j]Xanthene Derivatives in Presence of Effective Synergetic Catalytic System Bleaching Earth Clay and PEG-600. Catalysts. 2021; 11(11):1294. https://doi.org/10.3390/catal11111294
Chicago/Turabian StyleAtkore, Sandeep T., Giribala M. Bondle, Pranita V. Raithak, Vinod T. Kamble, Ravi Varala, Mufsir Kuniyil, Mohammad Rafe Hatshan, Baji Shaik, Syed Farooq Adil, and Mohammed Althaf Hussain. 2021. "Synthesis of 14-Substituted-14H-Dibenzo[a,j]Xanthene Derivatives in Presence of Effective Synergetic Catalytic System Bleaching Earth Clay and PEG-600" Catalysts 11, no. 11: 1294. https://doi.org/10.3390/catal11111294









