Effectively Synthesizing SO4/TiO2 Catalyst and Its Performance for Converting Ethanol into Diethyl Ether (DEE)
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Preparation of Catalysts
2.3. Characterization of Catalysts
- W0: weight of empty porcelains (g)
- W1: weight of crucible porcelain + sample before adsorption (g)
- W2: weight of crucible porcelain + sample after adsorption (g)
- MW NH3: molecular weight of NH3 (mol g−1)
2.4. Application of Catalyst
- methanol: mass of ethanol (g)
- Ei: peak area of DEE in GC chromatogram
- Etotal: total peak area in GC chromatogram
3. Results
3.1. Characterization of Catalyst
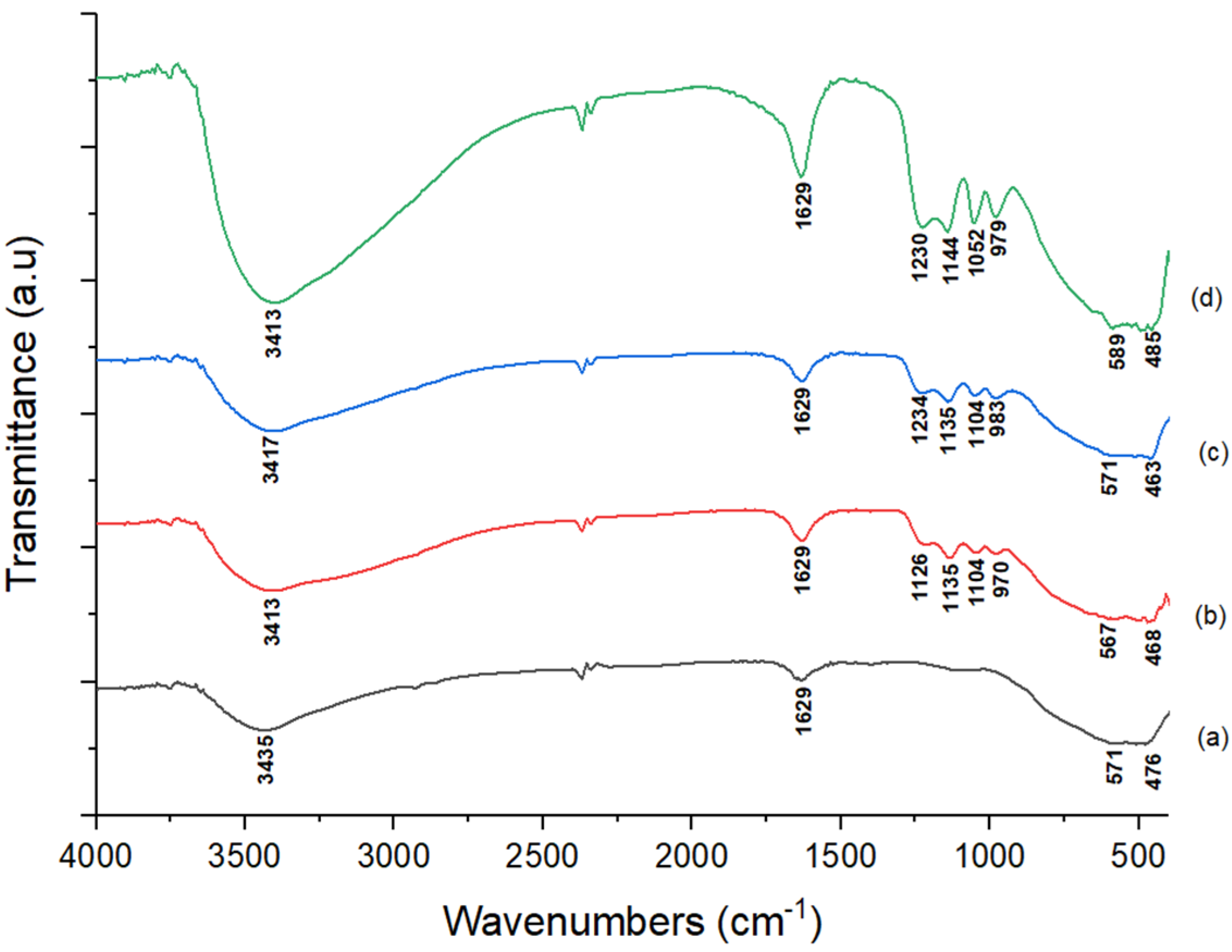
3.1.1. FTIR and Acidity Test
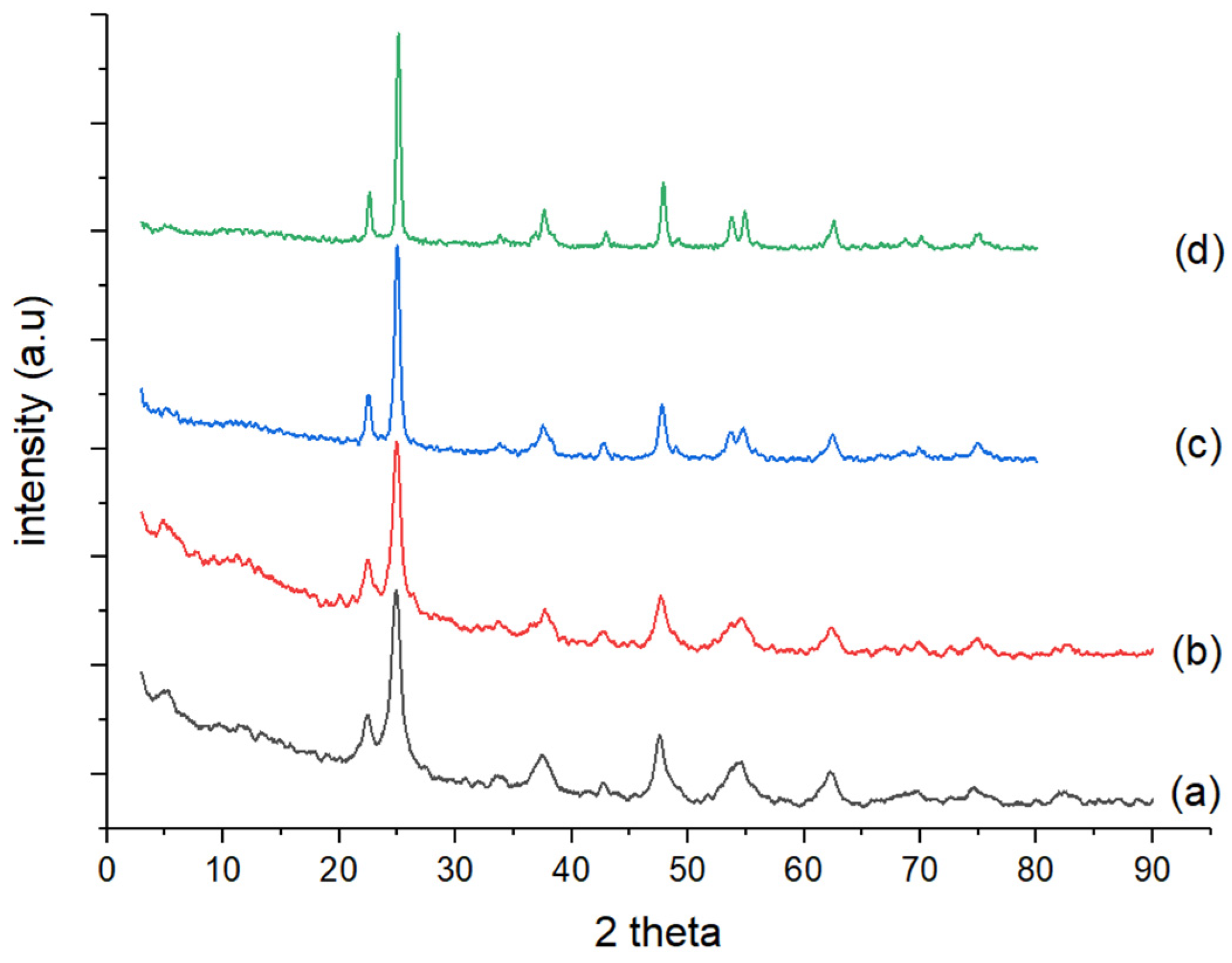
3.1.2. Characterization Using XRD
3.1.3. Characterization Using SEM-EDX
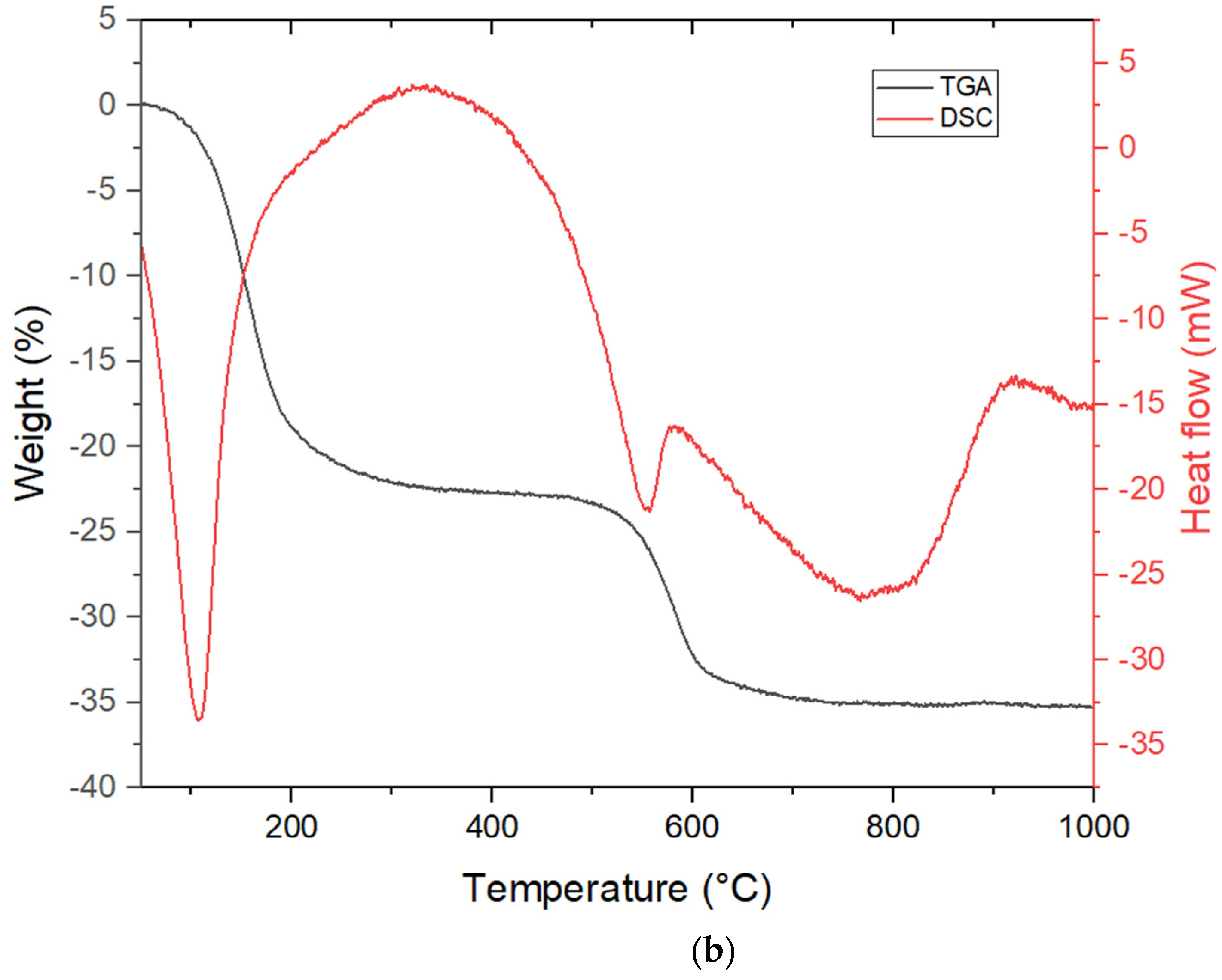
3.1.4. Characterization Using TGA/DSC
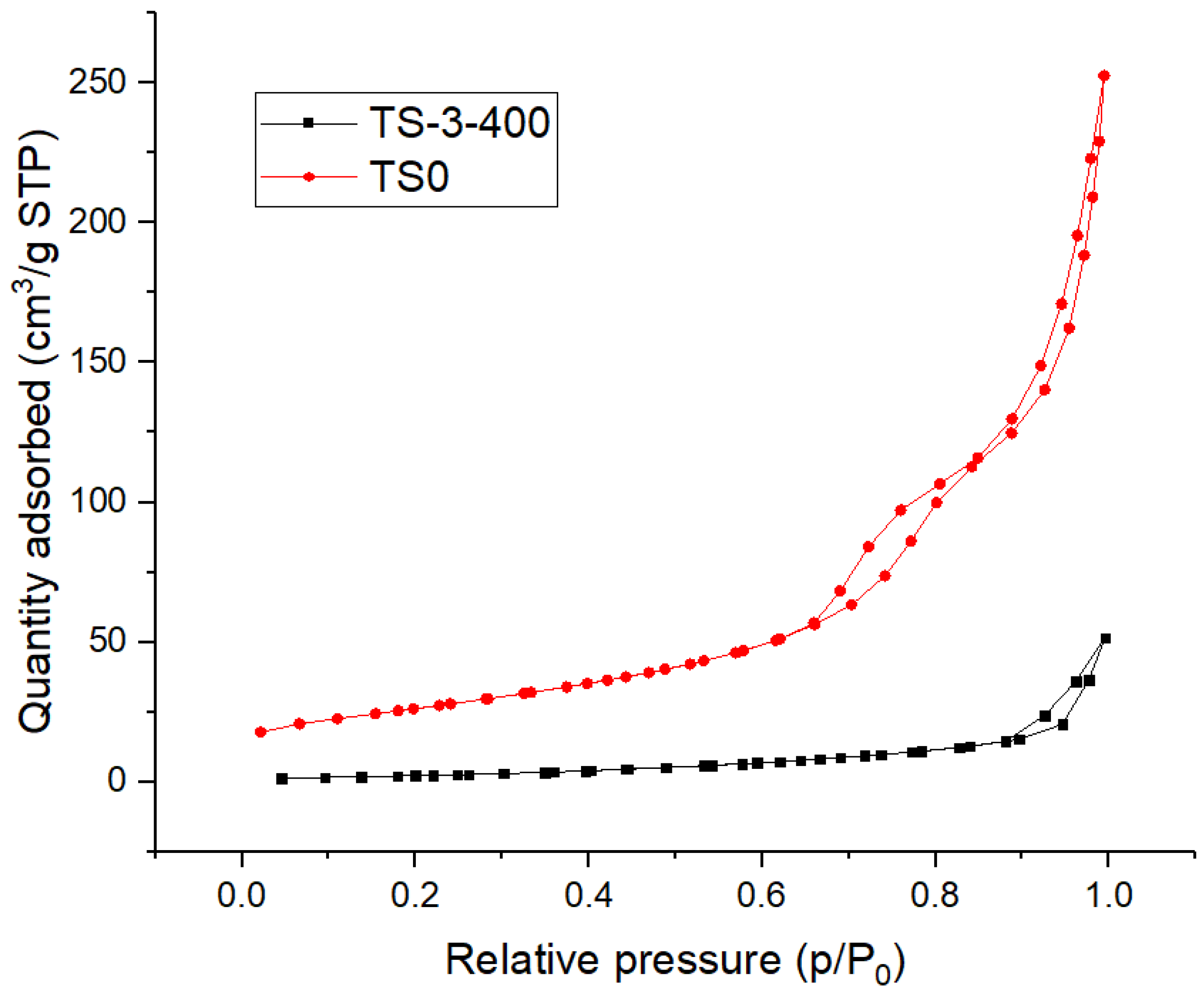
3.1.5. Characterization Using SAA
3.2. Application of Catalyst
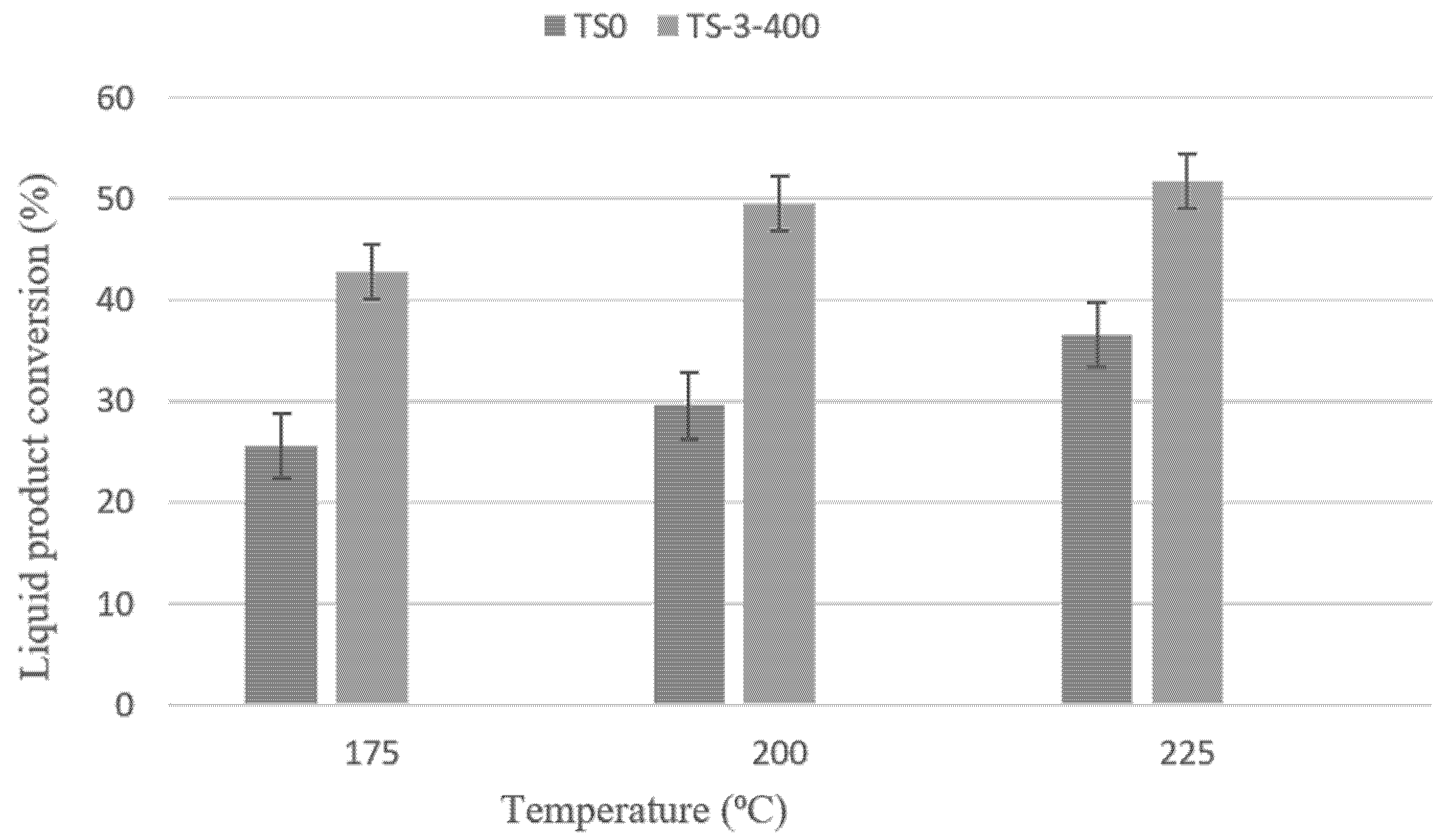
3.2.1. Test of Catalysts Activity toward Dehydrated Liquid Products
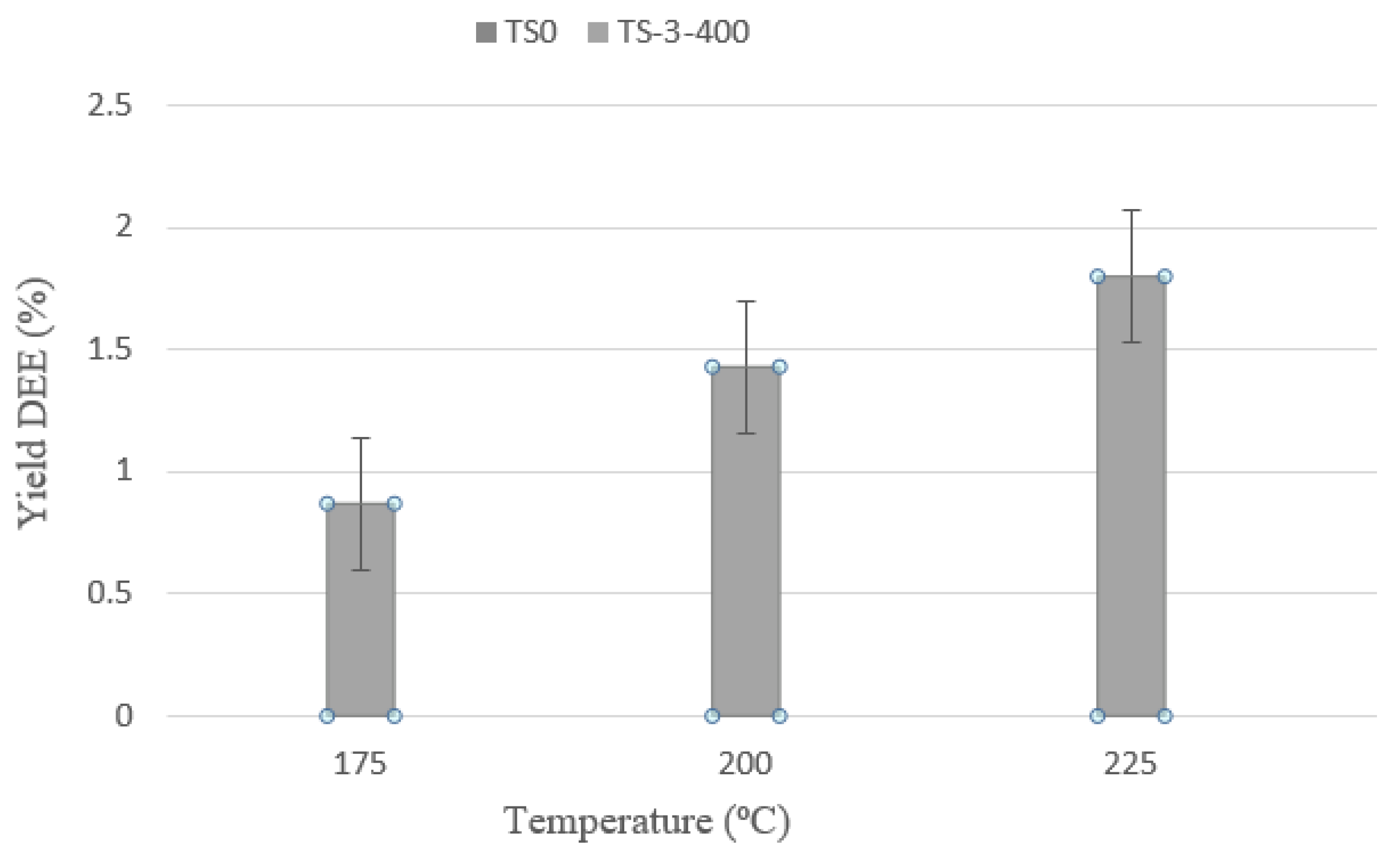
3.2.2. Selectivity Test for Dehydrated Liquid Products
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choopun, W.; Jitkarnka, S. Catalytic activity and stability of HZSM-5 zeolite and hierarchical uniform mesoporous MSU-S ZSM-5 material during bio-ethanol dehydration. J. Clean 2016, 135, 368–378. [Google Scholar] [CrossRef]
- Sousa, Z.; Veloso, C.; Henriques, C.; da Silva, V. Ethanol conversion into olefins andaromatics over HZSM-5 zeolite: Influence of reaction conditions and surface reaction studies. J. Mol. Catal. A 2016, 422, 266–274. [Google Scholar] [CrossRef]
- Varisli, D.; Dogu, T.; Dogu, G. Ethylene and diethyl-ether production by dehydration reaction of ethanol over different heteropolyacid catalysts. Chem. Eng. Sci. 2007, 62, 5349–5352. [Google Scholar] [CrossRef]
- Gucbilmez, Y.; Dogu, T.; Balci, S. Ethylene and acetalehyde production by selective oxidation of ethanol using mesoporous V-MCM-41 catalysts. Ind. Eng. Chem. Res. 2006, 45, 3496–3502. [Google Scholar] [CrossRef]
- Pereira, C.J. New avenues in ethylene synthesis. Science 1999, 285, 670–671. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Mahlia, T.M.I.; Ong, H.C.; Riayatsyah, T.M.I.; Kusumo, F.; Ibrahim, H.; Dharma, S.; Gumilang, D. A comparative study of biodiesel production methods for Reutealistrisperma. Energy Source Part A 2017, 39, 20. [Google Scholar] [CrossRef]
- Bailey, B.K. Perfomance of etanol as a transportations fuel. In Handbook on Bioetanol: Production Utilization; Wayman, C.E., Ed.; Taylor and Francis: Washington, DC, USA, 1996; pp. 37–60. [Google Scholar]
- El-Adawy, M.; Ibrahim, A.; El-Kassaby, M.M. An experimental evaluation of using waste cooking oil biodiesel in a diesel engine. Energy Technol. 2013, 1, 726–734. [Google Scholar] [CrossRef]
- Arcoumanis, C.; Bae, C.; Crookes, R.; Kinoshita, E. The potential of dimethyl ether (DME) as an alternative fuel for compression-ignition engines: A review. Fuel 2008, 87, 1014–1030. [Google Scholar] [CrossRef]
- Phung, T.K.; Busca, G. Ethanol dehydration on silica-aluminas: Active sites and ethylene/diethyl ether selectivities. Catal. Commun. 2015, 68, 110–115. [Google Scholar] [CrossRef]
- Kosaric, N.; Duvnjak, Z.; Farkas, A.; Sahm, H.; Bringer-Meyer, S.; Goebel, O.; Mayer, D. Ethanol in Ullmann’s Encyclopedia of Industrial Chemistry, 5th ed.; Verlag-Chemie: Weinheim, Germany, 1993; Volume A9, pp. 587–653. [Google Scholar]
- Chadwick, S.S. Ullmann’s Encyclopedia of Industrial Chemistry, 5th ed.; A (10); Verlagsgesellschaft: Weinhem, Germany, 1987. [Google Scholar]
- Chen, Y.; Wu, Y.; Tao, L.; Dai, B.; Yang, M.; Chen, Z.; Zhu, X. Dehydration reaction of bio-ethanol to ethylene over modified SAPO catalysts. J. Ind. Eng. Chem. 2010, 16, 717–722. [Google Scholar] [CrossRef]
- Phung, T.K.; Lagazzo, A.; Rivero Crespo, M.Á.; Sánchez Escribano, V.; Busca, G.A. Study of commercial transition aluminas and of their catalytic activity in the dehydration of ethanol. J. Catal. 2014, 311, 102–113. [Google Scholar] [CrossRef]
- Alharbi, W.; Brown, E.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Dehydration of ethanol over heteropoly acid catalysts in the gas phase. J. Catal. 2014, 319, 174–181. [Google Scholar] [CrossRef]
- Rahmanian, A.; Ghaziaskar, H.S. Continuous dehydration of ethanol to diethyl ether over aluminum phosphate–hydroxyapatite catalyst under sub and supercritical condition. J. Supercrit. Fluids 2014, 78, 34–41. [Google Scholar] [CrossRef]
- Duan, C.; Zhang, X.; Zhou, R.; Hua, Y.; Zhang, L.; Chen, J. Comparative studies of ethanol to propylene over HZSM-5/SAPO-34 catalysts prepared by hydrothermal synthesis and physical mixture. Fuel Process. Technol. 2003, 108, 31–40. [Google Scholar] [CrossRef]
- Phung, T.K.; Hernández, L.P.; Lagazzo, A.; Busca, G. Dehydration of ethanol over zeolites, silica alumina and alumina: Lewis acidity, Brønsted acidity and confinement effects. Appl. Catal. A 2015, 493, 77–89. [Google Scholar] [CrossRef]
- Golay, S.; Kiwi-Minsker, L.; Doepper, R.; Renken, A. Influence of the catalyst acid/base properties on the catalytic ethanol dehydration under steady state and dynamic conditions. In situ surface and gas phase analysis. Chem. Eng. Sci. 1999, 54, 3593–3598. [Google Scholar] [CrossRef] [Green Version]
- Zaki, T. Catalytic dehydration of ethanol using transition metal oxide catalysts. J. Colloid Interface Sci. 2005, 284, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, C.; Degennaro, L.; Luisi, R. Titanium dioxide as a catalyst in biodiesel production. Catalysts 2019, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- Arata, K.; Hino, M. Preparation of superacid by metal oxides and their catalytic action. Matter. Chem. Phys. 1990, 26, 213–237. [Google Scholar] [CrossRef]
- Gardi, J.; Ali, H.; Xiaojun, L.; Mukhtar, H.A. Synthesis of Ti(SO4)O solid acid nanocatalyst and its application for biodiesel production from used cooking oil. Appl. Catal A-Gen. 2016, 527, 81–95. [Google Scholar] [CrossRef]
- Ropero-Vega, J.; Aldana-Pérez, A.; Gómez, R.; Niño-Gómez, M.E.; Nino-Gomez, M.E. Sulfated titania (TiO2/SO42−) A very active solid acid catalyst for the esterfication of free fatty acid with ethanol. Appl. Catal. 2010, 379, 24–29. [Google Scholar] [CrossRef]
- Ahmed, I.; Khan, N.A.; Mishra, D.K.; Lee, J.S.; Hwang, J.S.; Jhung, S.H. Liquid-phase dehydration of sorbitol to isosorbide using sulfated titania as a solid acid catalyst. Chem. Eng. Sci. 2013, 93, 91–95. [Google Scholar] [CrossRef]
- Kamsuwan, T.; Praserthdam, P.; Jongsomjit, B. Diethyl ether production during catalytic dehydration of ethanol over Ru and Pt modified H-beta zeolite catalysts. J. Oleo Sci 2017, 66, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Guo, Y.; Liu, Y. Synthesis of high pure TiO2 nanoparticles from Ti(SO4) in presence of EDTA as complexing agent. China Part. 2005, 3, 240–242. [Google Scholar] [CrossRef]
- Aghilinategh, M.; Barati, M.; Hamadanian, M. Supercritical methanol for one Put biodiesel production from chlorella vulgaris microalgae in the presence of CaO/TiO2 nano-photocatalyst and subcritical water. Biomass Bioenergy 2019, 123, 34–40. [Google Scholar] [CrossRef]
- Sarvari, M.H.; Sodagar, E.; Doroodmand, M.M. Nano sulfated titania as solid acid catalyst in direct synthesis of fatty acid amides. J. Org. Chem. 2011, 76, 2853–2859. [Google Scholar] [CrossRef]
- Nagaraj, G.; Dhayal, R.A.; Albert, I.A.; Josephine, R.L. Turning the optical band gap of pure TiO2 via photon induced method. Optik 2019, 179, 889–894. [Google Scholar]
- Afshar, S.; Sadehvand, M.; Azad, A.; Dekamin, M.G.; Jalali-Heravi, M.; Mollahosseini, A.; Amani, M.; Tadjarodi, A. Optimization of catalytic activity of sulfated titania for efficient synthesis of isoamyl acetate by response surface methodology. Mon. Chem. 2015, 146, 1949–1957. [Google Scholar] [CrossRef]
- Bokhimi, X.; Morales, A.; Ortiz, E.; Lopez, T.; Gómez, R.; Navarrete, J. Sulfate ion in titania polymorphs. J. Sol-Gel Sci. Technol. 2004, 29, 31–40. [Google Scholar] [CrossRef]
- Yang, W.; Ok, Y.S.; Dou, X.; Zhang, Y.; Yang, M.; Wei, D.; Xu, P. Effectively Remediating Spiramycin from Production Wastewater through Hydrolyzing Functional Groups using Solid Superacid TiO2/SO4. Environ. Res. 2019, 175, 390–401. [Google Scholar] [CrossRef]
- Patel, A.; Brahmkhatri, V.; Singh, N. Biodiesel production by esterfication of free fatty acid over sulfated zirconia. Renew. Energy 2013, 51, 227–233. [Google Scholar] [CrossRef]
- Almeida, R.M.; Noda, L.C.; Goncalves, N.S.; Meneghetti, S.M.P.; Meneghetti, M.R. Transesterification reaction of vegetable oils, using superacid sulfated TiO2-base catalysts. Appl. Catal. A Gen. 2008, 347, 100–105. [Google Scholar] [CrossRef]
- Sarve, D.T.; Singh, S.K.; Ekhe, J.D. Kinetic and mechanistic study of ethanol dehydration to diethyl ether over Ni-ZSM-5 in a closed batch reactor. React. Kinet. Mech. Catal. 2020, 131, 261–281. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, H.; Lin, L. Biodiesel: An alternative to conventional fuel. Energy Procedia 2012, 16, 1874–1885. [Google Scholar] [CrossRef] [Green Version]
- Scott Fogler, H. Elements of Chemical Reaction Engineering, 1st ed.; Prentice Hall: Hoboken, NJ, USA, 1987. [Google Scholar]
- Widayat, A.; Rachimoellah, M. Diethyl Ether Production Process with Various Catalyst Type. Int. J. Sci. Eng. 2013, 4, 6–10. [Google Scholar] [CrossRef]
- Limlathong, M.; Chitpong, N.; Jongsomjit, B. Influence of Phosphoric Acid Modification on Catalytic Properties of γ−χ Al2O3 Catalysts for Dehydration of Ethanol to Diethyl Ether. Bull. Chem. React. 2019, 14, 1–8. [Google Scholar] [CrossRef] [Green Version]



| Catalysts | Total Acidity (mmol g−1) |
|---|---|
| TS-0 | 8.42 |
| TS-1 | 12.50 |
| TS-2 | 14.67 |
| TS-3 | 17.94 |
| Catalyst | Total Acidity (mmol g−1) |
|---|---|
| TS-3-400 | 11.35 |
| TS-3-500 | 6.19 |
| TS-3-600 | 3.3 |
| Sample | Crystal Size (nm) |
|---|---|
| TS0 | 25.99 |
| TS-3-400 | 10.03 |
| TS-3-500 | 17.25 |
| TS-4-600 | 19.73 |
| Element | Atom (%) | |
|---|---|---|
| TS0 | TS-3-400 | |
| Ti | 28.30 | 28.26 |
| O | 71.70 | 66.92 |
| S | - | 4.82 |
| Catalyst | Surface Area | Total Pore Volume | Pore Diameter |
|---|---|---|---|
| (m2g−1) | (ccg−1) | (nm) | |
| TS0 | 94.90 | 0.360 | 15.17 |
| TS-3-400 | 9.85 | 0.073 | 29.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijaya, K.; Putri, A.R.; Sudiono, S.; Mulijani, S.; Patah, A.; Wibowo, A.C.; Saputri, W.D. Effectively Synthesizing SO4/TiO2 Catalyst and Its Performance for Converting Ethanol into Diethyl Ether (DEE). Catalysts 2021, 11, 1492. https://doi.org/10.3390/catal11121492
Wijaya K, Putri AR, Sudiono S, Mulijani S, Patah A, Wibowo AC, Saputri WD. Effectively Synthesizing SO4/TiO2 Catalyst and Its Performance for Converting Ethanol into Diethyl Ether (DEE). Catalysts. 2021; 11(12):1492. https://doi.org/10.3390/catal11121492
Chicago/Turabian StyleWijaya, Karna, Alya Rahmadhani Putri, Sri Sudiono, Sri Mulijani, Aep Patah, Arief Cahyo Wibowo, and Wahyu Dita Saputri. 2021. "Effectively Synthesizing SO4/TiO2 Catalyst and Its Performance for Converting Ethanol into Diethyl Ether (DEE)" Catalysts 11, no. 12: 1492. https://doi.org/10.3390/catal11121492






