Preparation of Ti-Nb-Fe-O Nanotubes on Ti10NbxFe Alloy and the Application for Photocatalytic Degradation under Solar Irradiation
Abstract
:1. Introduction
2. Results and Discussion
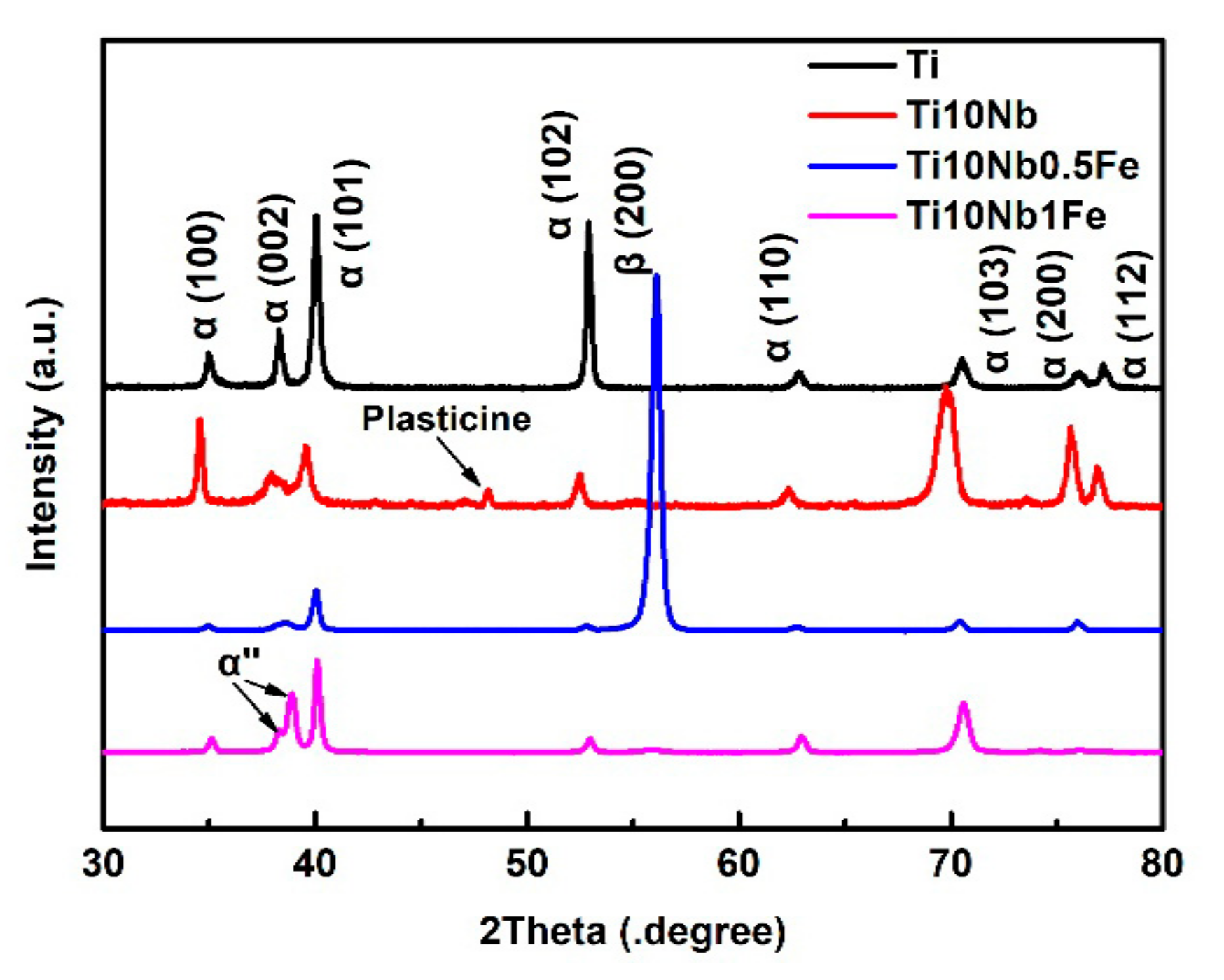
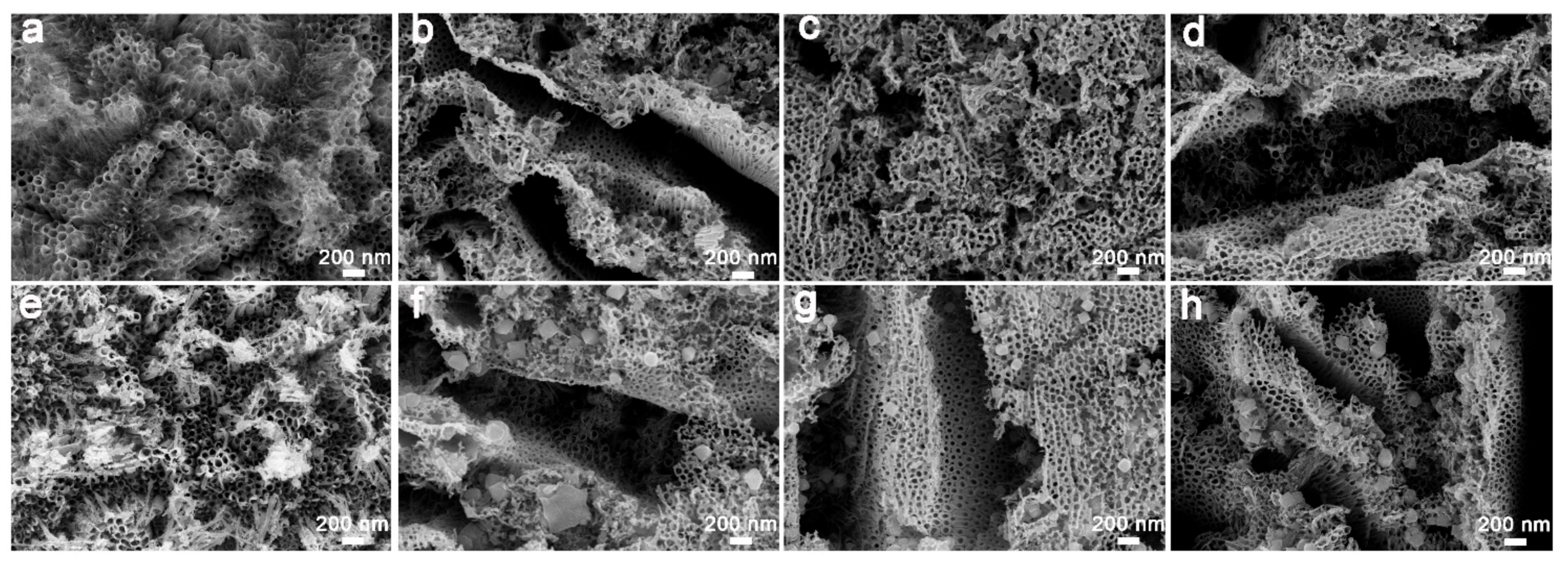
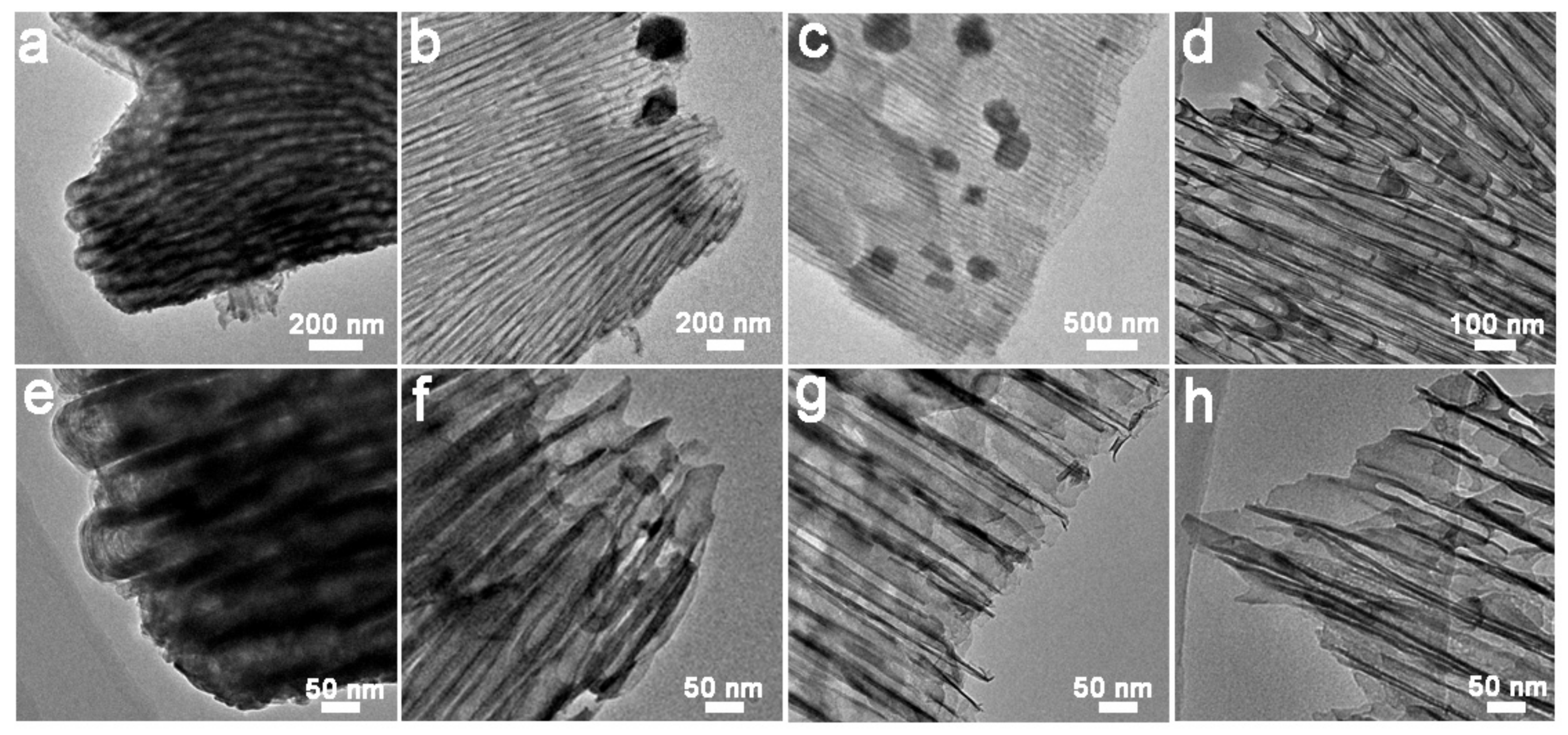
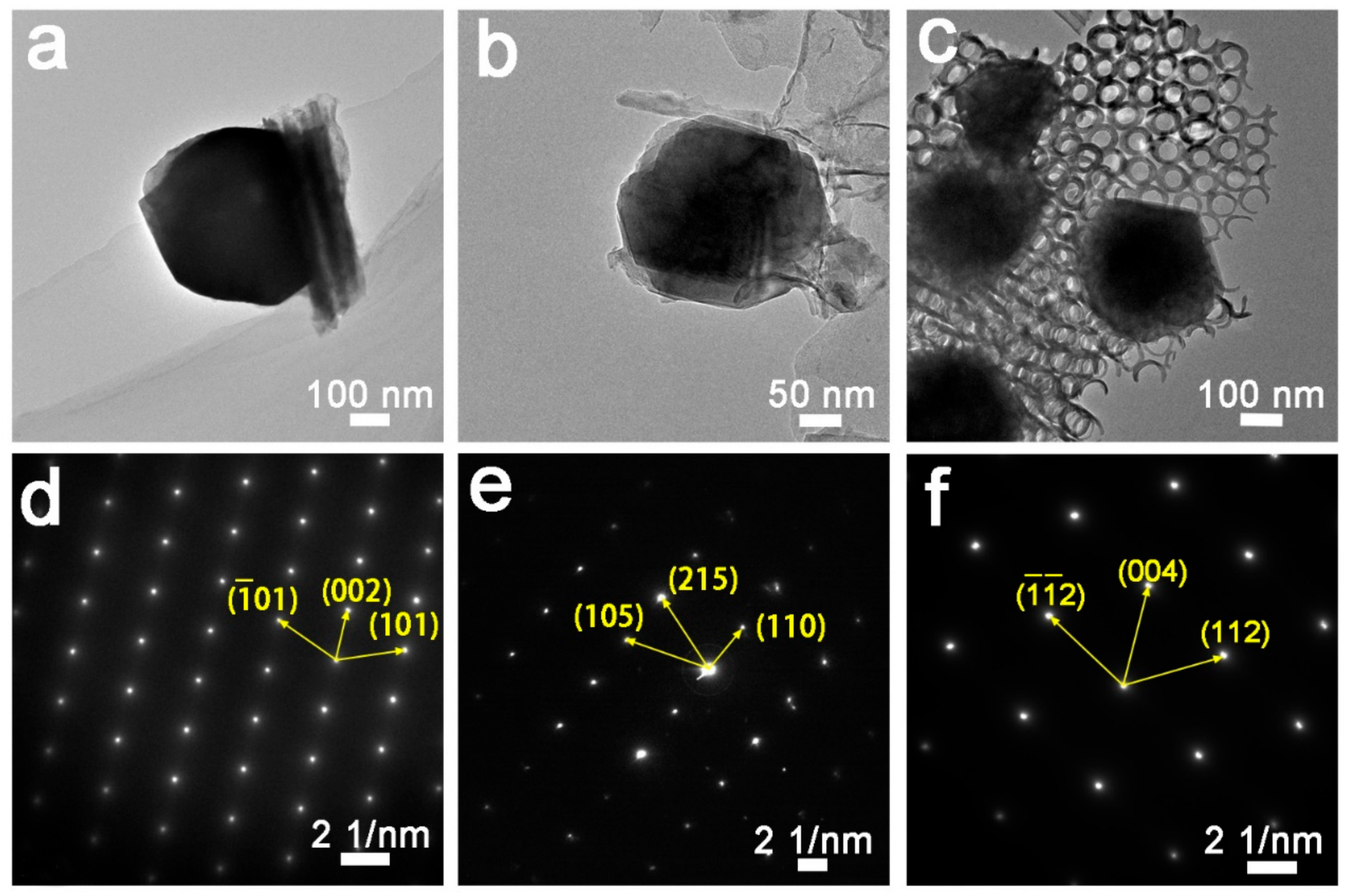
2.1. Characterization
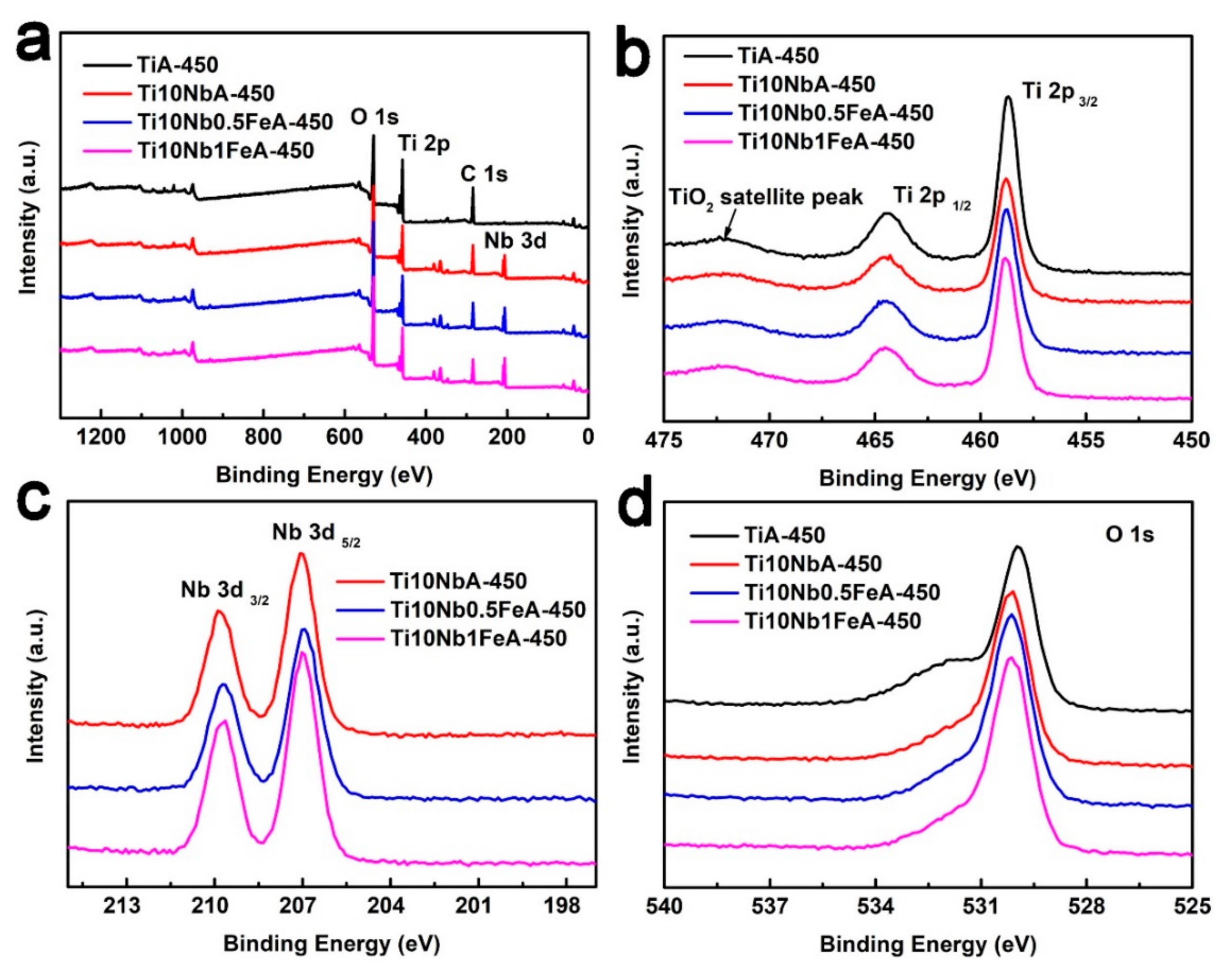
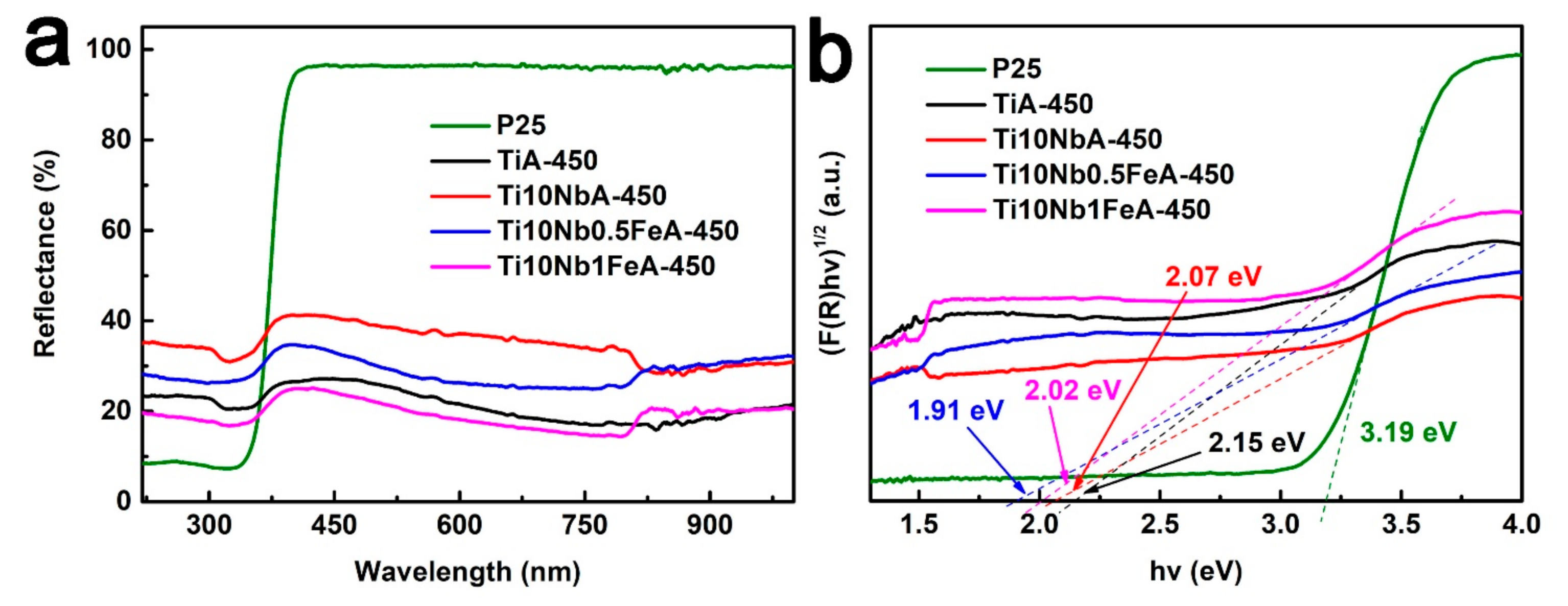
2.2. The Chemical Compositions (XPS) and Light Response (UV-vis) of As-Prepared Nanotubes
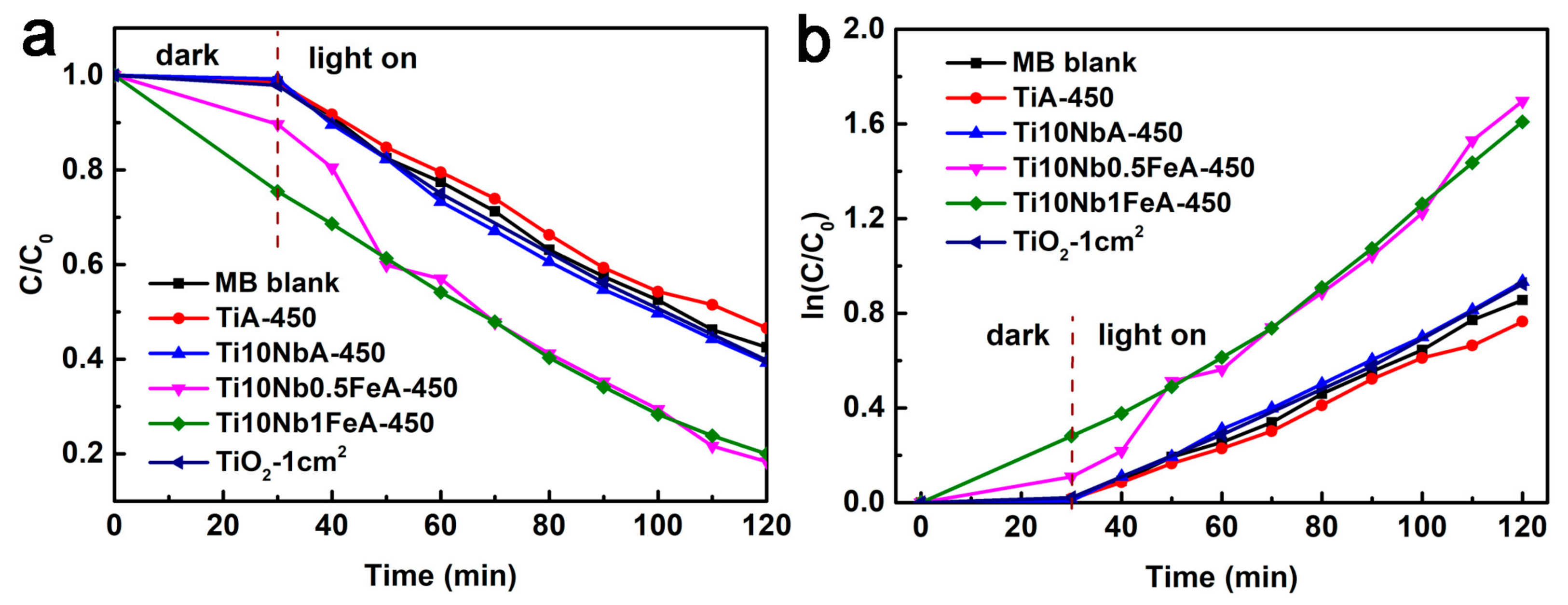
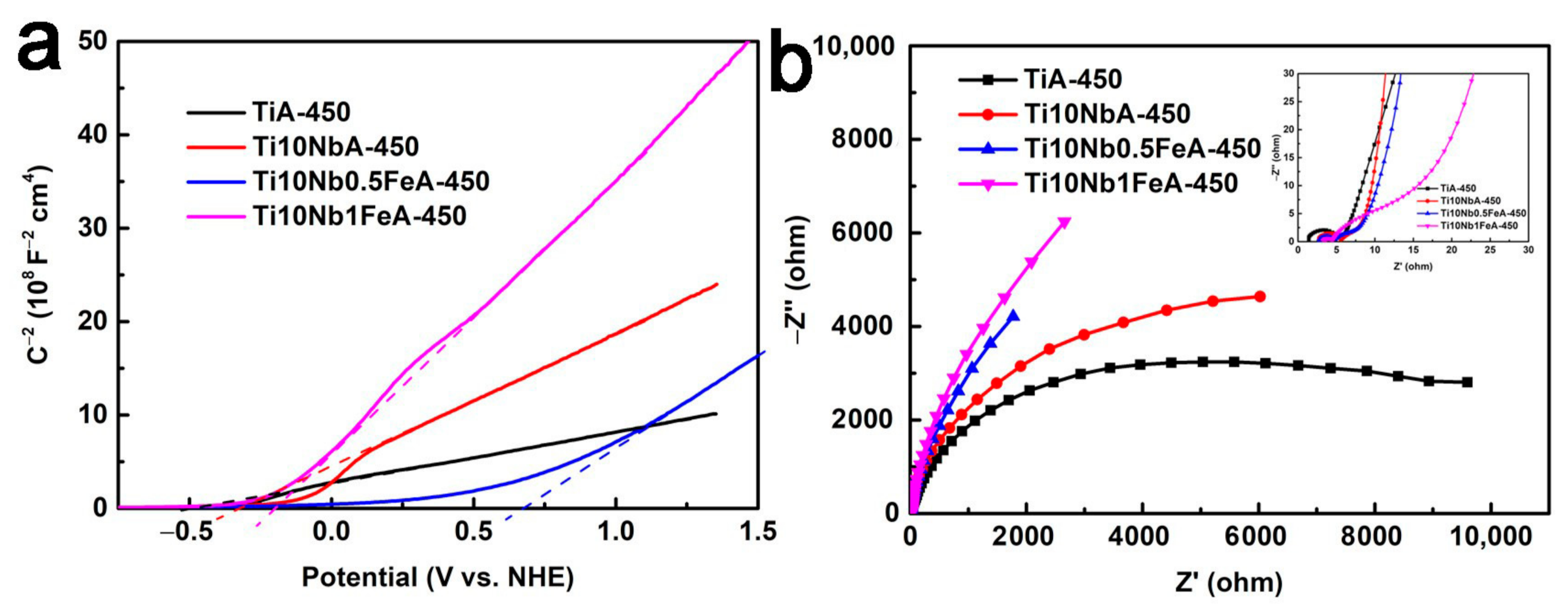
2.3. Photodegradation of MB and Electrochemical Performance
3. Materials and Methods
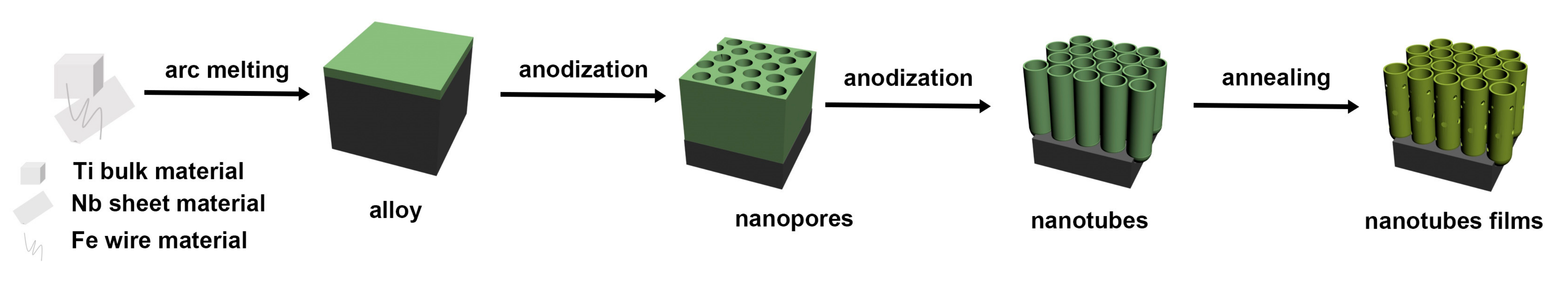
3.1. Alloy Preparation
3.2. Electrochemical Anodization
3.3. Characterization
3.4. MB Photocatalytic Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, X.; Cheng, Y.; Li, X.; Dong, J. High-efficiency and conveniently recyclable photo-catalysts for dye degradation based on urchin-like CuO microparticle/polymer hybrid composites. Appl. Surf. Sci. 2018, 439, 784–791. [Google Scholar] [CrossRef]
- Liu, X.; Gao, S.; Xu, H.; Lou, Z.; Wang, W.; Huang, B.; Dai, Y. Green synthetic approach for Ti3+ self-doped TiO(2-x) nanoparticles with efficient visible light photocatalytic activity. Nanoscale 2013, 5, 1870–1875. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Li, L.; Xu, Y.; Zuo, Y.; Li, G. Hybridization of brookite TiO2 with g-C3N4: A visible-light-driven photocatalyst for As3+ oxidation, MO degradation and water splitting for hydrogen evolution. J. Mater. Chem. A 2014, 2, 15774–15780. [Google Scholar] [CrossRef]
- Liu, M.; Qiu, X.; Hashimoto, K.; Miyauchi, M. Cu(ii) nanocluster-grafted, Nb-doped TiO2 as an efficient visible-light-sensitive photocatalyst based on energy-level matching between surface and bulk states. J. Mater. Chem. A 2014, 2, 13571–13579. [Google Scholar] [CrossRef] [Green Version]
- Tong, Z.; Yang, D.; Xiao, T.; Tian, Y.; Jiang, Z. Biomimetic fabrication of g-C3N4/TiO2 nanosheets with enhanced photocatalytic activity toward organic pollutant degradation. Chem. Eng. J. 2015, 260, 117–125. [Google Scholar] [CrossRef]
- Yuan, X.; Floresyona, D.; Aubert, P.-H.; Bui, T.-T.; Remita, S.; Ghosh, S.; Brisset, F.; Goubard, F.; Remita, H. Photocatalytic degradation of organic pollutant with polypyrrole nanostructures under UV and visible light. Appl. Catal. B Environ. 2019, 242, 284–292. [Google Scholar] [CrossRef]
- Chen, X.; Selloni, A. Introduction: Titanium dioxide (TiO2) nanomaterials. Chem. Rev. 2014, 114, 9281–9282. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Zhao, J.; Qiao, Z.-J.; Wang, J.-M.; Sun, S.-H.; Fu, W.-X.; Zhang, X.-Y.; Yu, Z.-Y.; Dou, Y.-H.; Kang, J.-L.; et al. Nonsolvent-induced phase separation-derived TiO2 nanotube arrays/porous Ti electrode as high-energy-density anode for lithium-ion batteries. Rare Met. 2020. [Google Scholar] [CrossRef]
- Alam, U.; Fleisch, M.; Kretschmer, I.; Bahnemann, D.; Muneer, M. One-step hydrothermal synthesis of Bi-TiO2 nanotube/graphene composites: An efficient photocatalyst for spectacular degradation of organic pollutants under visible light irradiation. Appl. Catal. B Environ. 2017, 218, 758–769. [Google Scholar] [CrossRef]
- Zhang, P.; Yu, Y.; Wang, E.; Wang, J.; Yao, J.; Cao, Y. Structure of nitrogen and zirconium co-doped titania with enhanced visible-light photocatalytic activity. ACS Appl. Mater. Interfaces 2014, 6, 4622–4629. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Prakash, J.; Misra, M.; Sharma, A.; Gupta, R.K. Dual Functional Ta-Doped Electrospun TiO2 Nanofibers with Enhanced Photocatalysis and SERS Detection for Organic Compounds. ACS Appl. Mater. Interfaces 2017, 9, 28495–28507. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; He, D.; Situ, Y.; Huang, H. Construction of heterostructured g-C(3)N(4)/Ag/TiO(2) microspheres with enhanced photocatalysis performance under visible-light irradiation. ACS Appl. Mater. Interfaces 2014, 6, 14405–14414. [Google Scholar] [CrossRef]
- Lee, A.; Libera, J.A.; Waldman, R.Z.; Ahmed, A.; Avila, J.R.; Elam, J.W.; Darling, S.B. Conformal Nitrogen-Doped TiO2Photocatalytic Coatings for Sunlight-Activated Membranes. Adv. Sustain. Syst. 2017, 1, 1600041. [Google Scholar]
- Hu, M.; Xing, Z.; Cao, Y.; Li, Z.; Yan, X.; Xiu, Z.; Zhao, T.; Yang, S.; Zhou, W. Ti3+ self-doped mesoporous black TiO2/SiO2/g-C3N4 sheets heterojunctions as remarkable visible-lightdriven photocatalysts. Appl. Catal. B Environ. 2018, 226, 499–508. [Google Scholar] [CrossRef]
- Li, X.; Liu, P.; Mao, Y.; Xing, M.; Zhang, J. Preparation of homogeneous nitrogen-doped mesoporous TiO2 spheres with enhanced visible-light photocatalysis. Appl. Catal. B Environ. 2015, 164, 352–359. [Google Scholar] [CrossRef]
- Liu, T.; Liu, B.; Yang, L.; Ma, X.; Li, H.; Yin, S.; Sato, T.; Sekino, T.; Wang, Y. RGO/Ag2S/TiO2 ternary heterojunctions with highly enhanced UV-NIR photocatalytic activity and stability. Appl. Catal. B Environ. 2017, 204, 593–601. [Google Scholar] [CrossRef]
- Li, Y.; Liu, G.; Zhang, J.; He, X. Preparation and optoelectronic properties of TiO2 thin films codoped with iron and molybdenum. Rare Met. 2011, 30, 238–242. [Google Scholar] [CrossRef]
- Kim, J.U.; Han, H.S.; Park, J.; Park, W.; Baek, J.H.; Lee, J.M.; Jung, H.S.; Cho, I.S. Facile and controllable surface-functionalization of TiO2 nanotubes array for highly-efficient photoelectrochemical water-oxidation. J. Catal. 2018, 365, 138–144. [Google Scholar] [CrossRef]
- Hang, R.; Zhao, Y.; Bai, L.; Liu, Y.; Gao, A.; Zhang, X.; Huang, X.; Tang, B.; Chu, P.K. Fabrication of irregular-layer-free and diameter-tunable Ni–Ti–O nanopores by anodization of NiTi alloy. Electrochem. Commun. 2017, 76, 10–14. [Google Scholar] [CrossRef]
- Kment, S.; Riboni, F.; Pausova, S.; Wang, L.; Wang, L.; Han, H.; Hubicka, Z.; Krysa, J.; Schmuki, P.; Zboril, R. Photoanodes based on TiO2 and alpha-Fe2O3 for solar water splitting—Superior role of 1D nanoarchitectures and of combined heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. [Google Scholar] [CrossRef]
- Sanabria-Arenas, B.E.; Mazare, A.; Yoo, J.; Nguyen, N.T.; Hejazi, S.; Bian, H.; Diamanti, M.V.; Pedeferri, M.P.; Schmuki, P. Intrinsic AuPt-alloy particles decorated on TiO2 nanotubes provide enhanced photocatalytic degradation. Electrochim. Acta 2018, 292, 865–870. [Google Scholar] [CrossRef]
- Jha, H.; Hahn, R.; Schmuki, P. ultrafast oxide nanotube formation on TiNb TiZr and TiTa alloys by rapid breakdown anodization. Electrochim. Acta 2010, 55, 8883–8887. [Google Scholar] [CrossRef]
- Sado, S.; Ueda, T.; Ueda, K.; Narushima, T. Formation of TiO2 layers on commercially pure Ti and Ti–Mo and Ti–Nb alloys by two-step thermal oxidation and their photocatalytic activity. Appl. Surf. Sci. 2015, 357, 2198–2205. [Google Scholar] [CrossRef]
- Allam, N.K.; Alamgir, F.; El-Sayed, M.A. Enhanced photoassisted water electrolysis using vertically oriented anodically fabricated Ti-Nb-Zr-O mixed oxide nanotube arrays. ACS Nano 2010, 4, 5819–5826. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, K.; Yin, J.; Yang, J.; Xu, J.; Gu, Y.; Liu, L.; Luo, J.; Li, Y.; Sun, L. A nanopump for low-temperature and efficient solar water evaporation. J. Mater. Chem. A 2019, 7, 24311–24319. [Google Scholar] [CrossRef]
- Li, G.-Z.; Tang, H.-P.; Zhang, W.-Y.; Li, G.; Yu, L.-L.; Li, Y.-N. Fabrication of multilayer Nb2O5 nanoporous film by anodization of niobium foils. Rare Met. 2013, 34, 77–80. [Google Scholar] [CrossRef]
- Byeon, I.-S.; Hwang, I.-J.; Choe, H.-C.; Brantley, W.A. Electrochemically-coated hydroxyapatite films on nanotubular Ti Nb alloys prepared in solutions containing Ca, P, and Zn ions. Thin Solid Films 2016, 620, 132–138. [Google Scholar] [CrossRef]
- Xu, Y.; Ahmed, R.; Klein, D.; Cap, S.; Freedy, K.; McDonnell, S.; Zangari, G. Improving photo-oxidation activity of water by introducing Ti3+ in self-ordered TiO2 nanotube arrays treated with Ar/NH3. J. Power Sources 2019, 414, 242–249. [Google Scholar] [CrossRef]
- Luz, A.R.; Lepienski, C.M.; Henke, S.L.; Grandini, C.R.; Kuromoto, N.K. Effect of microstructure on the nanotube growth by anodic oxidation on Ti-10Nb alloy. Mater. Res. Express 2017, 4, 076408. [Google Scholar] [CrossRef]
- Luz, A.R.; Santos, L.S.; Lepienski, C.M.; Kuroda, P.B.; Kuromoto, N.K. Characterization of the morphology, structure and wettability of phase dependent lamellar and nanotube oxides on anodized Ti-10Nb alloy. Appl. Surf. Sci. 2018, 448, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Ehtemam-Haghighi, S.; Prashanth, K.G.; Attar, H.; Chaubey, A.K.; Cao, G.H.; Zhang, L.C. Evaluation of mechanical and wear properties of Ti xNb 7Fe alloys designed for biomedical applications. Mater. Des. 2016, 111, 592–599. [Google Scholar] [CrossRef]
- Ehtemam-Haghighi, S.; Liu, Y.; Cao, G.; Zhang, L.-C. Phase transition, microstructural evolution and mechanical properties of Ti-Nb-Fe alloys induced by Fe addition. Mater. Des. 2016, 97, 279–286. [Google Scholar] [CrossRef]
- Ehtemam-Haghighi, S.; Liu, Y.; Cao, G.; Zhang, L.C. Influence of Nb on the beta-->alpha” martensitic phase transformation and properties of the newly designed Ti-Fe-Nb alloys. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 503–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-N.; Huang, W.; Zhang, Y.; Tang, B.; Xiao, H.; Zhao, G. Fabrication and enhanced visible-light photoelectrochemical performance of periodic hierarchical 3D Ti–Fe–O structure. Mater. Lett. 2016, 168, 24–27. [Google Scholar] [CrossRef]
- Zlámal, M.; Paušová, Š.; Kment, Š.; Hubička, Z.; Krýsa, J. Transparent α-Fe2O3/TiO2 nanotubular photoanodes. Catal. Today 2017, 287, 137–141. [Google Scholar] [CrossRef]
- Yu, J.; Wu, Z.; Gong, C.; Xiao, W.; Sun, L.; Lin, C. Fe3+-Doped TiO(2) Nanotube Arrays on Ti-Fe Alloys for Enhanced Photoelectrocatalytic Activity. Nanomaterials 2016, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- Riboni, F.; Nguyen, N.T.; So, S.; Schmuki, P. Aligned metal oxide nanotube arrays: Key-aspects of anodic TiO2nanotube formation and properties. Nanoscale Horiz. 2016, 1, 445–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R Rep. 2013, 74, 377–406. [Google Scholar] [CrossRef] [Green Version]
- Pan, D.; Huang, H.; Wang, X.; Wang, L.; Liao, H.; Li, Z.; Wu, M. C-axis preferentially oriented and fully activated TiO2 nanotube arrays for lithium ion batteries and supercapacitors. J. Mater. Chem. A 2014, 2, 11454–11464. [Google Scholar] [CrossRef]
- Robben, L.; Ismail, A.A.; Lohmeier, S.J.; Feldhoff, A.; Bahnemann, D.W.; Buhl, J.-C. Facile Synthesis of Highly Ordered Mesoporous and Well Crystalline TiO2: Impact of Different Gas Atmosphere and Calcination Temperatures on Structural Properties. Chem. Mater. 2012, 24, 1268–1275. [Google Scholar] [CrossRef]
- Jin, M.; Lu, X.; Qiao, Y.; Wang, L.-N.; Volinsky, A.A. Fabrication and characterization of anodic oxide nanotubes on TiNb alloys. Rare Met. 2016, 35, 140–148. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Zhang, Y.; Ding, Y.; Bi, Y. Ultrathin FeOOH Nanolayers with Abundant Oxygen Vacancies on BiVO4 Photoanodes for Efficient Water Oxidation. Angew. Chem. Int. Ed. Engl. 2018, 57, 2248–2252. [Google Scholar] [CrossRef]
- Hoang, S.; Berglund, S.P.; Hahn, N.T.; Bard, A.J.; Mullins, C.B. Enhancing visible light photo-oxidation of water with TiO2 nanowire arrays via cotreatment with H2 and NH3: Synergistic effects between Ti3+ and N. J. Am. Chem. Soc. 2012, 134, 3659–3662. [Google Scholar] [CrossRef] [PubMed]
- Folger, A.; Ebbinghaus, P.; Erbe, A.; Scheu, C. Role of Vacancy Condensation in the Formation of Voids in Rutile TiO2 Nanowires. ACS Appl. Mater. Interfaces 2017, 9, 13471–13479. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, L.C.; Gong, Y.; Niu, P.; Wang, J.Q.; Gu, L.; Chen, X.; Liu, G.; Wang, L.; Cheng, H.M. An Unusual Strong Visible-Light Absorption Band in Red Anatase TiO2 Photocatalyst Induced by Atomic Hydrogen-Occupied Oxygen Vacancies. Adv. Mater. 2018, 30, 1704479. [Google Scholar]
- Sun, Y.; Wang, H.; Xing, Q.; Cui, W.; Li, J.; Wu, S.; Sun, L. The pivotal effects of oxygen vacancy on Bi2MoO6: Promoted visible light photocatalytic activity and reaction mechanism. Chin. J. Catal. 2019, 40, 647–655. [Google Scholar] [CrossRef]
- Salari, S.; Ghodsi, F.E. A significant enhancement in the photoluminescence emission of the Mg doped ZrO2 thin films by tailoring the effect of oxygen vacancy. J. Lumin. 2017, 182, 289–299. [Google Scholar] [CrossRef]
- An, X.; Hu, C.; Liu, H.; Qu, J. Hierarchical Nanotubular Anatase/Rutile/TiO2(B) Heterophase Junction with Oxygen Vacancies for Enhanced Photocatalytic H2 Production. Langmuir 2018, 34, 1883–1889. [Google Scholar] [CrossRef]
- Das, T.K.; Ilaiyaraja, P.; Sudakar, C. Template assisted nanoporous TiO2 nanoparticles: The effect of oxygen vacancy defects on photovoltaic performance of DSSC and QDSSC. Sol. Energy 2018, 159, 920–929. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Q.; Ahmed, R.; Hoglund, E.R.; Zangari, G. Synthesis of TiO2-based nanocomposites by anodizing and hydrogen annealing for efficient photoelectrochemical water oxidation. J. Power Sources 2019, 410–411, 59–68. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Q.; Liu, Z.; Qiu, L.; Tian, X.; Zhang, S.; Gao, S. Fabrication and photoelectrochemical performance of Ag/AgBr sensitized TiO2 nanotube arrays for environmental and energy applications. Sep. Purif. Technol. 2019, 209, 782–788. [Google Scholar] [CrossRef]
- Sun, M.; Liu, X.; Zhao, G.; Kong, W.; Xuan, J.; Tan, S.; Sun, Y.; Wei, S.; Ren, J.; Yin, G. Sn4+ doping combined with hydrogen treatment for CdS/TiO2 photoelectrodes: An efficient strategy to improve quantum dots loading and charge transport for high photoelectrochemical performance. J. Power Sources 2019, 430, 80–89. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Li, Q.; Li, Y. Preparation of Ti-Nb-Fe-O Nanotubes on Ti10NbxFe Alloy and the Application for Photocatalytic Degradation under Solar Irradiation. Catalysts 2021, 11, 327. https://doi.org/10.3390/catal11030327
Zhao Y, Li Q, Li Y. Preparation of Ti-Nb-Fe-O Nanotubes on Ti10NbxFe Alloy and the Application for Photocatalytic Degradation under Solar Irradiation. Catalysts. 2021; 11(3):327. https://doi.org/10.3390/catal11030327
Chicago/Turabian StyleZhao, Yujie, Qiquan Li, and Yan Li. 2021. "Preparation of Ti-Nb-Fe-O Nanotubes on Ti10NbxFe Alloy and the Application for Photocatalytic Degradation under Solar Irradiation" Catalysts 11, no. 3: 327. https://doi.org/10.3390/catal11030327






