Highly CO Selective Trimetallic Metal-Organic Framework Electrocatalyst for the Electrochemical Reduction of CO2
Abstract
:1. Introduction
2. Result and Discussion
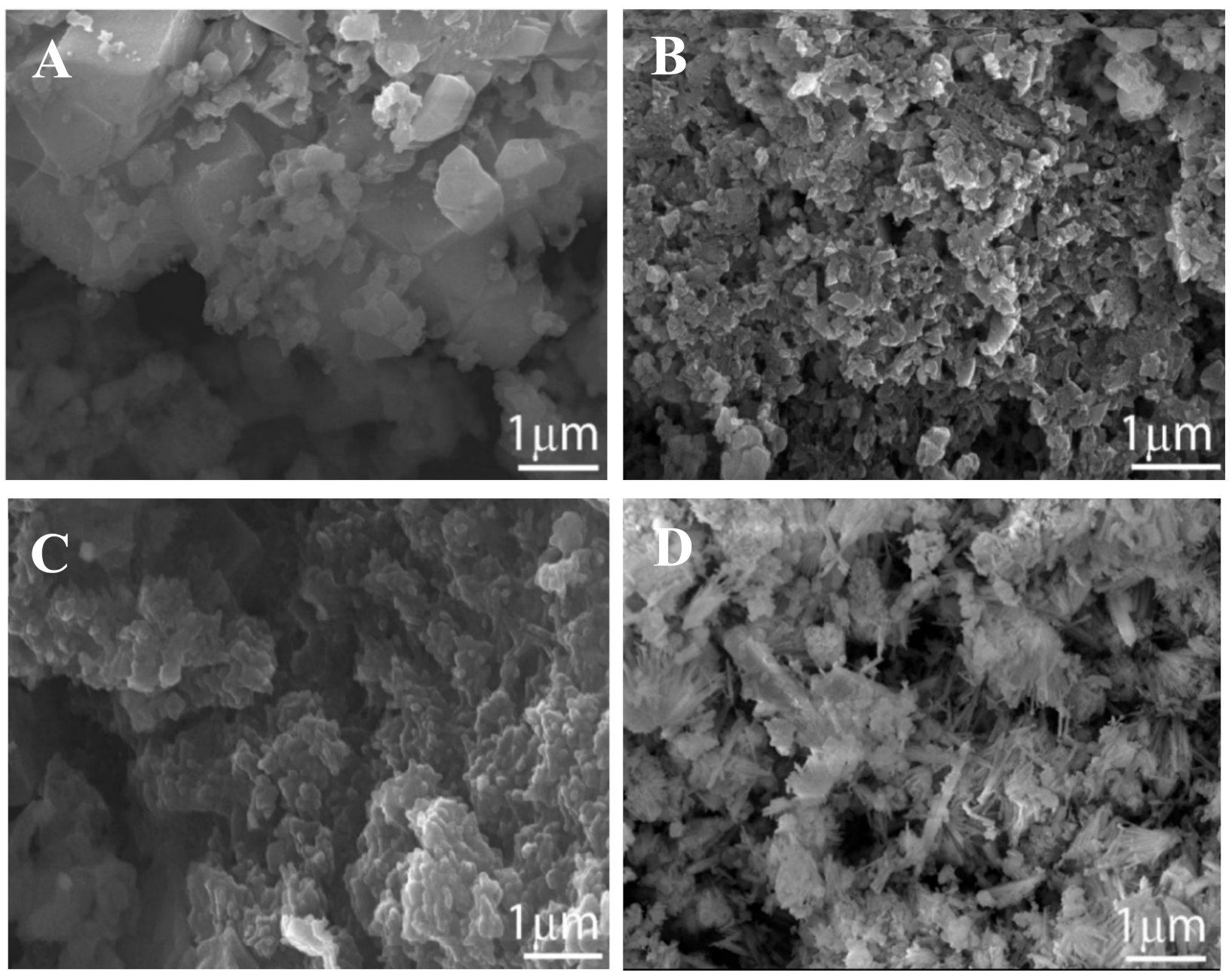
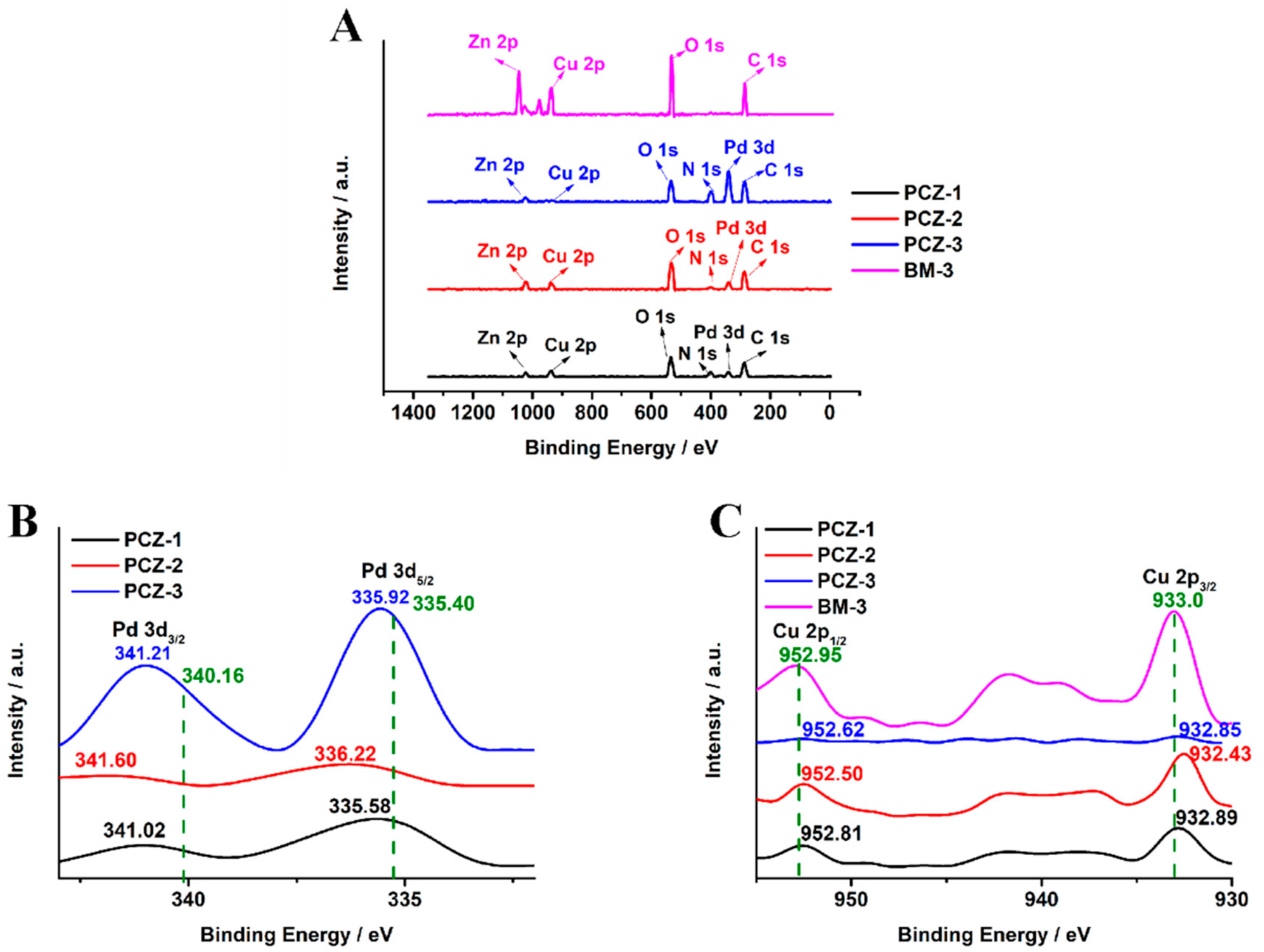
2.1. Characterization of the PCZs
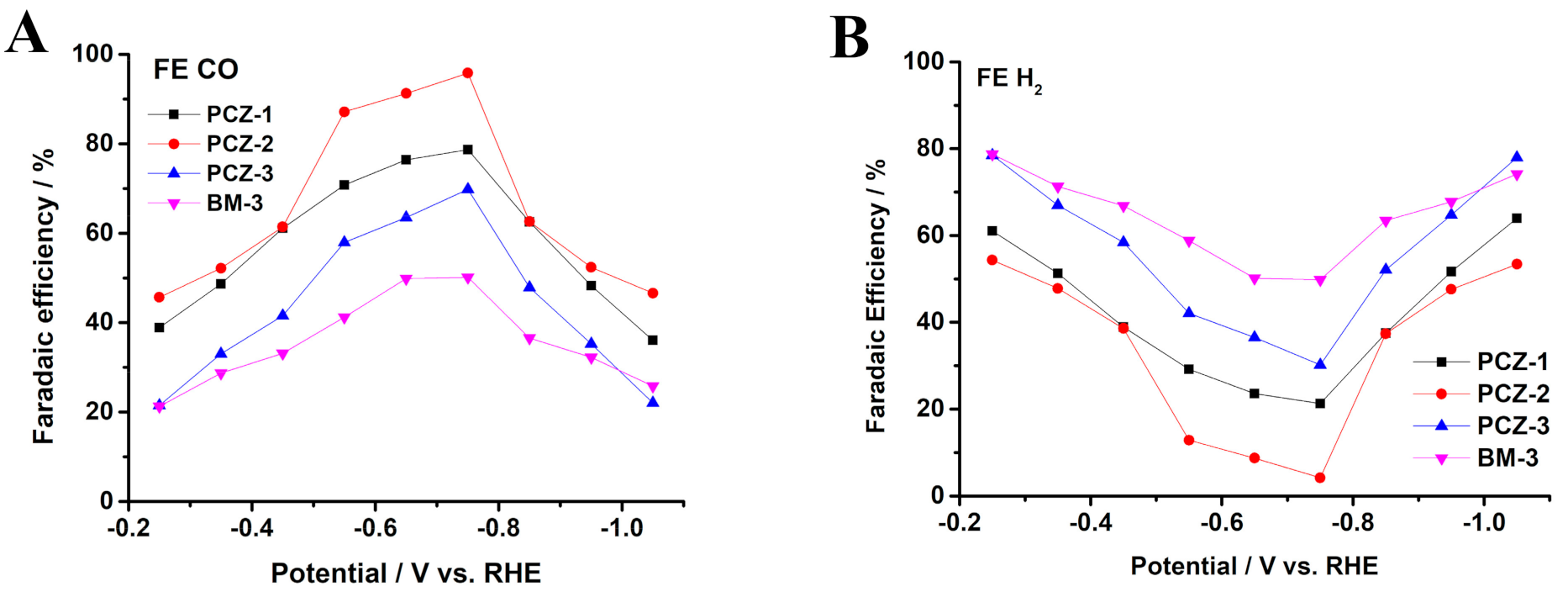
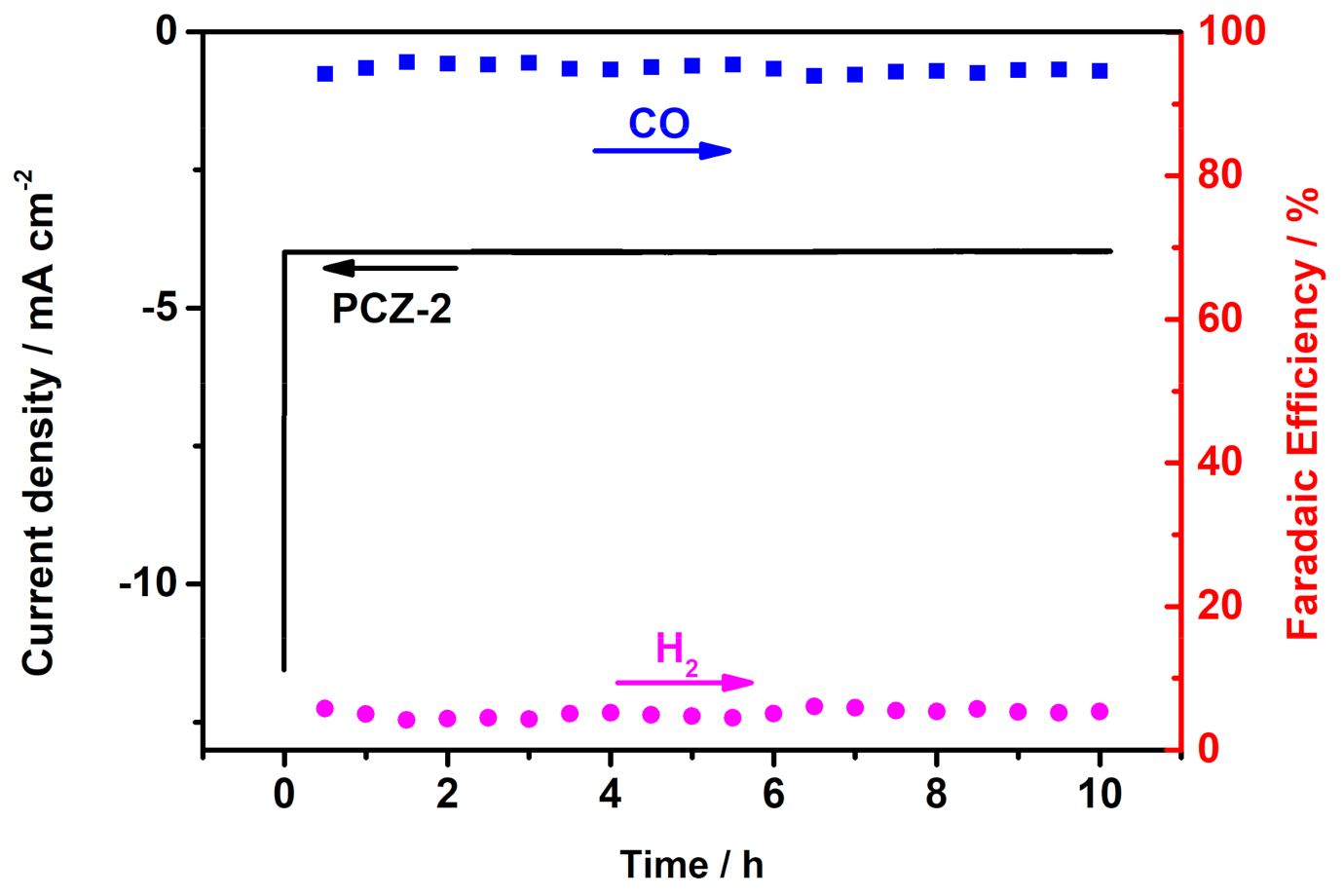
2.2. ERC Properties of PCZs and BM-3 Catalysts
3. Materials and Methods
3.1. Materials
3.2. Characterization Techniques
3.3. Synthesis of ZIF-8 and Pd, Cu, and Zn Trimetallic MOF Electrocatalysts (PCZs)
3.3.1. Synthesis ZIF-8
3.3.2. Synthesis of PCZs
3.4. Preparation of the Working Electrodes
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, C.W.; Ciston, J.; Kanan, M.W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 2014, 508, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Ozaki, T.; Terashima, C.; Fujishima, A.; Einaga, Y. High-Yield Electrochemical Production of Formaldehyde from CO2 and Seawater. Angew. Chem. Int. Ed. 2014, 53, 871–874. [Google Scholar] [CrossRef]
- Costentin, C.; Robert, M.; Savéant, J.-M. Catalysis of the electrochemical reduction of carbon dioxide. Chem. Soc. Rev. 2013, 42, 2423–2436. [Google Scholar] [CrossRef]
- Chen, B.; Ockwig, N.W.; Millward, A.R.; Contreras, D.S.; Yaghi, O.M. High H2 Adsorption in a Microporous Metal-Organic Framework with Open Metal Sites. Angew. Chem. Int. Ed. 2005, 44, 4745–4749. [Google Scholar] [CrossRef] [PubMed]
- Bae, T.-H.; Lee, J.S.; Qiu, W.; Koros, W.J.; Jones, C.W.; Nair, S. A High-Performance Gas-Separation Membrane Containing Submicrometer-Sized Metal-Organic Framework Crystals. Angew. Chem. Int. Ed. 2010, 49, 9863–9866. [Google Scholar] [CrossRef]
- Mason, J.A.; Veenstra, M.; Long, J.R. Evaluating metal-organic frameworks for natural gas storage. Chem. Sci. 2014, 5, 32–51. [Google Scholar] [CrossRef] [Green Version]
- Rodenas, T.; Luz, I.; Prieto, G.; Seoane, B.; Miro, H.; Corma, A.; Kapteijn, F.; Llabrés i Xamena, F.X.; Gascon, J. Metal-organic framework nanosheets in polymer composite materials for gas separation. Nat. Mater. 2015, 14, 48–55. [Google Scholar] [CrossRef]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydın, A.Ö.; Hupp, J.T. Metal-Organic Framework Materials with Ultrahigh Surface Areas: Is the Sky the Limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Wu, T.; Jiang, P.G.; Lin, J.H.; Yang, Y.X. Octamolybdate-Based Metal-Organic Framework with Unsaturated Coordinated Metal Center as Electrocatalyst for Generating Hydrogen from Water. Inorg. Chem. 2013, 52, 777–784. [Google Scholar] [CrossRef]
- Jiang, M.; Li, L.; Zhu, D.; Zhang, H.; Zhao, X. Oxygen reduction in the nanocage of metal-organic frameworks with an electron transfer mediator. J. Mater. Chem. A 2014, 2, 5323–5329. [Google Scholar] [CrossRef]
- Kornienko, N.; Zhao, Y.; Kley, C.S.; Zhu, C.; Kim, D.; Lin, S.; Chang, C.J.; Yaghi, O.M.; Yang, P. Metal-Organic Frameworks for Electrocatalytic Reduction of Carbon Dioxide. J. Am. Chem. Soc. 2015, 137, 14129–14135. [Google Scholar] [CrossRef] [Green Version]
- Van Phuc, T.; Kang, S.G.; Chung, J.S.; Hur, S.H. Highly CO selective Ca and Zn hybrid metal-organic framework electrocatalyst for the electrochemical reduction of CO2. Curr. Appl. Phys. 2021, 27, 31–37. [Google Scholar] [CrossRef]
- Van Phuc, T.; Kang, S.G.; Chung, J.S.; Hur, S.H. Highly selective metal-organic framework-based electrocatalyst for the electrochemical reduction of CO2 to CO. Mater. Res. Bull. 2021, 138, 111228. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, H.; Su, S.; Cai, J.; Dang, S.; Xiang, S.; Qian, G.; Zhang, H.; O’Keeffe, M.; Chen, B. A robust near infrared luminescent ytterbium metal-organic framework for sensing of small molecules. Chem. Comm. 2011, 47, 5551–5553. [Google Scholar] [CrossRef]
- Kung, C.-W.; Li, Y.-S.; Lee, M.-H.; Wang, S.-Y.; Chiang, W.-H.; Ho, K.-C. In situ growth of porphyrinic metal-organic framework nanocrystals on graphene nanoribbons for the electrocatalytic oxidation of nitrite. J. Mater. Chem. A 2016, 4, 10673–10682. [Google Scholar] [CrossRef]
- Torad, N.L.; Hu, M.; Ishihara, S.; Sukegawa, H.; Belik, A.A.; Imura, M.; Ariga, K.; Sakka, Y.; Yamauchi, Y. Direct Synthesis of MOF-Derived Nanoporous Carbon with Magnetic Co Nanoparticles toward Efficient Water Treatment. Small 2014, 10, 2096–2107. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Bansal, V.; Kim, K.-H.; Kwon, E.E. Metal-organic frameworks (MOFs) as futuristic options for wastewater treatment. J. Ind. Eng. Chem. 2018, 62, 130–145. [Google Scholar] [CrossRef]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef]
- Taylor-Pashow, K.M.L.; Della Rocca, J.; Xie, Z.; Tran, S.; Lin, W. Postsynthetic Modifications of Iron-Carboxylate Nanoscale Metal−Organic Frameworks for Imaging and Drug Delivery. J. Am. Chem. Soc. 2009, 131, 14261–14263. [Google Scholar] [CrossRef] [Green Version]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef]
- Huxford, R.C.; Della Rocca, J.; Lin, W. Metal-organic frameworks as potential drug carriers. Curr. Opin. Chem. Biol. 2010, 14, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.-L.; Li, C.; Yamauchi, Y. Nanostructured nonprecious metal catalysts for electrochemical reduction of carbon dioxide. Nano Today 2016, 11, 373–391. [Google Scholar] [CrossRef] [Green Version]
- Senthil Kumar, R.; Senthil Kumar, S.; Anbu Kulandainathan, M. Highly selective electrochemical reduction of carbon dioxide using Cu based metal organic framework as an electrocatalyst. Electrochem. Commun. 2012, 25, 70–73. [Google Scholar] [CrossRef]
- Kang, X.; Zhu, Q.; Sun, X.; Hu, J.; Zhang, J.; Liu, Z.; Han, B. Highly efficient electrochemical reduction of CO2 to CH4 in an ionic liquid using a metal-organic framework cathode. Chem. Sci. 2016, 7, 266–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palomino Cabello, C.; Gómez-Pozuelo, G.; Opanasenko, M.; Nachtigall, P.; Čejka, J. Metal-Organic Frameworks M-MOF-74 and M-MIL-100: Comparison of Textural, Acidic, and Catalytic Properties. ChemPlusChem 2016, 81, 828–835. [Google Scholar] [CrossRef]
- Hu, J.; Yang, Q.; Yang, L.; Zhang, Z.; Su, B.; Bao, Z.; Ren, Q.; Xing, H.; Dai, S. Confining Noble Metal (Pd, Au, Pt) Nanoparticles in Surfactant Ionic Liquids: Active Non-Mercury Catalysts for Hydrochlorination of Acetylene. ACS Catal. 2015, 5, 6724–6731. [Google Scholar] [CrossRef]
- Xing, Z.-M.; Gao, Y.-X.; Shi, L.-Y.; Liu, X.-Q.; Jiang, Y.; Sun, L.-B. Fabrication of gold nanoparticles in confined spaces using solid-phase reduction: Significant enhancement of dispersion degree and catalytic activity. Chem. Eng. Sci. 2017, 158, 216–226. [Google Scholar] [CrossRef]
- Karimi, B.; Khorasani, M.; Vali, H.; Vargas, C.; Luque, R. Palladium Nanoparticles Supported in the Nanospaces of Imidazolium-Based Bifunctional PMOs: The Role of Plugs in Selectivity Changeover in Aerobic Oxidation of Alcohols. ACS Catal. 2015, 5, 4189–4200. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, B.; Zhang, W.; Yu, M.; Cui, L.; Cao, X.; Liu, J. Super-light Cu@Ni nanowires/graphene oxide composites for significantly enhanced microwave absorption performance. Sci. Rep. 2017, 7, 1584. [Google Scholar] [CrossRef]
- Nguyen, D.L.T.; Jee, M.S.; Won, D.H.; Jung, H.; Oh, H.-S.; Min, B.K.; Hwang, Y.J. Selective CO2 Reduction on Zinc Electrocatalyst: The Effect of Zinc Oxidation State Induced by Pretreatment Environment. ACS Sustain. Chem. Eng. 2017, 5, 11377–11386. [Google Scholar] [CrossRef]
- Qin, B.; Li, Y.; Fu, H.; Wang, H.; Chen, S.; Liu, Z.; Peng, F. Electrochemical Reduction of CO2 into Tunable Syngas Production by Regulating the Crystal Facets of Earth-Abundant Zn Catalyst. ACS Appl. Mater. Interfaces 2018, 10, 20530–20539. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fu, J.; Zhu, W.; Chen, Z.; Shen, B.; Wu, L.; Xi, Z.; Wang, T.; Lu, G.; Zhu, J.-j.; et al. Tuning Sn-Catalysis for Electrochemical Reduction of CO2 to CO via the Core/Shell Cu/SnO2 Structure. J. Am. Chem. Soc. 2017, 139, 4290–4293. [Google Scholar] [CrossRef]
- Scholten, F.; Sinev, I.; Bernal, M.; Roldan Cuenya, B. Plasma-Modified Dendritic Cu Catalyst for CO2 Electroreduction. ACS Catal. 2019, 9, 5496–5502. [Google Scholar] [CrossRef] [Green Version]
- Arán-Ais, R.M.; Rizo, R.; Grosse, P.; Algara-Siller, G.; Dembélé, K.; Plodinec, M.; Lunkenbein, T.; Chee, S.W.; Cuenya, B.R. Imaging electrochemically synthesized Cu2O cubes and their morphological evolution under conditions relevant to CO2 electroreduction. Nat. Commun. 2020, 11, 3489. [Google Scholar] [CrossRef] [PubMed]
- Reske, R.; Mistry, H.; Behafarid, F.; Roldan Cuenya, B.; Strasser, P. Particle Size Effects in the Catalytic Electroreduction of CO2 on Cu Nanoparticles. J. Am. Chem. Soc. 2014, 136, 6978–6986. [Google Scholar] [CrossRef] [PubMed]
- Lupan, O.; Emelchenko, G.A.; Ursaki, V.V.; Chai, G.; Redkin, A.N.; Gruzintsev, A.N.; Tiginyanu, I.M.; Chow, L.; Ono, L.K.; Roldan Cuenya, B.; et al. Synthesis and characterization of ZnO nanowires for nanosensor applications. Mater. Res. Bull. 2010, 45, 1026–1032. [Google Scholar] [CrossRef]
- Saáedi, A.; Yousefi, R.; Jamali-Sheini, F.; Zak, A.K.; Cheraghizade, M.; Mahmoudian, M.R.; Baghchesara, M.A.; Dezaki, A.S. XPS studies and photocurrent applications of alkali-metals-doped ZnO nanoparticles under visible illumination conditions. Phys. E Low Dimens. Syst. Nanostruct. 2016, 79, 113–118. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V., Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Zhou, W.; Lee, J.Y. Particle Size Effects in Pd-Catalyzed Electrooxidation of Formic Acid. J. Phys. Chem. C 2008, 112, 3789–3793. [Google Scholar] [CrossRef]
- Hu, S.; Scudiero, L.; Ha, S. Electronic effect on oxidation of formic acid on supported Pd–Cu bimetallic surface. Electrochim. Acta 2012, 83, 354–358. [Google Scholar] [CrossRef]
- Takashima, T.; Suzuki, T.; Irie, H. Electrochemical carbon dioxide reduction on copper-modified palladium nanoparticles synthesized by underpotential deposition. Electrochim. Acta 2017, 229, 415–421. [Google Scholar] [CrossRef]
- Takashima, T.; Suzuki, T.; Irie, H. Electrochemical Reduction of Carbon Dioxide to Formate on Palladium-Copper Alloy Nanoparticulate Electrode. Electrochemistry 2019, 87, 134–138. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Sadakiyo, M.; Heima, M.; Luo, R.; Haasch, R.T.; Gold, J.I.; Yamauchi, M.; Kenis, P.J.A. Electroreduction of Carbon Dioxide to Hydrocarbons Using Bimetallic Cu–Pd Catalysts with Different Mixing Patterns. J. Am. Chem. Soc. 2017, 139, 47–50. [Google Scholar] [CrossRef]
- Chen, A.; Ostrom, C. Palladium-Based Nanomaterials: Synthesis and Electrochemical Applications. Chem. Rev. 2015, 115, 11999–12044. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Wang, J.; Li, P.; Chang, K.; Li, C.; Wang, T.; Jiang, B.; Zhang, H.; Liu, H.; Yamauchi, Y.; et al. Mesoporous palladium–copper bimetallic electrodes for selective electrocatalytic reduction of aqueous CO2 to CO. J. Mater. Chem. A 2016, 4, 4776–4782. [Google Scholar] [CrossRef]
- Zheng, X.; De Luna, P.; García de Arquer, F.P.; Zhang, B.; Becknell, N.; Ross, M.B.; Li, Y.; Banis, M.N.; Li, Y.; Liu, M.; et al. Sulfur-Modulated Tin Sites Enable Highly Selective Electrochemical Reduction of CO2 to Formate. Joule 2017, 1, 794–805. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Ji, Y.; Tang, J.; Wang, J.; Liu, B.; Steinrück, H.-G.; Lim, K.; Li, Y.; Toney, M.F.; Chan, K.; et al. Theory-guided Sn/Cu alloying for efficient CO2 electroreduction at low overpotentials. Nat. Catal. 2018. [Google Scholar] [CrossRef]
- Siahrostami, S.; Verdaguer-Casadevall, A.; Karamad, M.; Deiana, D.; Malacrida, P.; Wickman, B.; Escudero-Escribano, M.; Paoli, E.A.; Frydendal, R.; Hansen, T.W.; et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 2013, 12, 1137–1143. [Google Scholar] [CrossRef] [Green Version]
- Nørskov, J.K.; Bligaard, T.; Rossmeisl, J.; Christensen, C.H. Towards the computational design of solid catalysts. Nat. Chem. 2009, 1, 37–46. [Google Scholar] [CrossRef]
- Zhang, C.; Liao, P.; Wang, H.; Sun, J.; Gao, P. Preparation of novel bimetallic CuZn-BTC coordination polymer nanorod for methanol synthesis from CO2 hydrogenation. Mater. Chem. Phys. 2018, 215, 211–220. [Google Scholar] [CrossRef]
- Venna, S.R.; Jasinski, J.B.; Carreon, M.A. Structural Evolution of Zeolitic Imidazolate Framework-8. J. Am. Chem. Soc. 2010, 132, 18030–18033. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuc, T.-V.; Chung, J.-S.; Hur, S.-H. Highly CO Selective Trimetallic Metal-Organic Framework Electrocatalyst for the Electrochemical Reduction of CO2. Catalysts 2021, 11, 537. https://doi.org/10.3390/catal11050537
Phuc T-V, Chung J-S, Hur S-H. Highly CO Selective Trimetallic Metal-Organic Framework Electrocatalyst for the Electrochemical Reduction of CO2. Catalysts. 2021; 11(5):537. https://doi.org/10.3390/catal11050537
Chicago/Turabian StylePhuc, Tran-Van, Jin-Suk Chung, and Seung-Hyun Hur. 2021. "Highly CO Selective Trimetallic Metal-Organic Framework Electrocatalyst for the Electrochemical Reduction of CO2" Catalysts 11, no. 5: 537. https://doi.org/10.3390/catal11050537






