Highly Selective Vapor and Liquid Phase Transfer Hydrogenation of Diaryl and Polycyclic Ketones with Secondary Alcohols in the Presence of Magnesium Oxide as Catalyst
Abstract
:1. Introduction
2. Results and Discussion
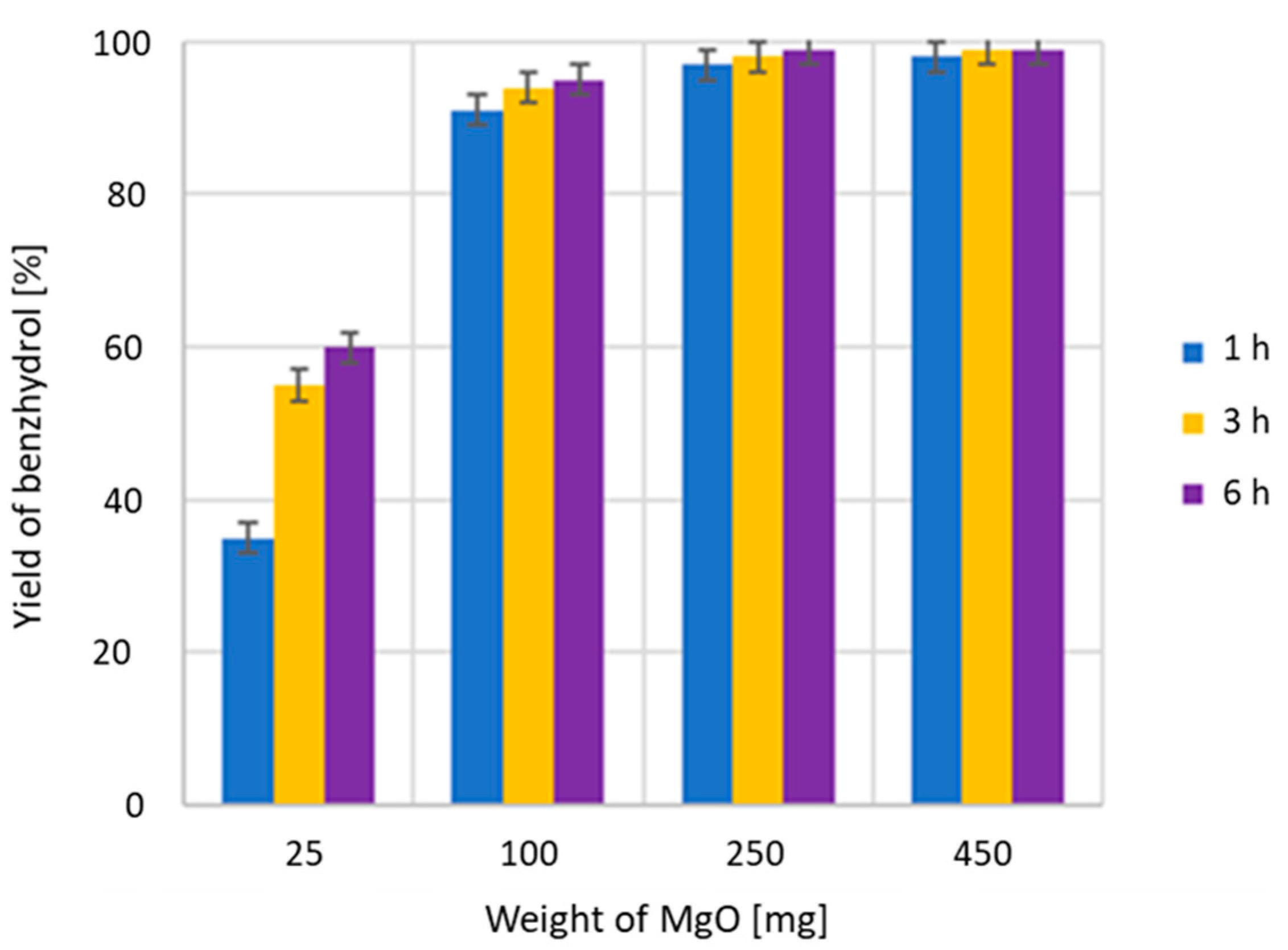
2.1. The Catalyst
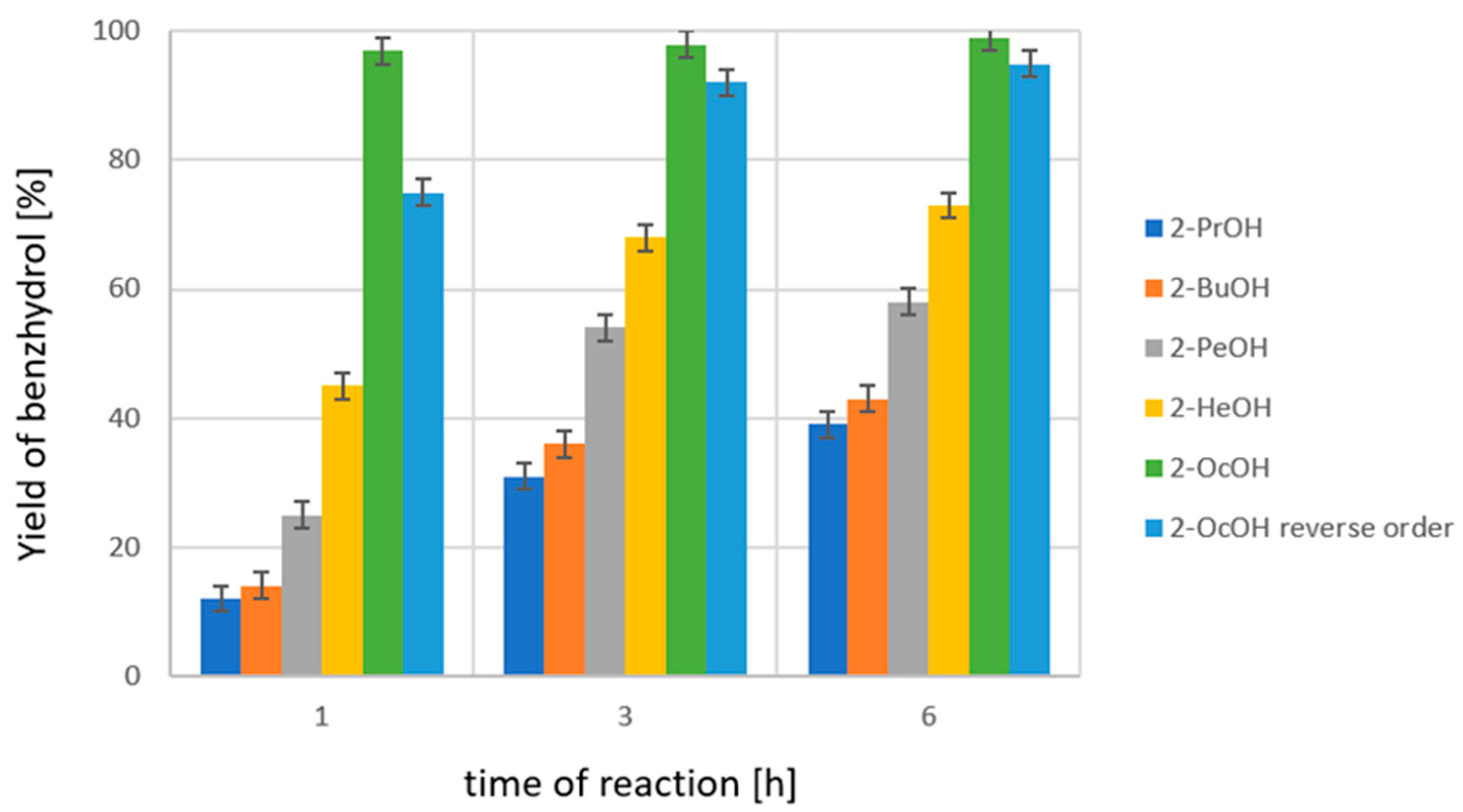
2.2. Liquid-Phase Transfer Tydrogenation of Diaryl Ketones


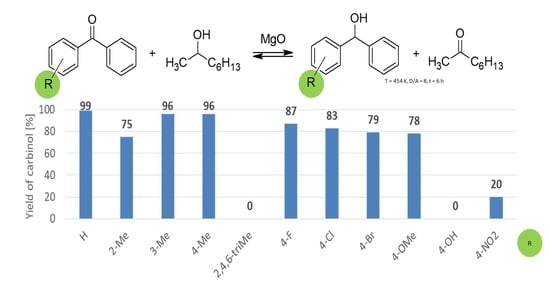
2.3. Vapour-Phase Transfer Hydrogenation of Diaryl Ketones

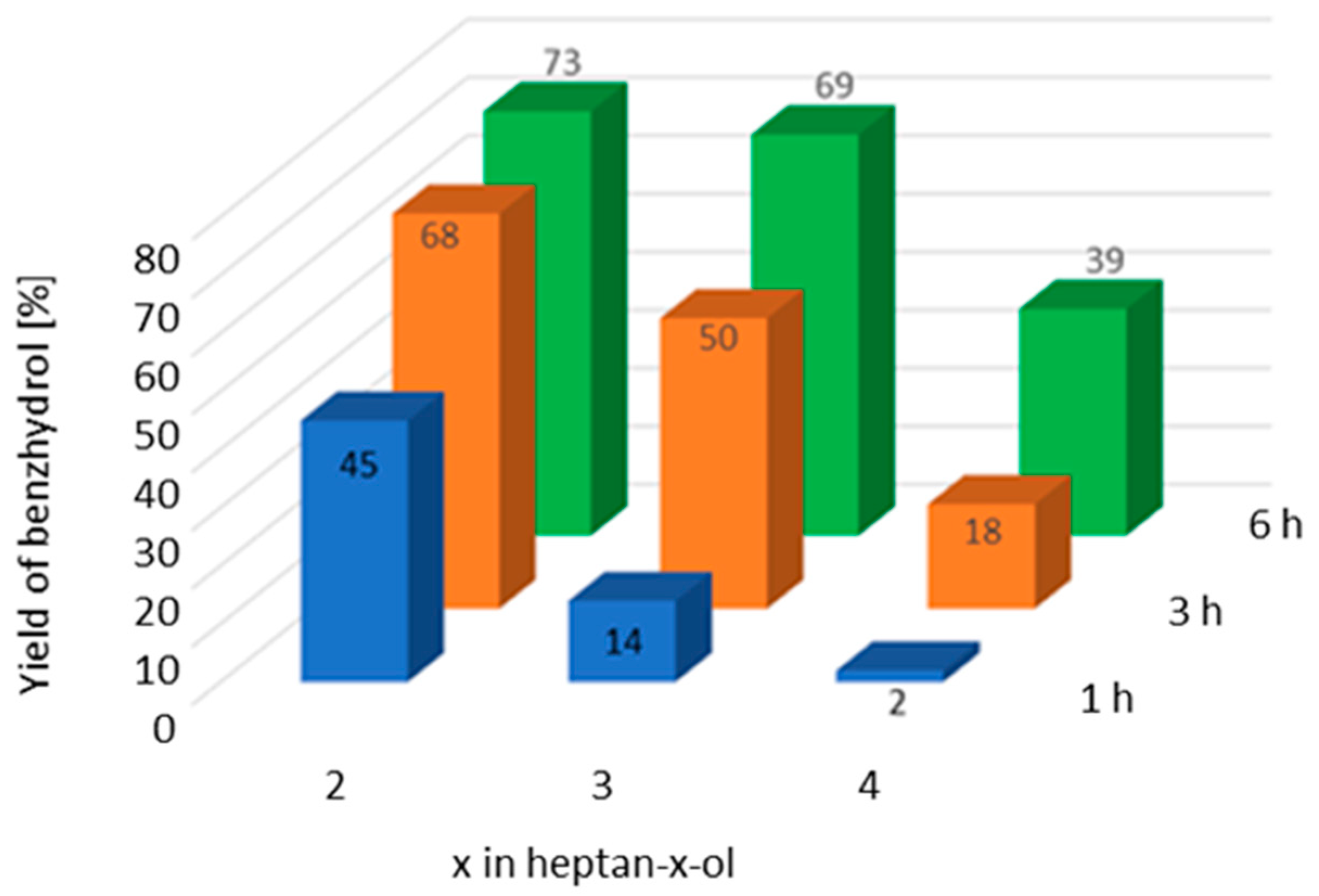
2.4. Liquid-Phase Transfer Hydrogenation of Polycyclic Ketones
- -
- cyclopentanone derivatives with one or two fused benzene rings: benzocyclopentenone (1-indanone) and dibenzocyclopentadienone (fluorenone);
- -
- cyclohexanone derivatives with one or two fused benzene rings: benzocyclohexenone (1-tetralone) and dibenzocyclohexadienone (anthrone).

3. Materials and Methods
3.1. Preparation of MgO and Its Characterization
3.2. Hydrogen Acceptors
3.3. Hydrogen Donors
3.4. Catalytic Activity Measurements
3.5. Analytical Determinations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lan, X.; Wang, T. Highly Selective Catalysts for the Hydrogenation of Unsaturated Aldehydes: A Review. ACS Catal. 2020, 10, 2764–2790. [Google Scholar] [CrossRef]
- Chen, H.-J.; Chiu, C.-C.; Wang, T.; Lee, D.-S.; Lu, T.-J. Bis-NHC–Ag/Pd(OAc)2 Catalytic System Catalyzed Transfer Hydrogenation Reaction. Catalysts 2021, 11, 8. [Google Scholar] [CrossRef]
- Putrakumar, B.; Seelam, P.K.; Srinivasarao, G.; Rajan, K.; Rajesh, R.; Rao, K.R.; Liang, T. High Performance and Sustainable Copper-Modified Hydroxyapatite Catalysts for Catalytic Transfer Hydrogenation of Furfural. Catalysts 2020, 10, 1045. [Google Scholar] [CrossRef]
- Li, F.; France, L.J.; Cai, Z.; Li, Y.; Liu, S.; Lou, H.; Long, J.; Li, X. Catalytic transfer hydrogenation of butyl levulinate to γ-valerolactone over zirconium phosphates with adjustable Lewis and Brønsted acid sites. Appl. Catal. B Environ. 2017, 214, 67–77. [Google Scholar] [CrossRef]
- Mihet, M.; Dan, M.; Barbu-Tudoran, L.; Lazar, D. CO2 Methanation Using Multimodal Ni/SiO2 Catalysts: Effect of Support Modification by MgO, CeO2, and La2O3. Catalysts 2021, 11, 443. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, P.; Wang, J.; Tan, T. Guerbet Reactions for Biofuel Production from ABE Fermentation Using Bifunctional Ni-MgO-Al2O3 Catalysts. Catalysts 2021, 11, 414. [Google Scholar] [CrossRef]
- Ishikawa, A.; Tateyama, Y. Hybrid Functional Study of H-Abstraction from Methane by Li-Doped, Pristine and Stepped MgO(100) and MgO(110) Surfaces. Catal. Lett. 2021, 151, 627–633. [Google Scholar] [CrossRef]
- Di Cosimo, J.I.; Acosta, A.; Apesteguia, C.R. Allylic alcohol synthesis by gas-phase hydrogen transfer reduction of unsaturated ketones. J. Mol. Catal. A Chem. 2005, 234, 111–120. [Google Scholar] [CrossRef]
- Gliński, M.; Ulkowska, U. Vapour phase transfer hydrogenation of α,β-unsaturated carbonyl compounds. Thermodynamic and experimental studies. Appl. Catal. A Gen. 2016, 511, 131–140. [Google Scholar] [CrossRef]
- Gliński, M.; Ulkowska, U. Description of the structure-chemoselectivity relationship in the transfer hydrogenation of α,β-unsaturated aldehydes and ketones with alcohols in the presence of magnesium oxide. Appl. Catal. A Gen. 2018, 554, 117–124. [Google Scholar] [CrossRef]
- Szöllösi, G.; Bartok, M. Vapour-phase heterogeneous catalytic transfer hydrogenation of alkyl methyl ketones on MgO: Prevention of the deactivation of MgO in the presence of carbon tetrachloride. Appl. Catal. A Gen. 1998, 169, 263–269. [Google Scholar] [CrossRef]
- Gliński, M.; Ulkowska, U. Reactivity of Alcohols in Chemoselective Transfer Hydrogenation of Acrolein over Magnesium Oxide as the Catalyst. Catal. Lett. 2011, 141, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Koppadi, K.S.; Chada, R.R.; Enumula, S.S.; Marella, R.K.; Rao, K.S.R.; Burri, D.R. Metal-Free Hydrogenation of Biomass Derived Furfural into Furfuryl Alcohol Over Carbon–MgO Catalysts in Continuous Mode. Catal. Lett. 2017, 147, 1278–1284. [Google Scholar] [CrossRef]
- Blair Vasquez, P.; Tabanelli, T.; Monti, E.; Albonetti, S.; Bonincontro, D.; Dimitratos, N.; Cavani, F. Gas-Phase Catalytic Transfer Hydrogenation of Methyl Levulinate with Ethanol over ZrO2. ACS Sustain. Chem. Eng. 2019, 7, 8317–8330. [Google Scholar] [CrossRef]
- Iglesias, J.; Melero, J.A.; Morales, G.; Paniagua, M.; Hernández, B.; Osatiashtiani, A.; Lee, A.F.; Wilson, K. ZrO2-SBA-15 catalysts for the one-pot cascade synthesis of GVL from furfural. Catal. Sci. Technol. 2018, 8, 4485–4493. [Google Scholar] [CrossRef] [Green Version]
- Shibagaki, M.; Takahashi, K.; Matsushita, H. The catalytic reduction of aldehydes and ketones with 2-propanol over hydrous zirconium oxide. Bull. Chem. Soc. Jpn. 1988, 61, 3283–3288. [Google Scholar] [CrossRef] [Green Version]
- Posner, G.H.; Runquist, A.W.; Chapdelaine, M.J. Organic reactions at alumina surfaces. 6. Isopropyl alcohol and diisopropylcarbinol on dehydrated alumina as reagents for very selective carbonyl reductions. J. Org. Chem. 1977, 42, 1202–1208. [Google Scholar] [CrossRef]
- Zhou, Y.; Chuah, G.; Janicke, S. Chemo- and regioselective Meerwein–Ponndorf–Verley and Oppenauer reactions catalyzed by Al-free Zr-zeolite beta. J. Catal. 2004, 227, 1–10. [Google Scholar] [CrossRef]
- Corma, A.; Domine, M.E.; Valencia, S. Water-resistant solid Lewis acid catalysts: Meerwein–Ponndorf–Verley and Oppenauer reactions catalyzed by tin-beta zeolite. J. Catal. 2003, 215, 294–304. [Google Scholar] [CrossRef]
- Ruiz, J.R.; Jimenez-Sanchidrian, C.; Hidalgo, J.M.; Marinas, J.M. Reduction of ketones and aldehydes to alcohols with magnesium–aluminium mixed oxide and 2-propanol. J. Mol. Catal. A Chem. 2006, 246, 190–194. [Google Scholar] [CrossRef]
- Aramendia, M.A.; Borau, V.; Jimenez, C.; Marinas, J.M.; Ruiz, J.R.; Urbano, F. Meerwein–Ponndorf–Verley reduction of cycloalkanones over magnesium-aluminium oxide. JCS Perkin Trans. 2002, 2, 1122–1125. [Google Scholar] [CrossRef]
- Aramendia, M.A.; Borau, V.; Jimenez, C.; Marinas, J.M.; Ruiz, J.R.; Urbano, F.J. Catalytic hydrogen transfer from 2-propanol to cyclohexanone over basic Mg-Al oxides. Appl. Catal. A Gen. 2003, 255, 301–308. [Google Scholar] [CrossRef]
- Jimenez-Sanchidrian, C.; Hidalgo, J.M.; Ruiz, J.R. Reduction of heterocyclic carboxaldehydes via Meerwein-Ponndorf-Verley reaction. Appl. Catal. A Gen. 2006, 303, 23–28. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Z.; Leng, X.; Zhu, H.; Liu, G.; Huang, Z. Transfer Hydrogenation of Alkenes Using Ethanol Catalyzed by a NCP Pincer Iridium Complex: Scope and Mechanism. J. Am. Chem. Soc. 2018, 140, 4417–4429. [Google Scholar] [CrossRef]
- Ramos, R.; Peixoto, A.F.; Arias-Serrano, B.I.; Soares, O.S.G.P.; Pereira, M.F.R.; Kubička, D.; Freire, C. Catalytic Transfer Hydrogenation of Furfural over Co3O4−Al2O3 Hydrotalcite-derived Catalyst. ChemCatChem 2019, 12, 1467–1475. [Google Scholar] [CrossRef]
- Aramendia, M.A.; Borau, V.; Jimenez, C.; Marinas, J.M.; Urbano, F.J. The Meerwein–Ponndorf–Verley–Oppenauer reaction between 2-hexanol and cyclohexanone on magnesium phosphates. Catal. Lett. 1999, 58, 53–58. [Google Scholar] [CrossRef]
- Ruiz, J.R.; Jimenez-Sanchidrian, C. Heterogeneous Catalysis in the Meerwein–Ponndorf–Verley Reduction of Carbonyl Compounds. Curr. Org. Chem. 2007, 11, 1113–1125. [Google Scholar] [CrossRef]
- Chuah, G.K.; Jaenicke, S.; Zhu, Y.Z.; Liu, S.H. Meerwein–Ponndorf–Verley Reduction over Heterogeneous Catalysts. Curr. Org. Chem. 2006, 10, 1639–1654. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Ohkuma, T.; Noyori, R. Hydrogenation of Carbonyl Groups in Comprehensive Asymmetric Catalysis; Jacobsen, E.N., Pfaltz, A., Yamamoto, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 199–246. [Google Scholar]
- Nishimura, S. Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis; Wiley-VCh: New York, NY, USA, 2001. [Google Scholar]
- Grauw, C.F.; Peters, J.A.; Van Bekkum, H.; Huskens, J. Meerwein–Ponndorf–Verley Reductions and Oppenauer Oxidations: An Integrated Approach. Synthesis 1994, 10, 1007–1017. [Google Scholar] [CrossRef]
- Gliński, M.; Gadomska, A. Catalytic hydrogen transfer over magnesia. IX. Reduction of long chain aliphatic ketones by 2-propanol. React. Kinet. Catal. Lett. 1998, 65, 121–129. [Google Scholar] [CrossRef]
- Gliński, M. Structure-reactivity relationship in transfer hydrogenation of aliphatic ketones over magnesium oxide. React. Kinet. Mechan. Catal. 2009, 97, 275–279. [Google Scholar] [CrossRef]
- Kumbhar, P.S.; Sanchez-Valente, J.; Lopez, J.; Figueras, F. Meerwein–Ponndorf–Verley reduction of carbonyl compounds catalysed by Mg–Al hydrotalcite. Chem. Commun. 1998, 535–536. [Google Scholar] [CrossRef]
- Gliński, M. Highly diastereoselective transfer hydrogenation of 4-t-butylcyclohexanone in the presence of magnesium oxide. React. Kinet. Mechan. Catal. 2010, 99, 93–98. [Google Scholar] [CrossRef]
- Ruiz, J.R.; Jimenez-Sanchidrian, C.; Hidalgo, J.M. Meerwein–Ponndorf–Verley reaction of acetophenones with 2-propanol over MgAl mixed oxide: The substituent effect. Catal. Commun. 2007, 8, 1036–1040. [Google Scholar] [CrossRef]
- Gliński, M. Catalytic hydrogen transfer over magnesia: Vapour and liquid phase reduction of various aralkyl ketones. Appl. Catal. A Gen. 2008, 349, 133–139. [Google Scholar] [CrossRef]
- Ueshima, M.; Shimasaki, Y. Regioselective Reduction of α,β-Unsaturated Carbonyl Compounds to Allylic Alcohols by Vapor Phase Hydrogen Transfer Reaction over MgO-B2O3 Catalyst. Chem. Lett. 1992, 1345–1348. [Google Scholar] [CrossRef]
- Aramendia, M.A.; Borau, V.; Jimenez, C.; Marinas, J.M.; Ruiz, J.R.; Urbano, F.J. Liquid-phase heterogeneous catalytic transfer hydrogenation of citral on basic catalysts. J. Mol. Catal. A Chem. 2001, 171, 153–158. [Google Scholar] [CrossRef]
- Aramendia, M.A.; Borau, V.; Jimenez, C.; Marinas, J.M.; Ruiz, J.R.; Urbano, F.J. Reduction of α,β-unsaturated aldehydes with basic MgO/M2O3 catalysts (M=Al, Ga, In). Appl. Catal. A Gen. 2003, 249, 1–9. [Google Scholar] [CrossRef]
- Brown, F.; Di Cosimo, J.I. Catalytic and spectroscopic study of the allylic alcohol synthesis by gas–phase hydrogen transfer reduction of unsaturated ketones on acid–base catalysts. Catal. Today 2006, 116, 206–215. [Google Scholar] [CrossRef]
- Ramana, D.V.; Pillai, C.N. Hydrogen transfer reactions. I. Reduction of carbonyl compounds by alcohols catalyzed by alumina. Can. J. Chem. 1969, 47, 3705–3707. [Google Scholar] [CrossRef]
- Jyothi, T.M.; Raja, T.; Rao, B.S. Catalytic transfer reduction (CTR) of alkyl alkyl, alkyl aryl, cyclic and unsaturated ketones over calcined Mg–Al hydrotalcites. J. Mol. Catal. A Chem. 2001, 168, 187–191. [Google Scholar] [CrossRef]
- Matsushita, H.; Ishiguro, S.; Ichinose, H.; Izumi, A.; Mizusaki, S. reduction of aldehydes and ketones to alcohols with hydrous zirconium oxide and 2-propanol. Chem. Lett. 1985, 731–735. [Google Scholar] [CrossRef]
- Iwanek, E.; Ulkowska, U.; Gliński, M. Surface studies of magnesium oxide—Based catalysts modified with X2 or MgX2 (X = Br, I). Surf. Interface Anal. 2015, 47, 1001–1008. [Google Scholar] [CrossRef]
- Meyer, K.H. Anthrone. Organic Syntheses Procedure. Org. Synth. 1928, 8, 8. [Google Scholar] [CrossRef]


| Line No. | Substituent | Yield of Alcohol a,b [%]/Reaction Time [h] | ||
|---|---|---|---|---|
| 1 | 3 | 6 | ||
| 1 | - | 97 | 98 | 99 |
| 2 | 2-Me | 5 | 27 | 75 |
| 3 | 3-Me | 74 | 88 | 96 |
| 4 | 4-Me | 81 | 94 | 99 |
| 5 | 2,4,6-triMe | 0 | 0 | 0 |
| 6 | 4-F c | 68 d | 88 | 87 e |
| 7 | 4-Cl c | 82 f | 85 | 83 g |
| 8 | 4-Br c | 87 h,i | 84 | 79 |
| 9 | j | 3 k | 10 | 18 |
| 10 | 4-OMe | 43 | 76 | 78 |
| 11 | 4-OH | 0 | 0 | 0 |
| 12 | 4-NO2 | - | 6 l,m | 20 l,n |
| 13 | 2-NpPh ° | 39 | 87 | 94 |
| Donor | T [K] | Yield of PhCH(OH)Ph-Br a [%]/Reaction Time [h] | ||
|---|---|---|---|---|
| 1 | 3 | 6 | ||
| 2-PrOH | 355 | 2.5/1.4 b | 10.2/1.7 | 17.9/2.0 |
| 2-PeOH | 393 | 31.1/2.3 b | 59.5/2.5 | 73.7/2.7 |
| 2-OcOH | 454 | 66.7/3.4 b | 70.7/3.8 | 66.5/4.4 |
| Line No. | Substituents | Conversion [%]/Yield of Alcohol [%] a | |||
|---|---|---|---|---|---|
| 573 K | 623 K | 673 K | 723 K | ||
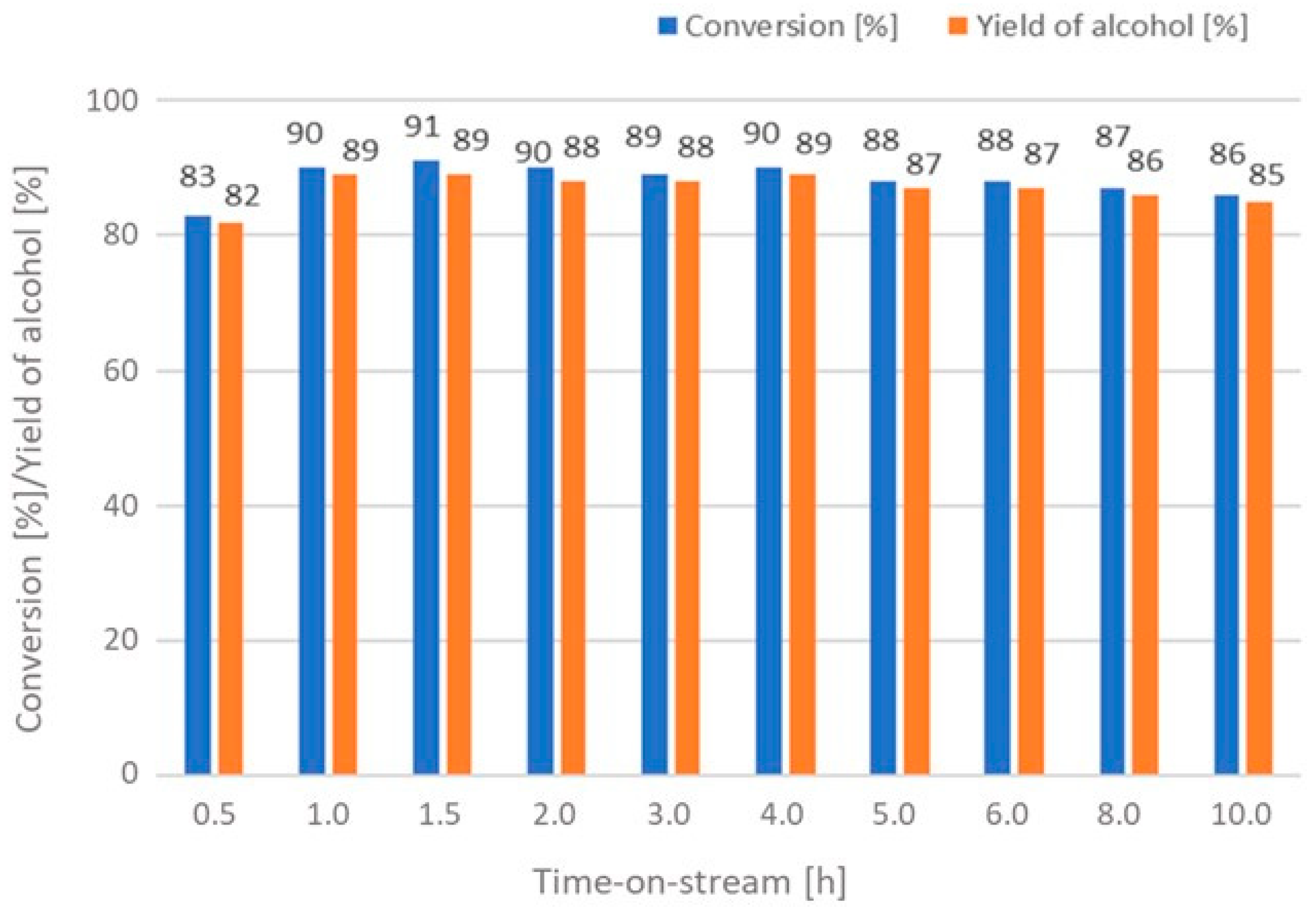
| 1 | - | 90/89 | 90/89 | 76/68 | 72/43 |
| 2 | - b | 89/88 | 91/89 | - | - |
| 3 | - c | 62/62 | 68/67 | 54/51 | 39/16 |
| 4 | - d | 52/52 | 61/57 | 66/59 | 60/44 |
| 5 | 2-Me | 75/74 | 75/73 | 69/62 | 67/44 |
| 6 | 3-Me | 90/89 | 88/83 | 81/78 | 74/46 |
| 7 | 4-Me | 89/88 | 85/80 | 78/56 | 78/41 |
| Ketone | Donor | Yield of Alcohol [%] a/Reaction Time [h] | ||
|---|---|---|---|---|
| 1 | 3 | 4 | ||
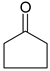 | 2-PrOH | 1 b | 2 | 3 |
 | 2-PrOH | 8 c | 12 | 14 |
| 2-OcOH | 43 c | 45 | 45 | |
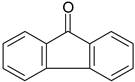 | 2-PrOH | 49 d | 74 c | 78 |
| 2-OcOH | 82 c | 82 | 78 | |
| Ketone | Donor | Yield of Alcohol [%] a/Reaction Time [h] | ||
|---|---|---|---|---|
| 1 | 3 | 4 | ||
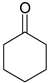 | 2-PrOH | 52 b | 58 | 61 |
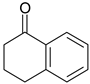 | 2-PrOH | 10 c | 13 | 14 |
| 2-OcOH | 53 c | 58 | 59 | |
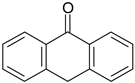 | 2-PrOH | 0 | - | 0 |
| 2-OcOH | 0 | - | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gliński, M.; Markowska, A.; Wrońska, L.; Jerzak, A.; Tarkowska, M. Highly Selective Vapor and Liquid Phase Transfer Hydrogenation of Diaryl and Polycyclic Ketones with Secondary Alcohols in the Presence of Magnesium Oxide as Catalyst. Catalysts 2021, 11, 574. https://doi.org/10.3390/catal11050574
Gliński M, Markowska A, Wrońska L, Jerzak A, Tarkowska M. Highly Selective Vapor and Liquid Phase Transfer Hydrogenation of Diaryl and Polycyclic Ketones with Secondary Alcohols in the Presence of Magnesium Oxide as Catalyst. Catalysts. 2021; 11(5):574. https://doi.org/10.3390/catal11050574
Chicago/Turabian StyleGliński, Marek, Anna Markowska, Laura Wrońska, Anna Jerzak, and Magdalena Tarkowska. 2021. "Highly Selective Vapor and Liquid Phase Transfer Hydrogenation of Diaryl and Polycyclic Ketones with Secondary Alcohols in the Presence of Magnesium Oxide as Catalyst" Catalysts 11, no. 5: 574. https://doi.org/10.3390/catal11050574