Hollow-Shell-Structured Mesoporous Silica-Supported Palladium Catalyst for an Efficient Suzuki-Miyaura Cross-Coupling Reaction
Abstract
:1. Introduction
2. Results and Discussion
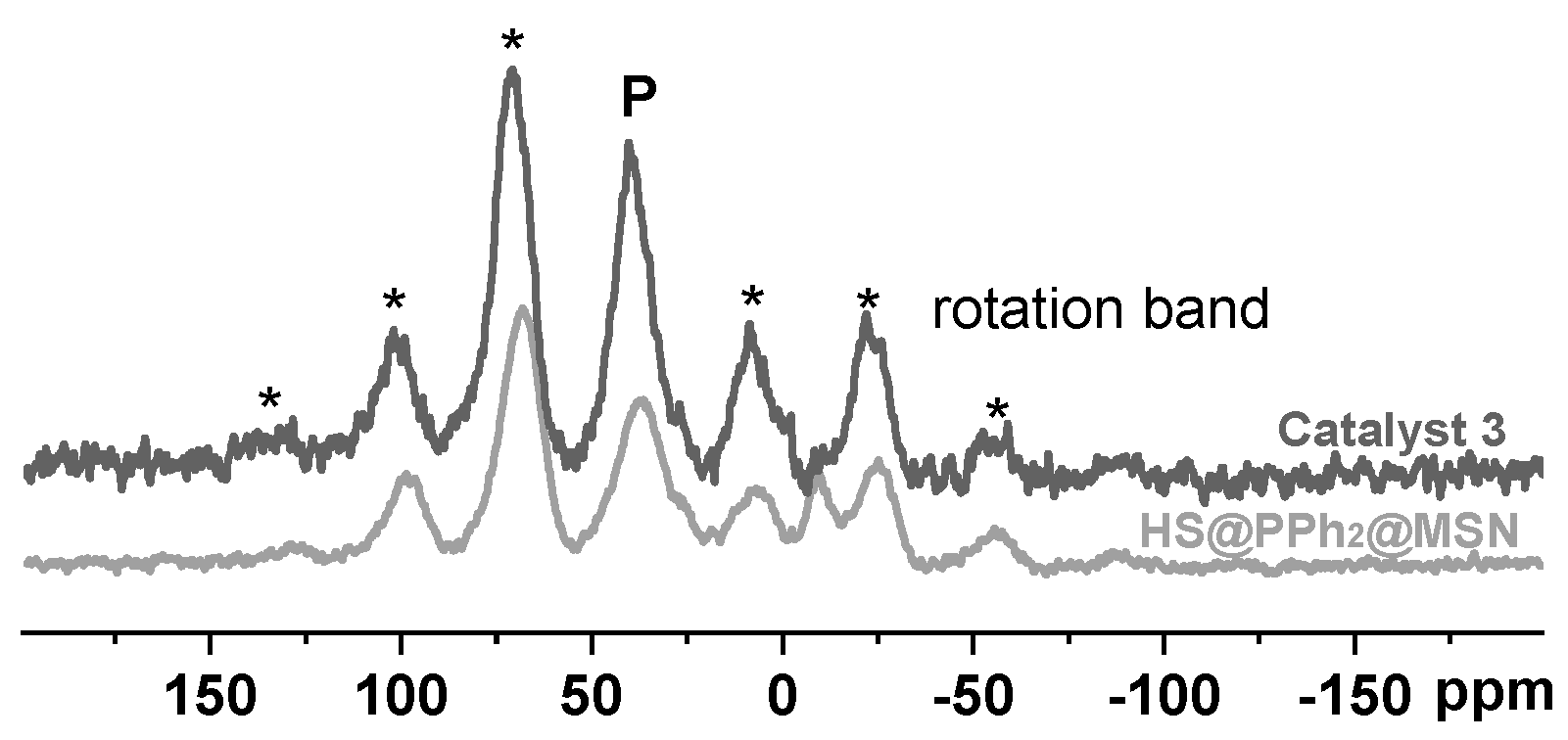
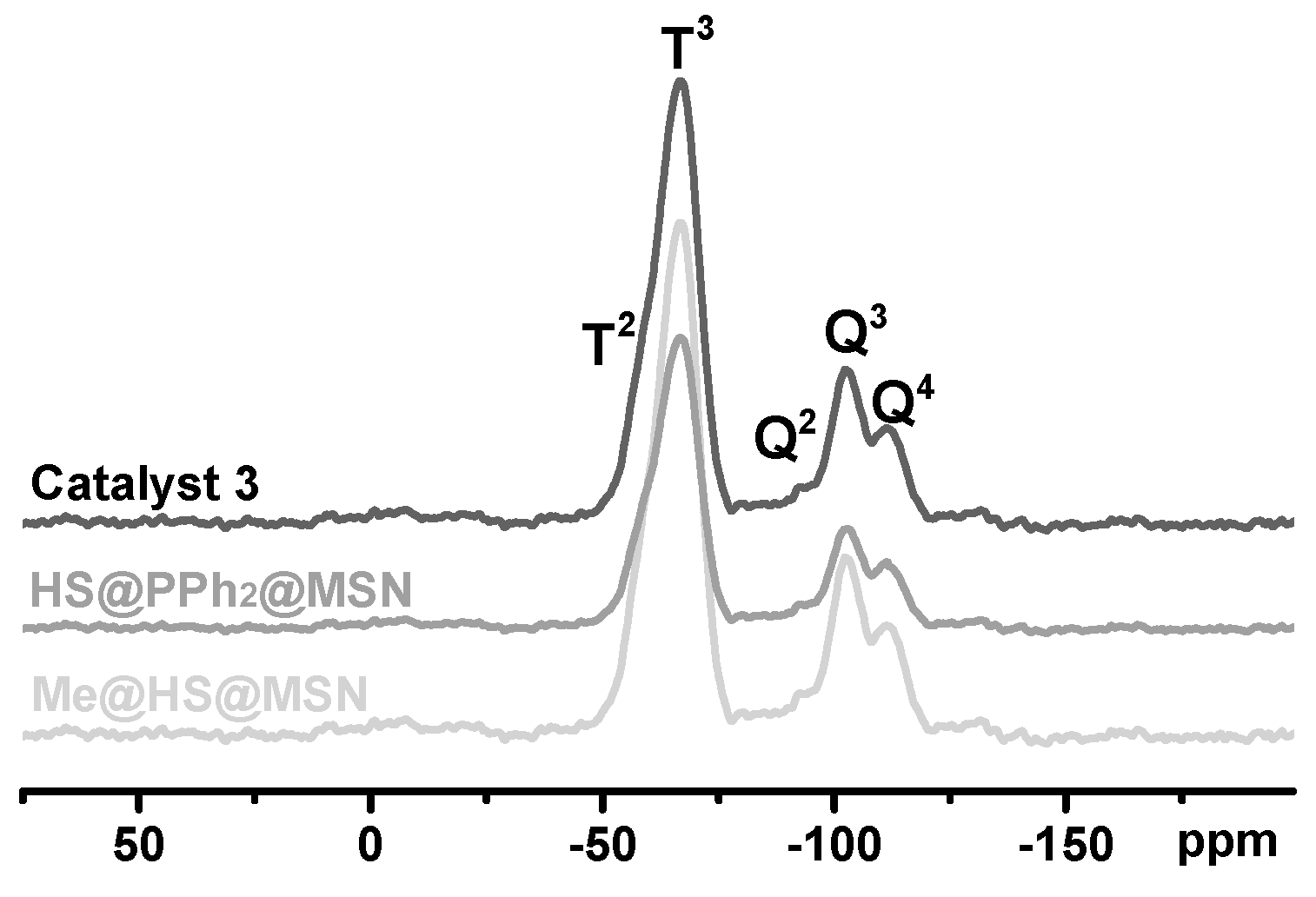
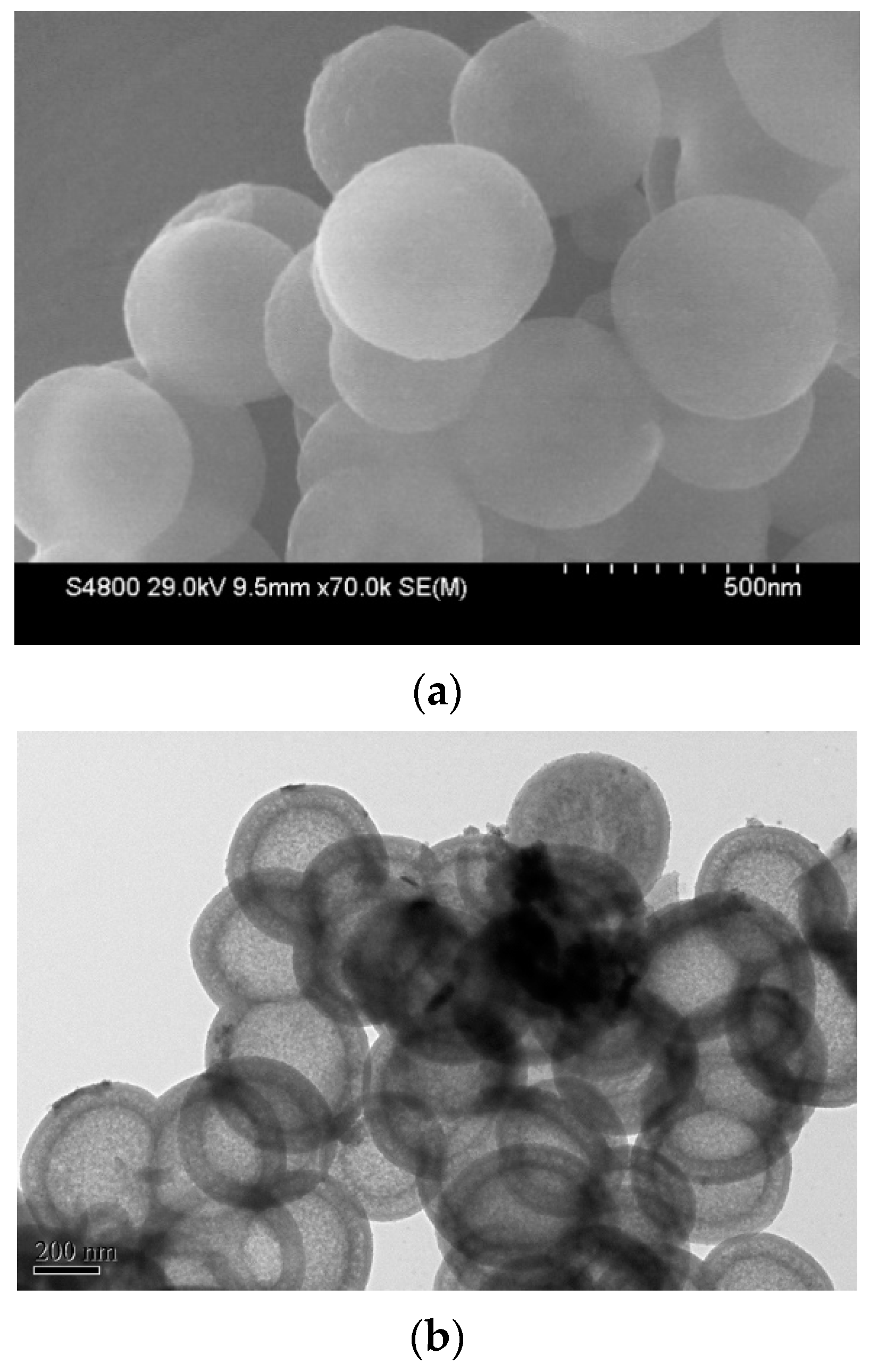
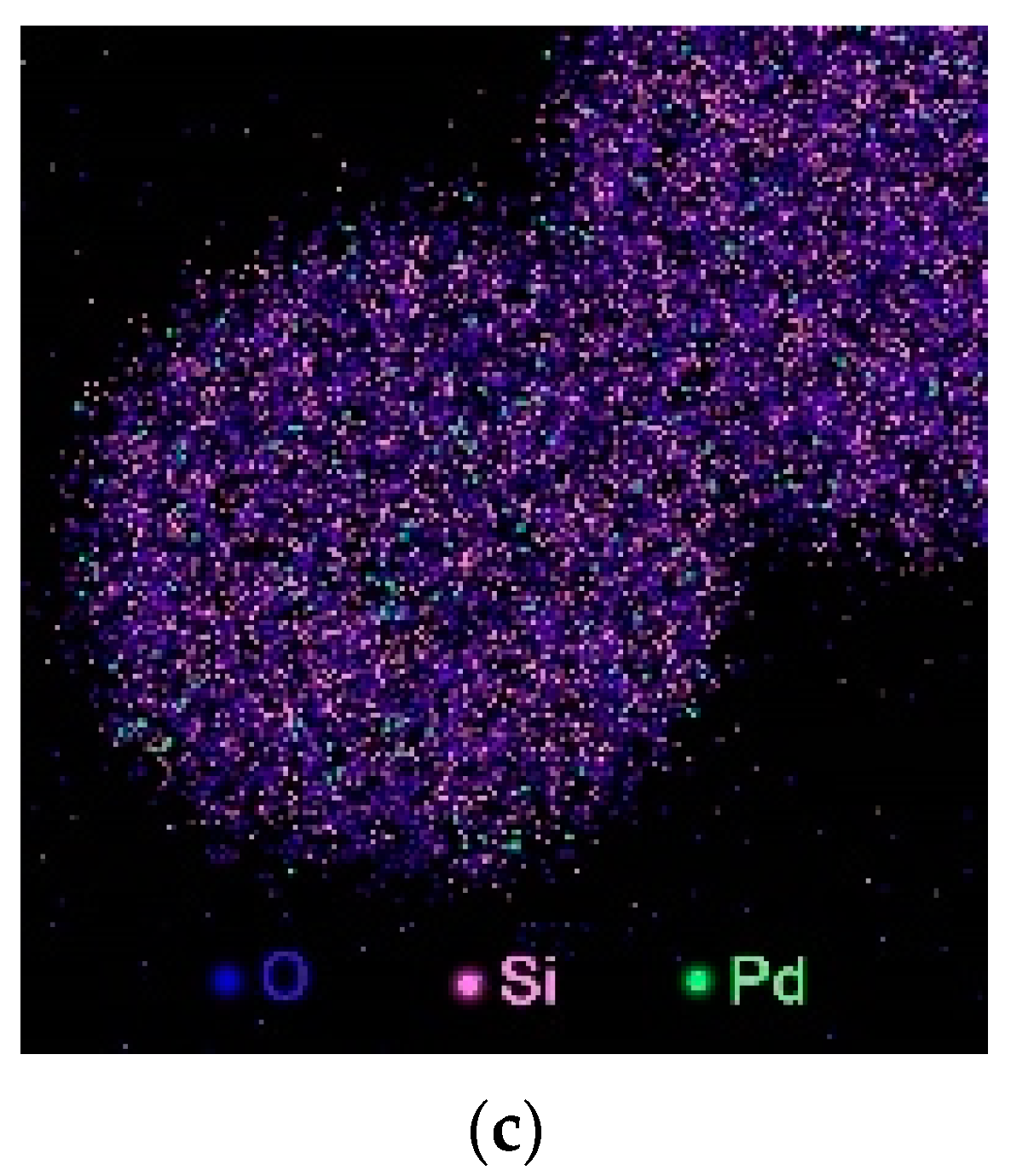
2.1. Synthesis and Structural Characterization of the Hollow-Shell Catalyst
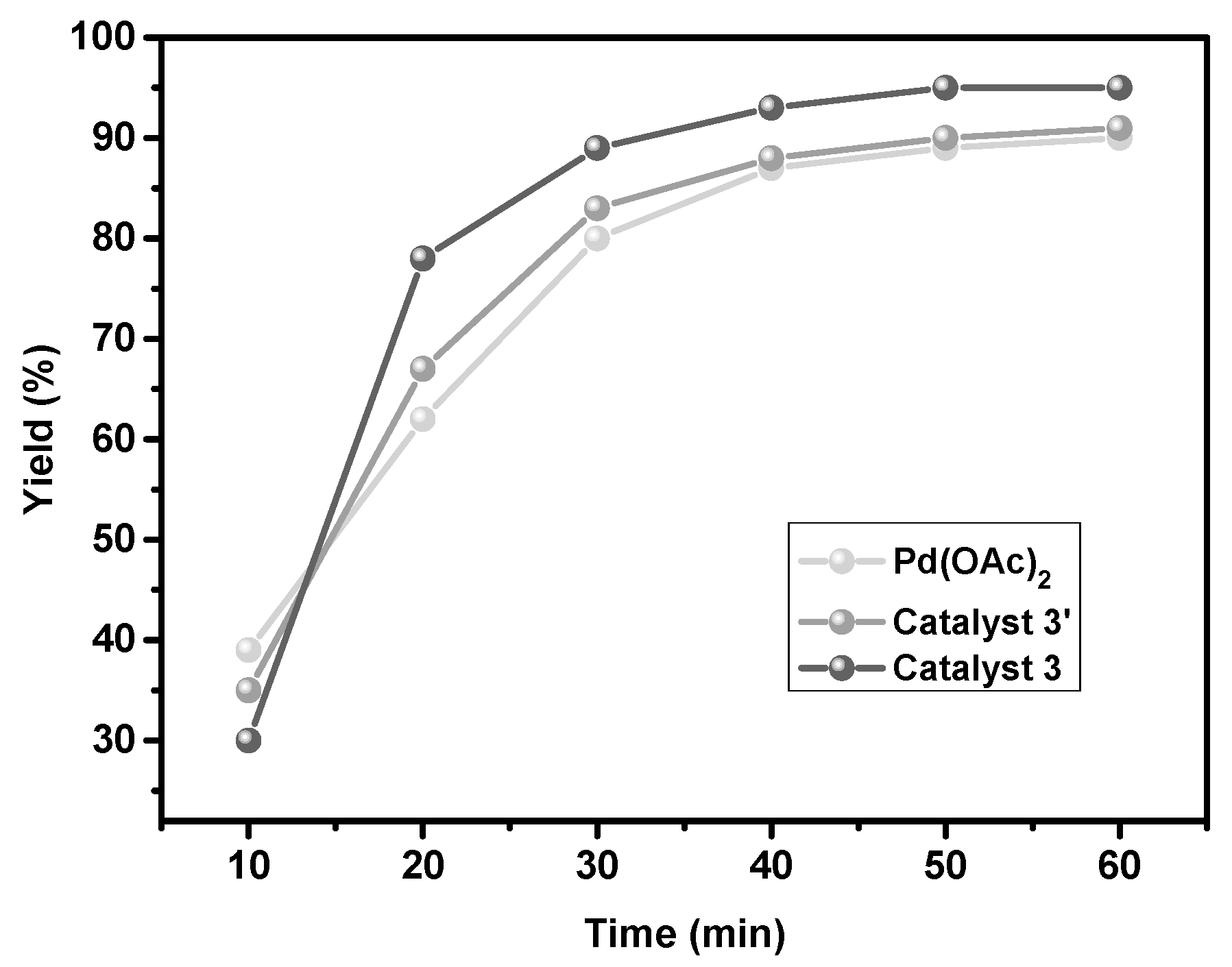
2.2. Catalytic Performance of the Heterogeneous Catalyst
3. Experimental
3.1. Characterization
3.2. Preparation of the Catalyst 3
3.3. General Procedure for the Suzuki-Miyaura Cross-Coupling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chen, B.W.J.; Xu, L.; Mavrikakis, M. Computational Methods in Heterogeneous Catalysis. Chem. Rev. 2021, 121, 1007–1048. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, N.; Misono, M. Heterogeneous Catalysis. Chem. Rev. 1998, 98, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Garcí, H.; Xamena, F.X.L.I. Engineering Metal-Organic Framework for Heterogeneous Catalysis. Chem. Rev. 2010, 110, 4606. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef] [Green Version]
- Nicolaou, K.C.; Bulger, P.G.; Sarlah, D. Palladium-Catalyzed Cross-Coupling Reactions in Total Synthesis. Angew. Chem. Int. Ed. 2005, 44, 4442–4489. [Google Scholar] [CrossRef] [PubMed]
- Leblond, C.R.; Andrews, A.T.; Sun, Y.; Sowa, J.R. Activation of Aryl Chlorides for Suzuki Cross-Coupling by Ligandless, Heterogeneous Palladium. Org. Lett. 2001, 3, 1555–1557. [Google Scholar] [CrossRef]
- Seechurn, C.C.C.J.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef]
- Kotha, S.; Lahiri, K.; Kashinath, D. Recent applications of the Suzuki–Miyaura cross-coupling reaction in organic synthesis. Tetrahedron 2002, 58, 9633–9695. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.; Ward, T.R. Recent Advances in the Palladium Catalyzed Suzuki–Miyaura Cross-Coupling Reaction in Water. Catal. Lett. 2016, 146, 820–840. [Google Scholar] [CrossRef] [Green Version]
- Marin, R.; Buchwald, S.L. Palladium-Catalyzed Suzuki-Miyaura Cross Coupling Reactions Employing Dialkylbiaryl Phosphine Ligands. Acc. Chem. Res. 2008, 41, 1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lennox, A.J.J.; Lloyd-Jones, G.C. Selection of boron reagents for Suzuki–Miyaura coupling. Chem. Soc. Rev. 2014, 43, 412–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maluenda, I.; Navarro, O. Recent Developments in the Suzuki-Miyaura Reaction: 2010–2014. Molecules 2015, 20, 7528–7557. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Jones, G.O.; Watson, D.A.; Bhayana, B.; Buchwald, S.L. Enantioselective Synthesis of Axially Chiral Biaryls by the Pd-Catalyzed Suzuki−Miyaura Reaction: Substrate Scope and Quantum Mechanical Investigations. J. Am. Chem. Soc. 2010, 132, 11278–11287. [Google Scholar] [CrossRef] [Green Version]
- Lebel, H.; Janes, M.K.; Charette, A.A.B.; Nolan, S.P. Structure and Reactivity of “Unusual” N-Heterocyclic Carbene (NHC) Palladium Complexes Synthesized from Imidazolium Salts. J. Am. Chem. Soc. 2004, 126, 5046–5047. [Google Scholar] [CrossRef] [PubMed]
- Chalker, J.M.; Wood, C.S.C.; Davis, B.G. A Convenient Catalyst for Aqueous and Protein Suzuki−Miyaura Cross-Coupling. J. Am. Chem. Soc. 2009, 131, 16346–16347. [Google Scholar] [CrossRef] [Green Version]
- Lima, C.F.R.A.C.; Rodrigues, A.S.M.C.; Silva, A.M.S.; Santos, L. Role of the Base and Control of Selectivity in the Suzuki-Miyaura Cross-Coupling Reaction. ChemCatChem 2014, 6. [Google Scholar] [CrossRef]
- Zapf, A.; Ehrentraut, A.; Beller, M. A New Highly Efficient Catalyst System for the Coupling of Nonactivated and Deactivated Aryl Chlorides with Arylboronic Acid. Angew. Chem. Int. Ed. 2000, 39, 4153. [Google Scholar] [CrossRef]
- Wünsche, M.A.; Mehlmann, P.; Witteler, T.; Buß, F.; Rathmann, P.; Dielmann, F. Imidazolin-2-ylidenaminophosphines as Highly Electron-Rich Ligands for Transition-Metal Catalysts. Angew. Chem. Int. Ed. 2015, 54, 11857–11860. [Google Scholar] [CrossRef]
- Fujihara, T.; Yoshida, S.; Ohta, H.; Tsuji, Y. Triarylphosphanes with Dendritically Arranged Tetraethylene Glycol Moieties at the Periphery: An Efficient Ligand for the Palladium-Catalyzed Suzuki-Miyaura Coupling Reaction. Angew. Chem. Int. Ed. 2008, 47, 8310–8314. [Google Scholar] [CrossRef]
- Botella, L.; Naájera, C. A Convenient Oxime-CarbapalladacycleCatalyzed Suzuki Cross-Coupling of Aryl Chlorides in Water. Angew. Chem. Int. Ed. 2002, 41, 179. [Google Scholar] [CrossRef]
- Snelders, D.J.M.; van Koten, G.; Gebbink, R.J.M.K. Hexacationic Dendriphos Ligands in the Pd-Catalyzed Suzuki−Miyaura Cross-Coupling Reaction: Scope and Mechanistic Studies. J. Am. Chem. Soc. 2009, 131, 11407–11416. [Google Scholar] [CrossRef] [PubMed]
- George, S.M. Introduction: Heterogeneous Catalysis. Chem. Rev. 1995, 95, 475–476. [Google Scholar] [CrossRef]
- Crudden, C.M.; Sateesh, M.; Lewis, R. Mercaptopropyl-Modified Mesoporous Silica: A Remarkable Support for the Preparation of a Reusable, Heterogeneous Palladium Catalyst for Coupling Reactions. J. Am. Chem. Soc. 2005, 127, 10045–10050. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.-J.; Lee, D.-H. A Practical Heterogeneous Catalyst for the Suzuki, Sonogashira, and Stille Coupling Reactions of Unreactive Aryl Chlorides. Angew. Chem. Int. Ed. 2010, 49, 1119–1122. [Google Scholar] [CrossRef]
- Qiu, H.; Sarkar, S.M.; Lee, D.-H.; Jin, M.-J. Highly effective silica gel-supported N-heterocyclic carbene–Pd catalyst for Suzuki–Miyaura coupling reaction. Green Chem. 2008, 10, 37–40. [Google Scholar] [CrossRef]
- Liu, Y.; Lou, B.; Shangguan, L.; Cai, J.; Zhu, H.; Shi, B. Pillar[5]arene-Based Organometallic Cross-Linked Polymer: Synthesis, Structure Characterization, and Catalytic Activity in the Suzuki-Miyaura Coupling Reaction. Macromolecules 2018, 51, 1351. [Google Scholar] [CrossRef]
- Noh, T.H.; Hong, W.; Lee, H.; Jung, O.-S. Indistinguishability and distinguishability between amide and ester moieties in the construction and properties of M 6 L 8 octahedral nanocages. Dalton Trans. 2014, 44, 787–794. [Google Scholar] [CrossRef]
- Iwai, T.; Harada, T.; Hara, K.; Sawamura, M. Threefold Cross-Linked Polystyrene-Triphenylphosphane Hybrids: Mono-P-Ligating Behavior and Catalytic Applications for Aryl Chloride Cross-Coupling and C(sp3)-H Borylation. Angew. Chem. Int. Ed. 2013, 52, 12322–12326. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.-J.; Liu, Y.; Ma, H.; Yang, X.-L.; Xu, H.-B. Main-Chain Organometallic Microporous Polymers Based on Triptycene: Synthesis and Catalytic Application in the Suzuki-Miyaura Coupling Reaction. Chem. A Eur. J. 2013, 19, 5004–5008. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Zhu, D.; Li, T. Porphyrin-based polymer-supported palladium as an excellent and recyclable catalyst for Suzuki-Miyaura coupling reaction in water. Appl. Organomet. Chem. 2018, 32, e3996. [Google Scholar] [CrossRef]
- Yuan, B.; Pan, Y.; Li, Y.; Yin, B.; Jiang, H. A Highly Active Heterogeneous Palladium Catalyst for the Suzuki-Miyaura and Ullmann Coupling Reactions of Aryl Chlorides in Aqueous Media. Angew. Chem. Int. Ed. 2010, 49, 4054–4058. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Wei, Z.; Chen, C.; Wang, D.; Cao, C.; Qiu, Q.; Jiang, J.; Wang, H.; Su, C. Self-Generation of Surface Roughness by Low-Surface-Energy Alkyl Chains for Highly Stable Superhydrophobic/Superoleophilic MOFs with Multiple Functionalities. Angew. Chem. 2019, 131, 17189–17196. [Google Scholar] [CrossRef]
- Li, G.; Yang, H.; Lia, W.; Zhang, G. Rationally designed palladium complexes on a bulky N-heterocyclic carbene-functionalized organosilica: An efficient solid catalyst for the Suzuki–Miyaura coupling of challenging aryl chlorides. Green Chem. 2011, 13, 2939. [Google Scholar] [CrossRef]
- Lou, X.W.; Archer, L.A.; Yang, Z. Hollow Micro-/Nanostructures: Synthesis and Applications. Adv. Mater. 2008, 20, 3987–4019. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.C.; Song, H. A Nanoreactor Framework of a Au@SiO2 Yolk/Shell Structure for Catalytic Reduction of p-Nitrophenol. Adv. Mater. 2008, 20, 1523. [Google Scholar] [CrossRef]
- Van Gough, D.; Wolosiuk, A.; Braun, P.V. Mesoporous ZnS Nanorattles: Programmed Size Selected Access to Encapsulated Enzymes. Nano Lett. 2009, 9, 1994–1998. [Google Scholar] [CrossRef] [PubMed]
- Kamata, K.; Lu, Y.; Xia, Y. Synthesis and Characterization of Monodispersed Core−Shell Spherical Colloids with Movable Cores. J. Am. Chem. Soc. 2003, 125, 2384–2385. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.Z.; Hartono, S.B.; Lu, G.Q. (Max) Monodisperse Yolk-Shell Nanoparticles with a Hierarchical Porous Structure for Delivery Vehicles and Nanoreactors. Angew. Chem. Int. Ed. 2010, 49, 4981–4985. [Google Scholar] [CrossRef]
- Teng, Z.; Su, X.; Zheng, Y.; Sun, J.; Chen, G.; Tian, C.; Wang, J.; Li, H.; Zhao, Y.; Lu, G. Mesoporous Silica Hollow Spheres with Ordered Radial Mesochannels by a Spontaneous Self-Transformation Approach. Chem. Mater. 2013, 25, 98–105. [Google Scholar] [CrossRef]
- Liu, J.; Xia, H.; Xue, D.F.; Lu, L. Double-Shelled Nanocapsules of V2O5-Based Composites as High-Performance Anode and Cathode Materials for Li Ion Batteries. J. Am. Chem. Soc. 2009, 131, 12086. [Google Scholar] [CrossRef]
- Lou, X.W.; Li, C.M.; Archer, L.A. Designed Synthesis of Coaxial SnO2@carbon Hollow Nanospheres for Highly Reversible Lithium Storage. Adv. Mater. 2009, 21, 2536–2539. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.T.; Jung, Y.S.; Oh, S.M. Synthesis of Tin-Encapsulated Spherical Hollow Carbon for Anode Material in Lithium Secondary Batteries. J. Am. Chem. Soc. 2003, 125, 5652–5653. [Google Scholar] [CrossRef]
- Zhang, W.M.; Hu, J.S.; Guo, Y.G.; Zheng, S.F.; Zhong, L.S.; Song, W.G.; Wan, L.J. Tin-Nanoparticles Encapsulated in Elastic Hollow Carbon Speheres for High-Performance Anode Material in Lithium-Ion Batteries. Adv. Mater. 2008, 20, 1160. [Google Scholar] [CrossRef]
- Li, H.X.; Bian, Z.F.; Zhu, J.; Huo, Y.N.; Li, H.X.; Lu, Y.F. Mesoporous Titania Spheres with Tunable Chamber Stucture and Enhanced Photocatalytic Activity. J. Am. Chem. Soc. 2007, 129, 8406. [Google Scholar] [CrossRef]
- Chang, F.; Wang, S.; Zhao, Z.; Wang, L.; Cheng, T.; Liu, G. Enantioselective Dual-Catalysis: A Sequential Michael Addition/Asymmetric Transfer Hydrogenation of α-Nitrosulfone and Enones. ACS Catal. 2020, 10, 10381. [Google Scholar] [CrossRef]
- Meng, J.; Chang, F.; Su, Y.; Liu, R.; Cheng, T.; Liu, G. Switchable Catalysts Used to Control Suzuki Cross-Coupling and Aza–Michael Addition/Asymmetric Transfer Hydrogenation Cascade Reactions. ACS Catal. 2019, 9, 8693–8701. [Google Scholar] [CrossRef]
- Woo, H.; Lee, K.; Park, K.H. Optimized Dispersion and Stability of Hybrid Fe3O4/Pd Catalysts in Water for Suzuki Coupling Reactions: Impact of Organic Capping Agents. ChemCatChem 2014, 6, 1635–1640. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Xu, S.; Yang, Y.; Liu, J.; Wei, Y.; Yang, Q. Polystyrene sulphonic acid resins with enhanced acid strength via macromolecular self-assembly within confined nanospace. Nat. Commun. 2014, 5, 3170. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, X.; Li, X.; Zhao, J.; Bai, S.; Liu, J.; Yang, Q. A Yolk-Shell Nanoreactor with a Basic Core and an Acidic Shell for Cascade Reactions. Angew. Chem. Int. Ed. 2012, 51, 9164–9168. [Google Scholar] [CrossRef]
- Baer, H.H.; Urbas, L. The Chemistry of Nitro and Nitroso Groups; Patai, S., Ed.; Interscience: New York, NY, USA, 1970; p. 117. [Google Scholar]
- Krőcher, O.; Kőppel, O.A.; Frőba, M.; Baiker, A. Silica Hybrid Gel Catalysts Containing Group(VIII) Transition Metal Complexes: Preparation, Structural, and Catalytic Properties in the Synthesis of N,N-Dimethylformamide and Methyl Formate from Supercritical Carbon Dioxide. J. Catal. 1998, 178, 284. [Google Scholar] [CrossRef]
- Yang, S.; He, J. Heterogeneous asymmetric Henry–Michael one-pot reaction synergically catalyzed by grafted chiral bases and inherent achiral hydroxyls on mesoporous silica surface. Chem. Commun. 2012, 48, 10349. [Google Scholar] [CrossRef] [PubMed]
- Shiju, N.R.; Alberts, A.H.; Khalid, S.; Brown, D.R.; Rothenberg, G. Mesoporous Silica with Site-Isolated Amine and Phosphotungstic Acid Groups: A Solid Catalyst with Tunable Antagonistic Functions for One-Pot Tandem Reactions. Angew. Chem. 2011, 123, 9789. [Google Scholar] [CrossRef] [Green Version]



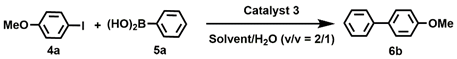
| Entry. | Solvent | Base | Time (h) | Temperature (°C) | Yield (%) b |
|---|---|---|---|---|---|
| 1 | MeOH/H2O | K2CO3 | 1 | 35 | 95 |
| 2 | MeOH/H2O | NaHCO3 | 1 | 35 | 50 |
| 3 | MeOH/H2O | Et3N | 1 | 35 | 40 |
| 4 | MeOH/H2O | NaOH | 1 | 35 | - |
| 5 | MeOH/H2O | DABCO | 1 | 35 | - |
| 6 | MeOH/H2O | DBU | 1 | 35 | - |
| 7 | EtOH/H2O | K2CO3 | 1 | 35 | 90 |
| 8 | iPrOH/H2O | K2CO3 | 1.5 | 35 | 90 |
| 9 | Acetone/H2O | K2CO3 | 1.5 | 35 | 90 |
| 10 | DCM/H2O | K2CO3 | 2 | 35 | 10 |
| 11 | EA/H2O | K2CO3 | 2 | 35 | 36 |
| 12 | 1,4-dioxane | K2CO3 | 2 | 35 | 79 |
| 13 | MeOH/H2O | K2CO3 | 1 | 25 | 60 |
| 14 | MeOH/H2O | K2CO3 | 1 | 45 | 95 |
| 15 | MeOH/H2O | K2CO3 | 1 | 55 | 95 |
| 16 | MeOH/H2O | K2CO3 | 1 | 65 | 96 |
| 17 c | MeOH/H2O | Pd(OAc)2 | 1 | 35 | 90 |
| 18 d | MeOH/H2O | Pd(OAc)2 + PPh3 | 1 | 35 | 50 |
| 19 e | MeOH/H2O | 3′ | 1 | 35 | 90 |
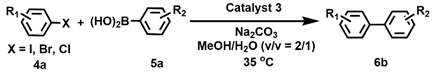
| Entry | X | R1 | R2 | Time (h) | Yield (%) b |
|---|---|---|---|---|---|
| 1 | I | 4-COMe | H | 1 | 95 |
| 2 | I | 4-Cl | H | 1 | 90 |
| 3 | I | 4-Me | H | 1 | 90 |
| 4 | Br | 4-NO2 | H | 1 | 85 |
| 5 | Br | 4-Cl | H | 1 | 95 |
| 6 | Br | 4-OMe | H | 1 | 90 |
| 7 | Br | 2-Me | H | 1 | 90 |
| 8 | Br | 4-CN | H | 1 | 92 |
| 9 | Br | 4-CH3 | H | 1 | 92 |
| 10 | Br | 4-Cl | 4-CF3 | 1 | 95 |
| 11 | Br | 4-CN | 4-CF3 | 4 | 85 |
| 12 | Br | 4-Me | 4-CF3 | 1 | 93 |
| 13 | Br | 4-OMe | 4-OMe | 1.5 | 90 |
| 14 | Br | 4-CN | 4-OMe | 1 | 95 |
| 15 | Br | 4-Me | 4-OMe | 1.5 | 94 |
| 16 | Br | 4-Cl | 4-OMe | 1 | 92 |
| 17 | Br | 4-OH | H | 1 | 95 |
| 18 | Br | H | 4-OMe | 1 | 95 |
| 19 | Br | H | 4-CF3 | 1 | 95 |
| 20 | Br | COMe | H | 1 | 95 |
| 21 | Br | COMe | 4-OMe | 1 | 95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, A.T.A.; Wang, L.; Jin, R.; Liu, G.; Tan, C. Hollow-Shell-Structured Mesoporous Silica-Supported Palladium Catalyst for an Efficient Suzuki-Miyaura Cross-Coupling Reaction. Catalysts 2021, 11, 582. https://doi.org/10.3390/catal11050582
Mohammed ATA, Wang L, Jin R, Liu G, Tan C. Hollow-Shell-Structured Mesoporous Silica-Supported Palladium Catalyst for an Efficient Suzuki-Miyaura Cross-Coupling Reaction. Catalysts. 2021; 11(5):582. https://doi.org/10.3390/catal11050582
Chicago/Turabian StyleMohammed, Abdulelah Taher Ali, Lijian Wang, Ronghua Jin, Guohua Liu, and Chunxia Tan. 2021. "Hollow-Shell-Structured Mesoporous Silica-Supported Palladium Catalyst for an Efficient Suzuki-Miyaura Cross-Coupling Reaction" Catalysts 11, no. 5: 582. https://doi.org/10.3390/catal11050582






