Catalytic Hydrogenation of Carbon Dioxide over Magnetic Nanoparticles: Modification in Fixed-Bed Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Finger-Projected Fixed-Bed Reactor and Reaction Setup
2.2. Synthesis of Magnetic Nanoparticles
2.3. Synthesis of Bismuth-Doped MnFe2O4 (Bi-MnFe2O4)
2.4. Characterization
2.5. Hydrogenation of CO2
3. Results and Discussions
3.1. Finger-Projected Fixed-Bed Reactor
3.2. Catalyst Characterization
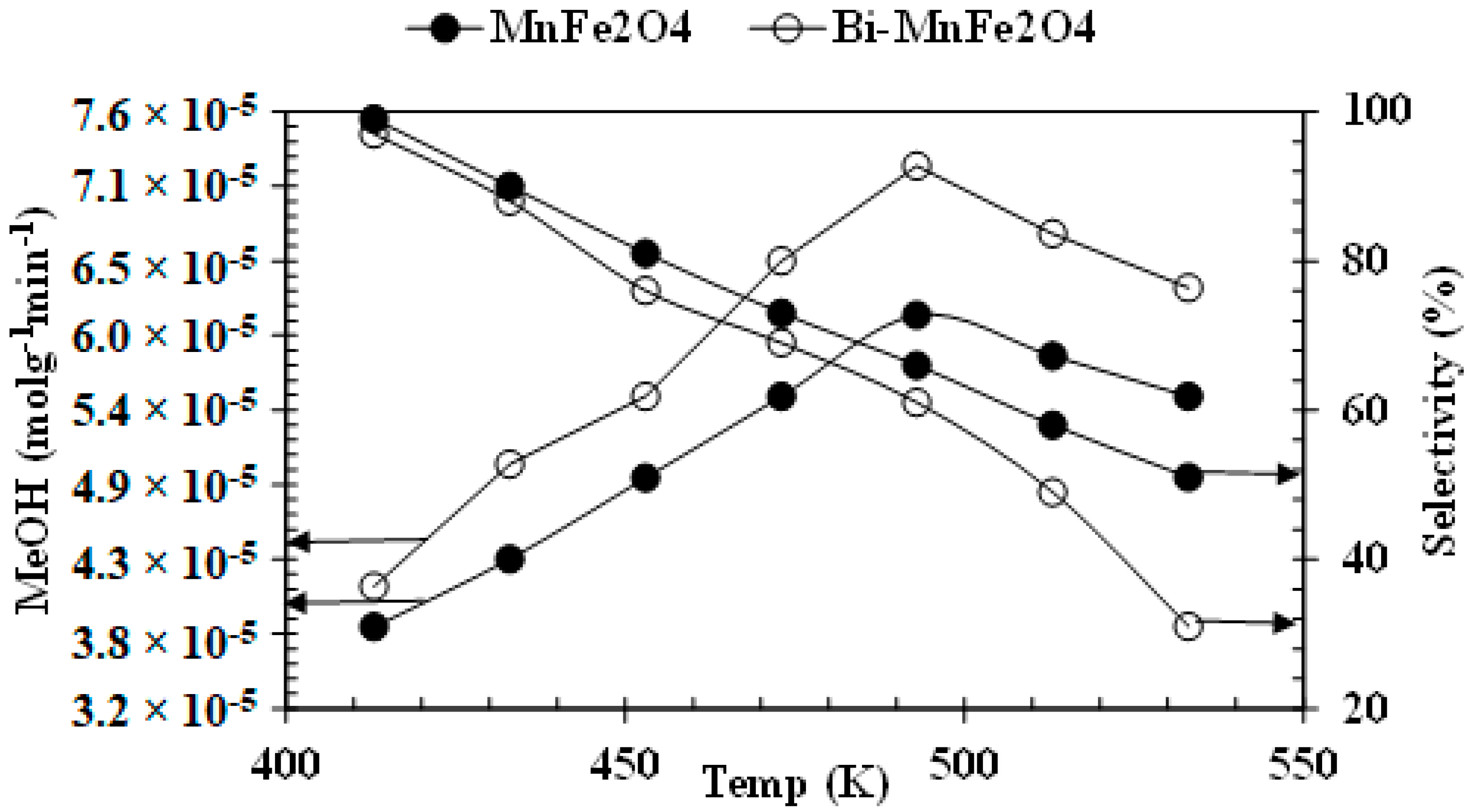
3.3. Catalytic Activity
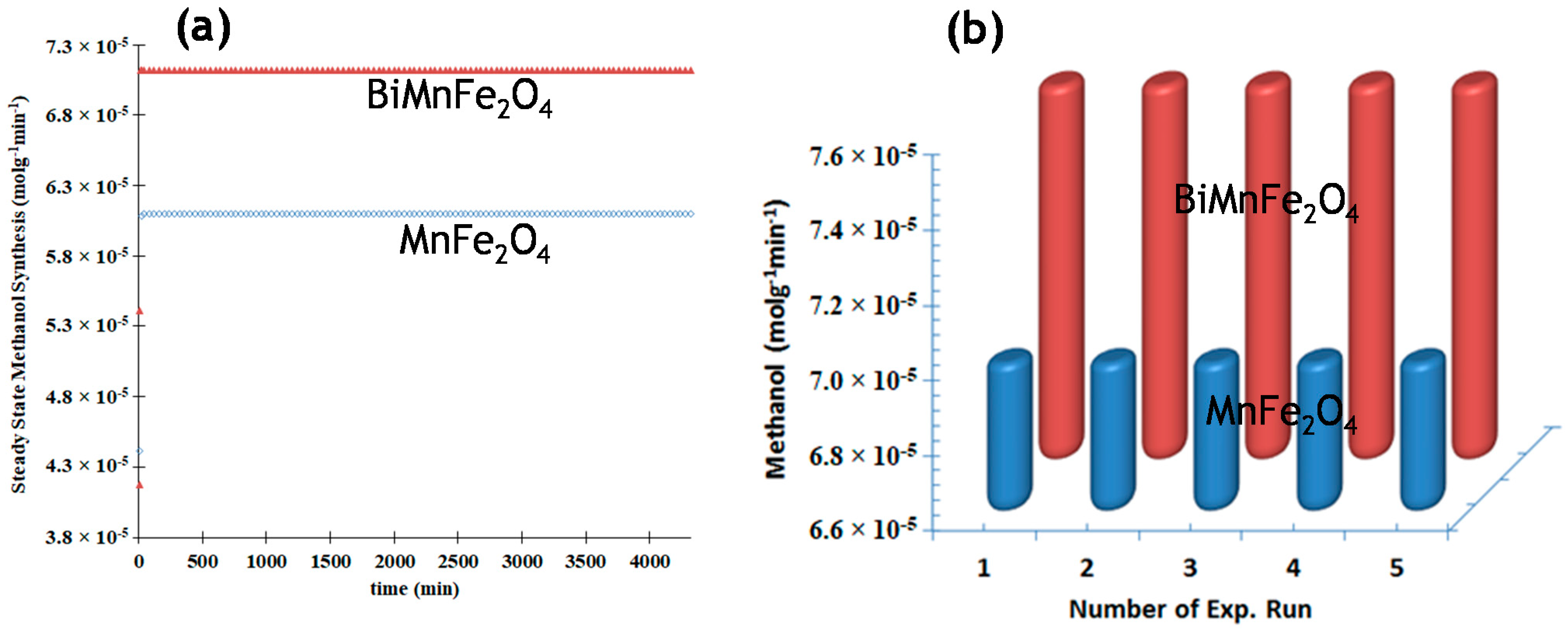
3.4. Stability of Catalysts
3.5. Comparative Study of Different Reactors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aresta, M.; DiBenedetto, A. Utilisation of CO2 as a chemical feedstock: Opportunities and challenges. Dalton Trans. 2007, 28, 2975–2992. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, G.; Elkamel, A.; Lohi§, A. Dynamic Optimization Strategies of a Heterogeneous Reactor for CO2 Conversion to Methanol. Energy Fuels 2007, 21, 2977–2983. [Google Scholar] [CrossRef]
- Ateka, A.; Pérez-Uriarte, P.; Gamero, M.; Ereña, J.; Aguayo, A.T.; Bilbao, J. Aguayo and J. Bilbao A comparative thermodynamic study on the CO2 con-version in the synthesis of methanol and of DME. Energy 2017, 120, 796–804. [Google Scholar] [CrossRef]
- von der Assen, N.; Müller, L.J.; Steingrube, A.; Voll, P.; Bardow, A. Selecting CO2 sources for CO2 utilization by environmental-merit-order curves. Environ. Sci. Technol. 2016, 50, 1093–1101. [Google Scholar] [CrossRef]
- Ahmad, K.; Upadhyayula, S. Greenhouse gas CO2 hydrogenation to fuels: A thermodynamic analysis. Environ. Prog. Sustain. Energy 2019, 38, 98–111. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; With, P.; Srivastava, V.C.; Gläser, R.; Mishra, I.M. Conversion of carbon dioxide along with methanol to dimethyl car-bonate over ceria catalyst. J. Environ. Chem. Eng. 2015, 3, 2943–2947. [Google Scholar] [CrossRef]
- Wisaijorn, W.; Poo-Arporn, Y.; Marin, P.; Ordóňez, S.; Assabumrungrat, S.; Praserthdam, P.; Saebea, D.; Soisuwan, S. Reduction of carbon dioxide via catalytic hydrogenation over copper-based catalysts modified by oyster shell-derived calcium oxide. J. Environ. Chem. Eng. 2017, 5, 3115–3121. [Google Scholar] [CrossRef]
- Akhter, P.; Farkhondehfal, M.A.; Hernández, S.; Hussain, M.; Fina, A.; Saracco, G.; Khan, A.U.; Russo, N. Environmental issues regarding CO2 and recent strategies for alternative fuels through photocatalytic reduction with titania-based materials. J. Environ. Chem. Eng. 2016, 4, 3934–3953. [Google Scholar] [CrossRef]
- Ranjbar, A.; Irankhah, A.; Aghamiri, S.F. Reverse water gas shift reaction and CO2 mitigation: Nanocrystalline MgO as a support for nickel based catalysts. J. Environ. Chem. Eng. 2018, 6, 4945–4952. [Google Scholar] [CrossRef]
- Samimi, F.; Hamedi, N.; Rahimpour, M.R. Green methanol production process from indirect CO2 conversion: RWGS reactor versus RWGS membrane reactor. J. Environ. Chem. Eng. 2019, 7, 102813. [Google Scholar] [CrossRef]
- Rahimpour, M. A two-stage catalyst bed concept for conversion of carbon dioxide into methanol. Fuel Process. Technol. 2008, 89, 556–566. [Google Scholar] [CrossRef]
- Liu, X.-M.; Lu, G.Q.; Yan, Z.-F.; Beltramini, J. Recent Advances in Catalysts for Methanol Synthesis via Hydrogenation of CO and CO2. Ind. Eng. Chem. Res. 2003, 42, 6518–6530. [Google Scholar] [CrossRef]
- Din, I.U.; Usman, M.; Khan, S.; Helal, A.; Alotaibi, M.A.; Alharthi, A.I.; Centi, G. Prospects for a green methanol thermo-catalytic process from CO2 by using MOFs based materials: A mini-review. J. CO2 Util. 2021, 43, 101361. [Google Scholar] [CrossRef]
- Bukhtiyarova, M.; Lunkenbein, T.; Kähler, K.; Schlögl, R. Methanol Synthesis from Industrial CO2 Sources: A Contribution to Chemical Energy Conversion. Catal. Lett. 2017, 147, 416–427. [Google Scholar] [CrossRef] [Green Version]
- Tursunov, O.; Kustov, L.; Kustov, A. A Brief Review of Carbon Dioxide Hydrogenation to Methanol Over Copper and Iron Based Catalysts. Oil & Gas Sci. Technol. Rev. d’IFP Energ. Nouv. 2017, 72, 30. [Google Scholar] [CrossRef] [Green Version]
- Bansode, A.; Tidona, B.; von Rohr, P.R.; Urakawa, A. Impact of K and Ba promoters on CO2 hydrogenation over Cu/Al2O3 catalysts at high pressure. Catal. Sci. Technol. 2013, 3, 767–778. [Google Scholar] [CrossRef]
- Arab, S.; Commenge, J.-M.; Portha, J.-F.; Falk, L. Methanol synthesis from CO2 and H2 in multi-tubular fixed-bed reactor and multi-tubular reactor filled with monoliths. Chem. Eng. Res. Des. 2014, 92, 2598–2608. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Dalai, A.K. Effects of metal content on activity and stability of Ni-Co bimetallic catalysts for CO2 reforming of CH4. Appl. Catal. A Gen. 2008, 339, 121–129. [Google Scholar] [CrossRef]
- Sugawa, S.; Sayama, K.; Okabe, K.; Arakawa, H. Methanol synthesis from CO2 and H2 over silver catalyst. Energy Convers. Manag. 1995, 36, 665–668. [Google Scholar] [CrossRef]
- Hong, Z.S.; Cao, Y.; Deng, J.F.; Fan, K.N. CO2 hydrogenation to methanol over Cu/ZnO/Al2O3 catalysts prepared by a novel gel-network-coprecipitation method. Catal. Lett. 2002, 82, 37–44. [Google Scholar] [CrossRef]
- Chary, K.V.; Kumar, C.P.; Rao, P.V.R.; Rao, V.V. Dispersion and reactivity of V2O5 catalysts supported on Al2O3–ZrO2. Catal. Commun. 2004, 5, 479–484. [Google Scholar] [CrossRef]
- Dominguez, J.; Hernandez, J.; Sandoval, G. Surface and catalytic properties of Al2O3–ZrO2 solid solutions prepared by sol–gel methods. Appl. Catal. A Gen. 2000, 197, 119–130. [Google Scholar] [CrossRef]
- Yoon, T.-J.; Lee, W.; Oh, Y.-S.; Lee, J.-K. Magnetic nanoparticles as a catalyst vehicle for simple and easy recycling. New J. Chem. 2003, 27, 227–229. [Google Scholar] [CrossRef]
- Venkataraju, C.; Satishkumar, G.; Sivakumar, K. Effect of bismuth on the properties of Mn ferrite nanoparticles prepared by co-precipitation method. J. Mater. Sci. Mater. Electron. 2012, 23, 1163–1168. [Google Scholar] [CrossRef]
- Prabhakaran, T.; Mangalaraja, R.; Denardin, J.C.; Jiménez, J.A. The effect of calcination temperature on the structural and magnetic properties of co-precipitated CoFe2O4 nanoparticles. J. Alloys Compd. 2017, 716, 171–183. [Google Scholar] [CrossRef]
- Zhang, Y.; Fei, J.; Yu, Y.; Zheng, X. Study of CO2 Hydrogenation to Methanol over Cu-V/γ-Al2O3 Catalyst. J. Nat. Gas Chem. 2007, 16, 12–15. [Google Scholar] [CrossRef]
- Zhou, X.; Su, T.; Jiang, Y.; Qin, Z.; Ji, H.; Guo, Z. CuO-Fe2O3-CeO2/HZSM-5 bifunctional catalyst hydrogenated CO2 for enhanced dimethyl ether synthesis. Chem. Eng. Sci. 2016, 153, 10–20. [Google Scholar] [CrossRef]
- Han, A.; Liao, J.; Ye, M.; Li, Y.; Peng, X. Preparation of nano-MnFe2O4 and its catalytic performance of thermal decomposition of ammonium perchlorate. Chin. J. Chem. Eng. 2011, 19, 1047–1051. [Google Scholar] [CrossRef]
- Jacintha, A.M.; Umapathy, V.; Neeraja, P.; Rajkumar, S.R.J. Synthesis and comparative studies of MnFe2O4 nanoparticles with different natural polymers by sol–gel method: Structural, morphological, optical, magnetic, catalytic and biological activities. J. Nanostruct. Chem. 2017, 7, 375–387. [Google Scholar] [CrossRef] [Green Version]
- Abraham, S.D.; David, S.T.; Bennie, R.B.; Joel, C.; Kumar, D.S. Eco-friendly and green synthesis of BiVO4 nanoparticle using microwave irradiation as photocatalayst for the degradation of Alizarin Red S. J. Mol. Struct. 2016, 1113, 174–181. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Gao, P.; Wang, H.; Li, X.; Zhong, L.; Wei, W.; Sun, Y. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons. Catal. Sci. Technol. 2017, 7, 4580–4598. [Google Scholar] [CrossRef]
- Díez-Ramírez, J.; Dorado, F.; De La Osa, A.R.; Valverde, J.L.; Sánchez, P. Hydrogenation of CO2 to Methanol at Atmospheric Pressure over Cu/ZnO Catalysts: Influence of the Calcination, Reduction, and Metal Loading. Ind. Eng. Chem. Res. 2017, 56, 1979–1987. [Google Scholar] [CrossRef]
- Toyir, J.; De La Piscina, P.R.; Llorca, J.; Fierro, J.-L.G.; Homs, N. Methanol synthesis from CO2 and H2 over gallium promoted copper-based supported catalysts. Effect of hydrocarbon impurities in the CO2/H2 source. Phys. Chem. Chem. Phys. 2001, 3, 4837–4842. [Google Scholar] [CrossRef]
- Iwasa, N.; Masuda, S.; Ogawa, N.; Takezawa, N. Steam reforming of methanol over Pd/ZnO: Effect of the formation of PdZn alloys upon the reaction. Appl. Catal. A Gen. 1995, 125, 145–157. [Google Scholar] [CrossRef]
- Wang, Y.; Kattel, S.; Gao, W.; Li, K.; Liu, P.; Chen, J.G.; Wang, H. Exploring the ternary interactions in Cu–ZnO–ZrO2 catalysts for efficient CO2 hydrogenation to methanol. Nat. Commun. 2019, 10, 1166. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mims, C.A.; Mei, D.; Peden, C.H.; Campbell, C.T. Mechanistic studies of methanol synthesis over Cu from CO/CO2/H2/H2O mixtures: The source of C in methanol and the role of water. J. Catal. 2013, 298, 10–17. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Zhang, B.; Liu, F. Density functional theory study on the heterogeneous reaction between HgO and HCl over spinel-type MnFe2O4. Chem. Eng. J. 2017, 308, 897–903. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, H.; Zhang, C.; Feng, J.; Pu, S.; Ren, Y.; Wang, Y. Enhanced catalytic ozonation treatment of dibutyl phthalate enabled by porous magnetic Ag-doped ferrospinel MnFe2O4 materials: Performance and mechanism. Chem. Eng. J. 2018, 354, 42–52. [Google Scholar] [CrossRef]



| Entry | Catalysts | Morphology | Size (nm) * | BET (m2/g) |
|---|---|---|---|---|
| 1 | MnFe2O4 | Spinel | 64.1 | 32 |
| 2 | BiMnFe2O4 | Hexagonal/spinel | 79.3 | 29 |
| * VPs | Conditions: 1 Cat, 2 FR, 3 T, 4 P, 5 GFR | a Conv/b Sel | Ref |
|---|---|---|---|
| H3COCH3 CH3OH | CuO-ZnO-Al2O3-ZrO2+HZSM-5 (1), 3/1, 473, 49.3, 100 | 15.8/74 5.4/25 | [31] |
| CO CH4 CH3OH | 20CuZnO-350-200 (0.8), 9/1, 498, 1,100 | 14/77.3 14/17 14/4.4 | [32] |
| CO CH3OH | 5%Pd/ZnO (0.5), 3/1, 523, 19.7, 30 | 10.7/39 10.7/60 | [34] |
| CH3OH | Cu/ZnO/Al2O3 (1), 7/2, 473, 29.6, 150 | 4/68 | [14] |
| CO H3COCH3 C2H4O2 CH3OH | Cu/Al2O3 (0.17), 3.8/1, 553, 355, 0.011 Cu/Al2O3 (0.17), 3.8/1, 553, 98.7, 0.011 Cu/Al2O3 (0.17), 3.8/1, 443, 355.2, 0.011 Cu/Al2O3 (0.17), 3.8/1, 473, 355.2, 0.011 | 29/91.7 21/8.5 3/3.1 8/48 | [16] |
| CH3OH C2H4O2 H3COCH3 | Bi-MnFe2O4, (2/chamber), 3/1, 493, 1, 40 Bi-MnFe2O4, (2/chamber), 3/1, 563, 1, 40 Bi-MnFe2O4, (2/chamber), 3/1, 543, 1, 40 | 22/61 6/33 4/19 | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bibi, M.; Ullah, R.; Sadiq, M.; Sadiq, S.; Khan, I.; Saeed, K.; Zia, M.A.; Iqbal, Z.; Ullah, I.; Iqbal, Z.; et al. Catalytic Hydrogenation of Carbon Dioxide over Magnetic Nanoparticles: Modification in Fixed-Bed Reactor. Catalysts 2021, 11, 592. https://doi.org/10.3390/catal11050592
Bibi M, Ullah R, Sadiq M, Sadiq S, Khan I, Saeed K, Zia MA, Iqbal Z, Ullah I, Iqbal Z, et al. Catalytic Hydrogenation of Carbon Dioxide over Magnetic Nanoparticles: Modification in Fixed-Bed Reactor. Catalysts. 2021; 11(5):592. https://doi.org/10.3390/catal11050592
Chicago/Turabian StyleBibi, Mehnaz, Rasheed Ullah, Muhammad Sadiq, Saima Sadiq, Idrees Khan, Khalid Saeed, Muhammad Abid Zia, Zaffar Iqbal, Inam Ullah, Zahoor Iqbal, and et al. 2021. "Catalytic Hydrogenation of Carbon Dioxide over Magnetic Nanoparticles: Modification in Fixed-Bed Reactor" Catalysts 11, no. 5: 592. https://doi.org/10.3390/catal11050592







