Performance of Sulfide-Driven Fuel Cell Aerated by Venturi Tube Ejector
Abstract
:1. Introduction
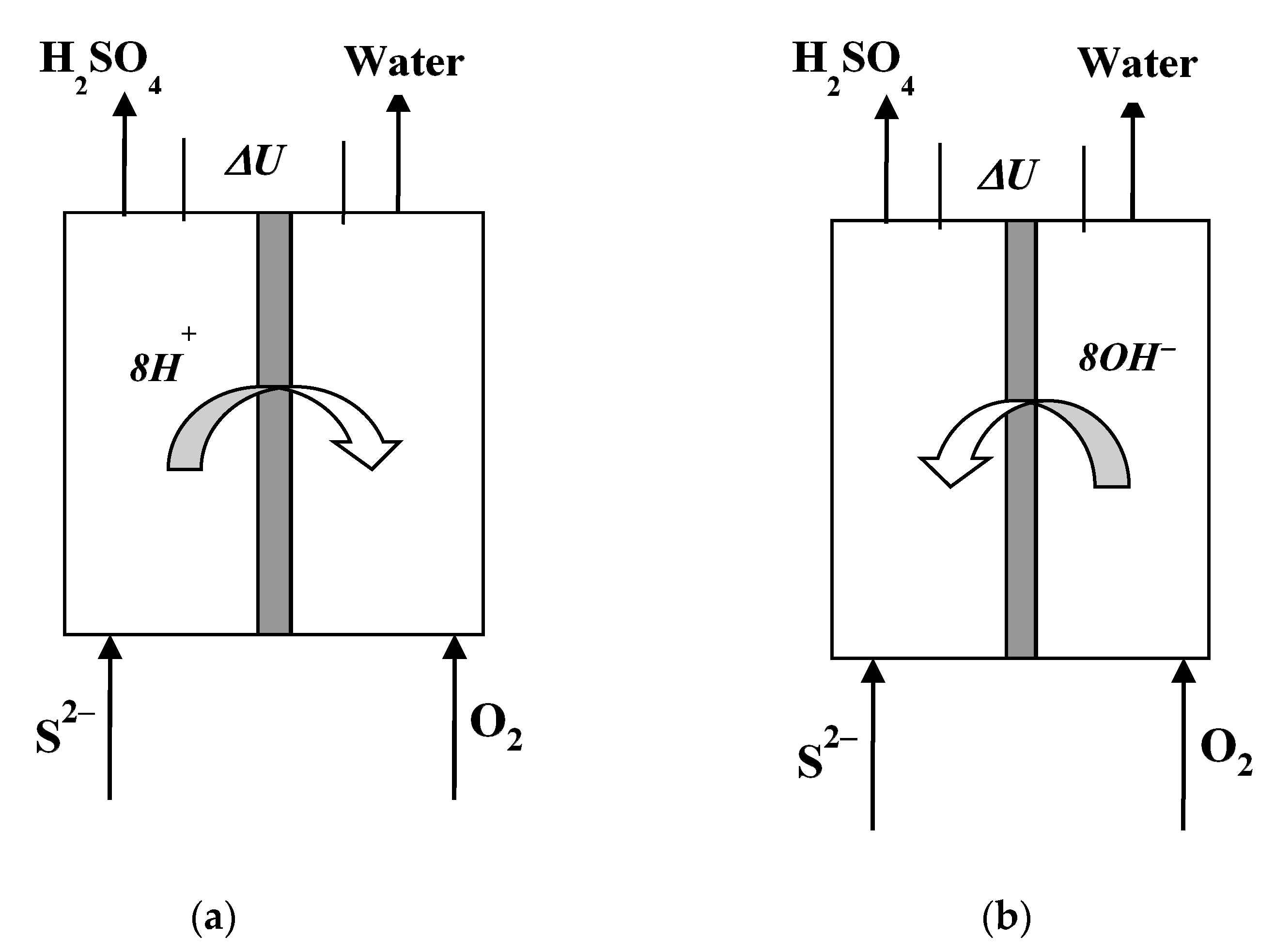
2. Results and Discussion
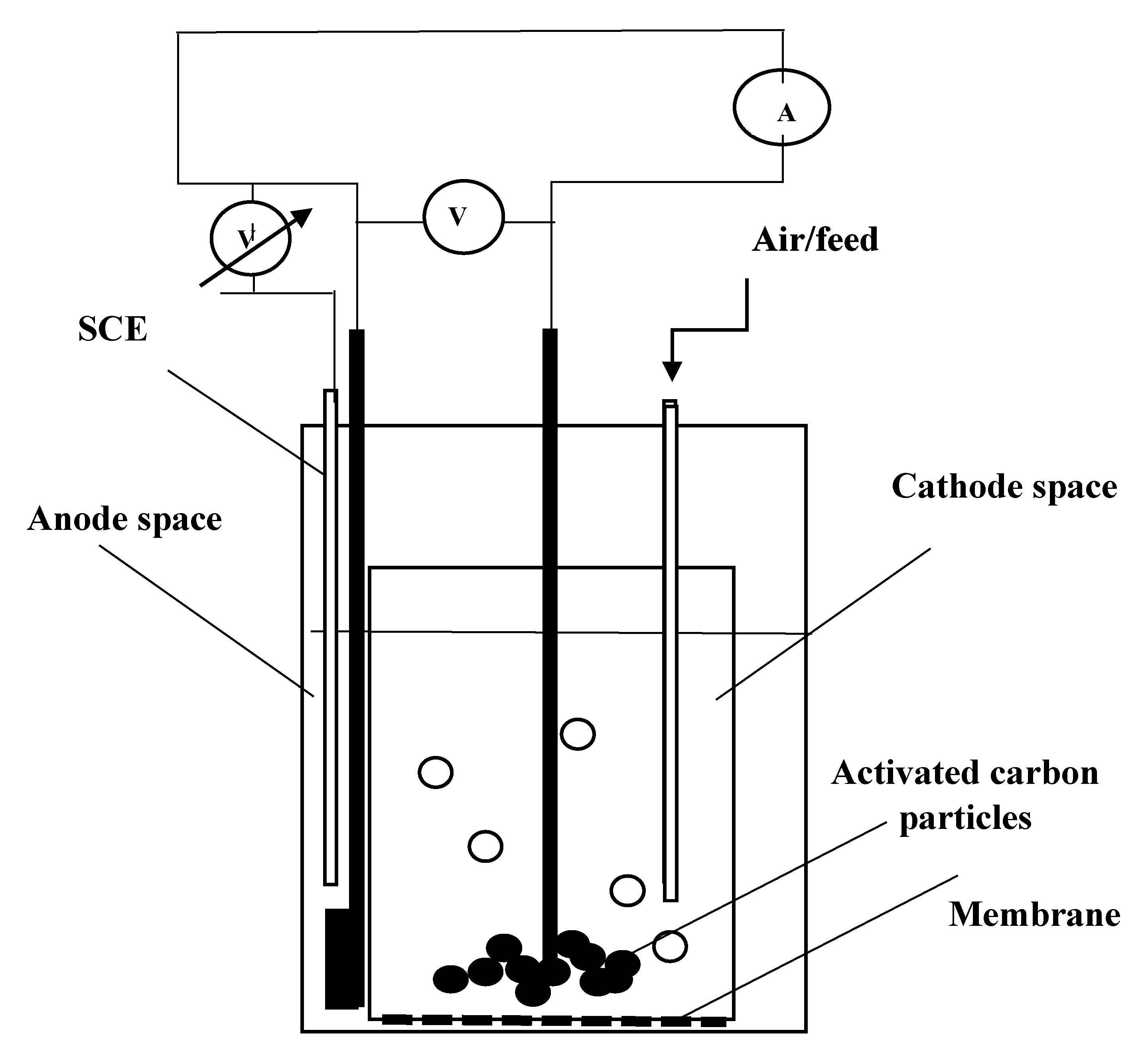
2.1. Cycle Voltammetric Studies
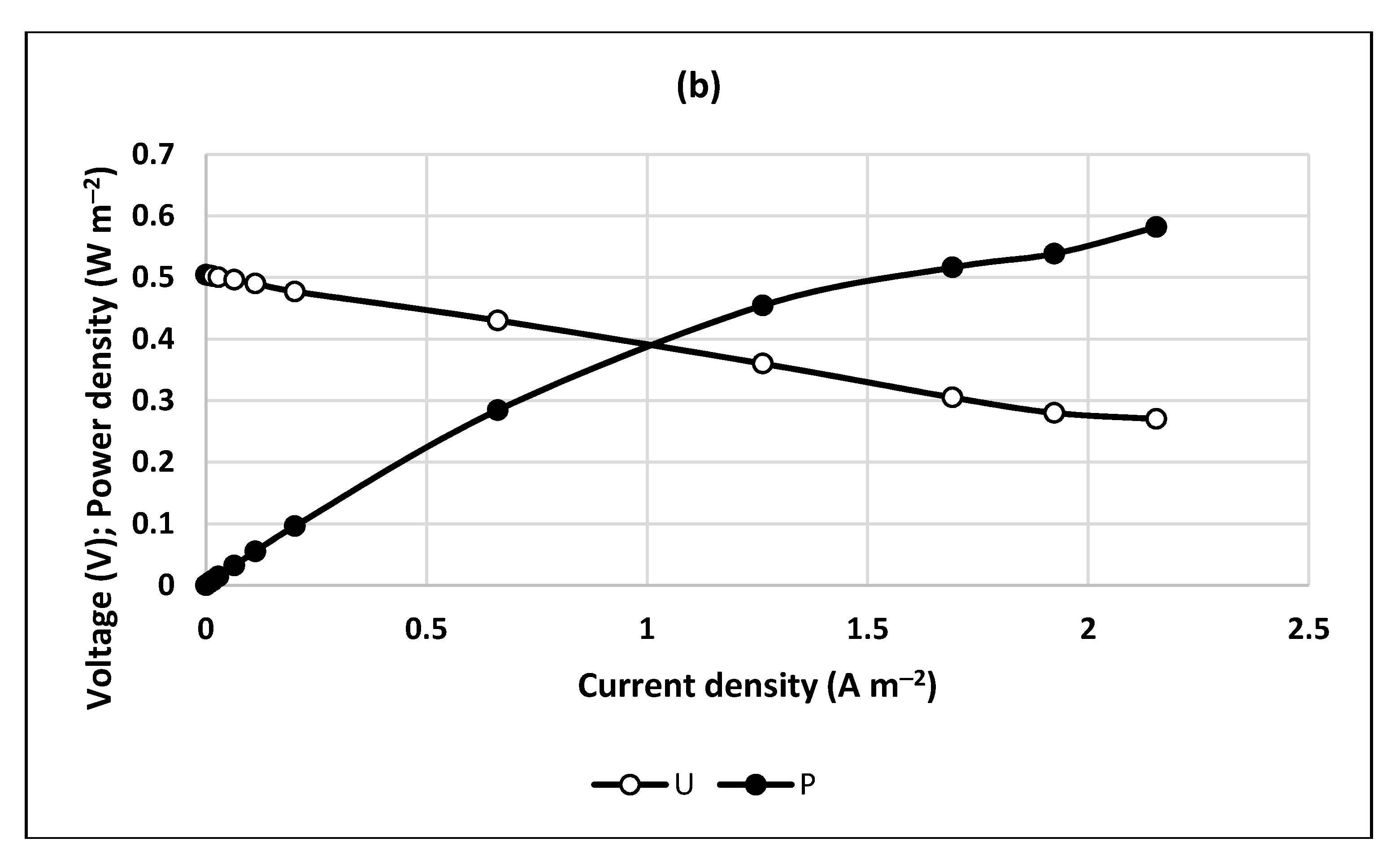
2.2. Polarization Curves
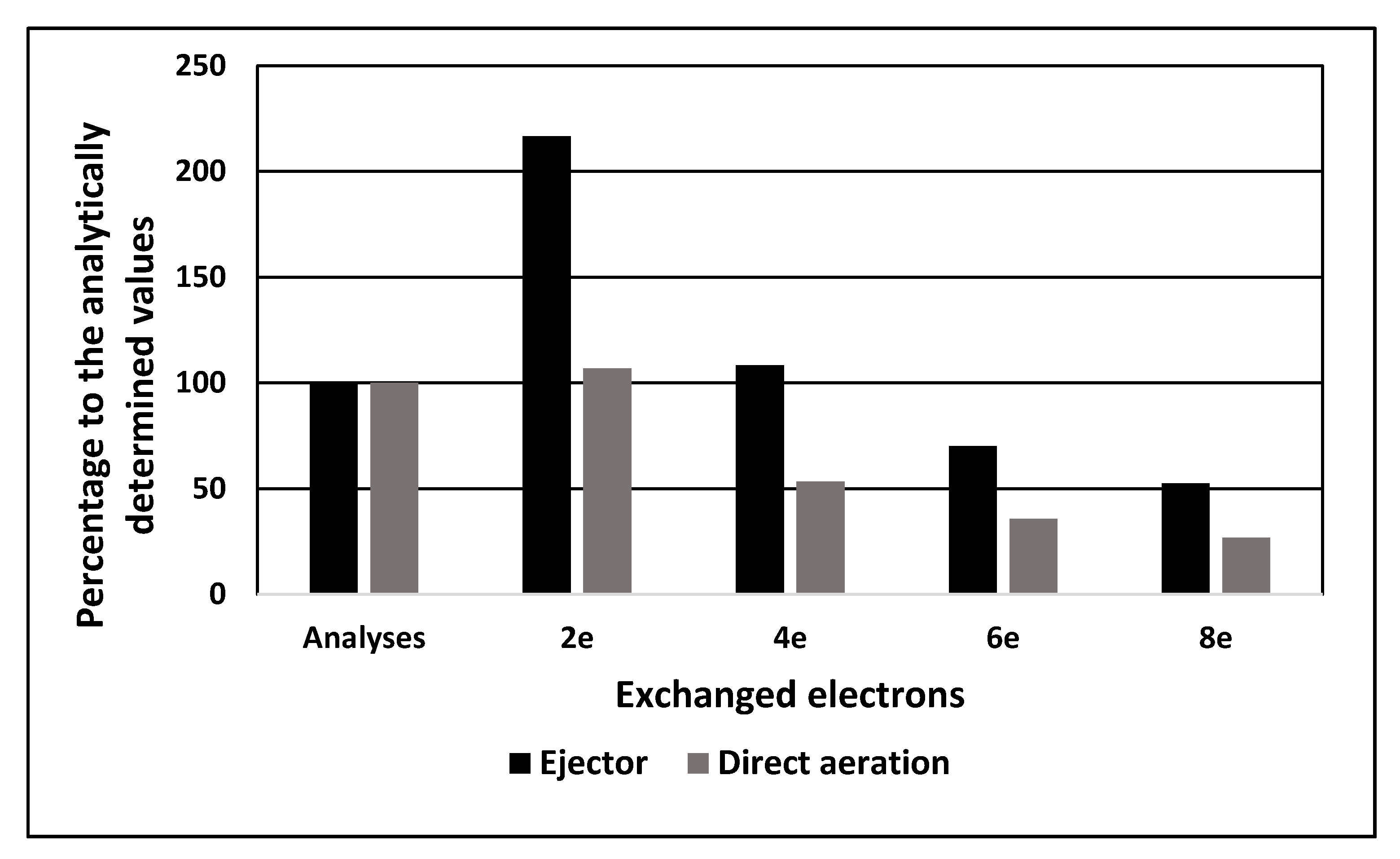
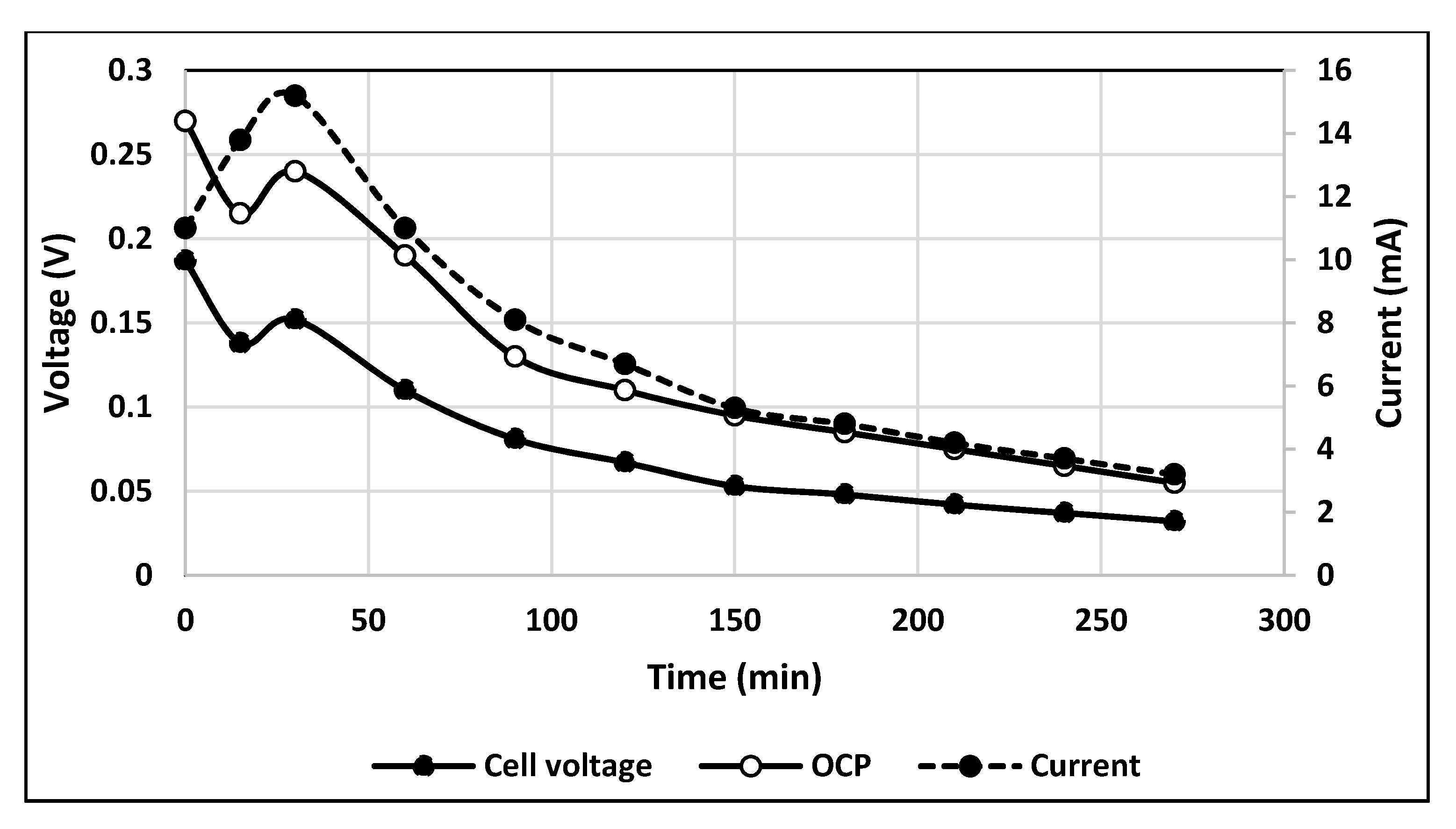
2.3. Results on Fuel Cell Discharge at Different Aeration Modes
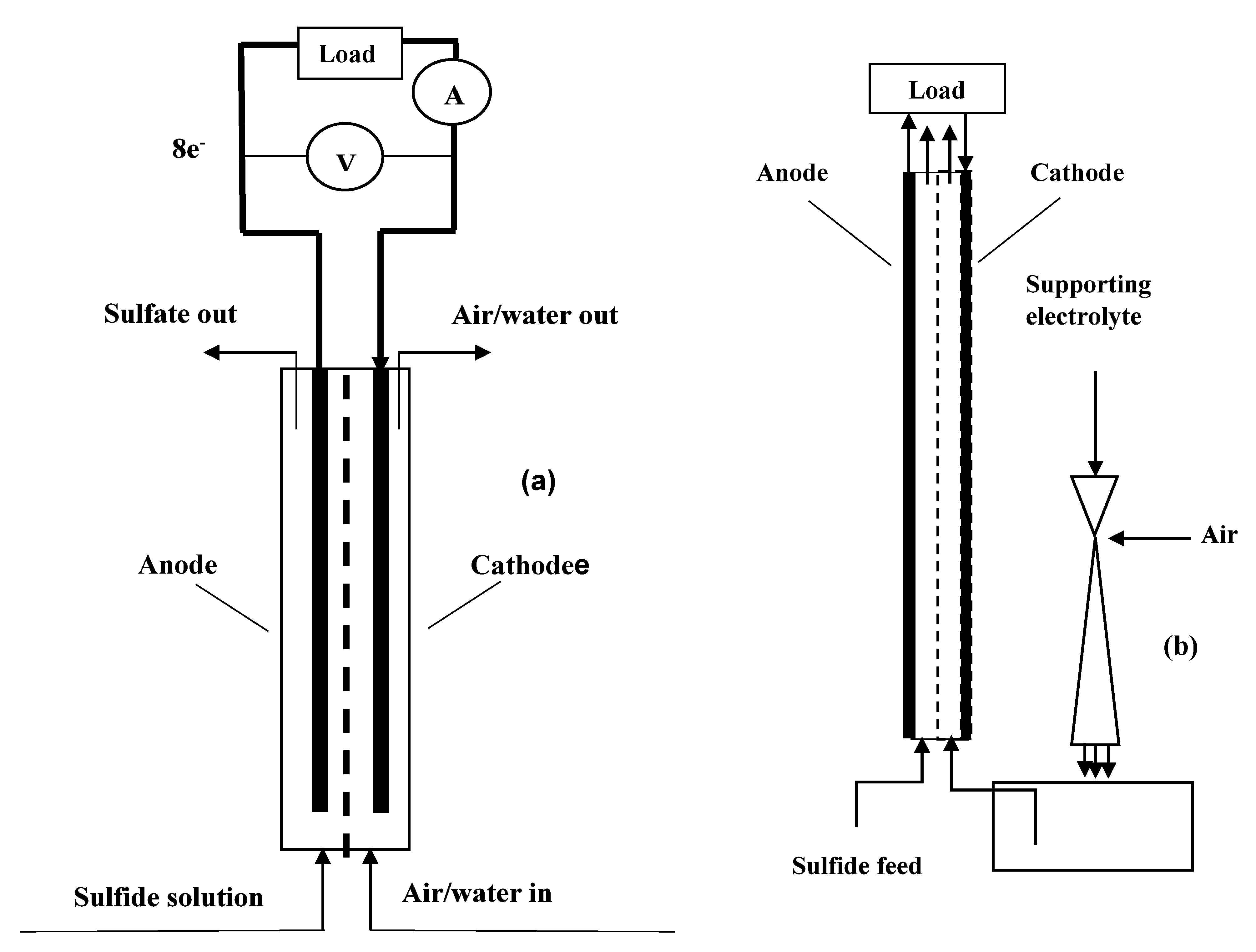
2.3.1. Direct Aeration
2.3.2. Aeration by Venturi Tube Ejector
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Voltammetric Studies
3.4. Fuel Cell Discharge Experiments
3.5. Analyses
4. Conclusions
- (1)
- It is possible to produce energy from hydrogen sulfide in marine water as a fuel. Its enthalpy of combustion is comparable to methane and hydrogen. The proposed approach enables direct production of electricity without intermediate processes, with sulfate ions as a product. The latter is compatible with the marine environment and, therefore, the method can be considered as waste-free.
- (2)
- The main drawback of the proposed method is the low current and power densities because of the low oxygen transfer rate and oxygen concentration in the cathode space. Hence, oxygen reduction appears to be the rate-determining step in the overall electrochemical process. This problem has to be solved by increasing oxygen partial pressure in the air and by introducing a suitable catalyst for oxygen reduction.
- (3)
- Another problem is due to the variety of reactions of sulfide oxidation. The present data show that the oxidation of sulfide to sulfate in the bulk is successful but there are parasite reactions of sulfide oxidation. Oxidation of sulfide to sulfite is also observed. That is why the anodic process should be carried out under oxygen-free conditions.
- (4)
- The practical application of this process will be promoted if suitable catalysts for selective sulfide to sulfate oxidation are developed and when the process of oxygen reduction is enhanced properly.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Petrov, K.; Baykara, S.Z.; Ebrasu, D.; Gulin, M.; Veziroglu, A. An assessment of electrolytic hydrogen production from H2S in Black Sea waters. Int. J. Hydrog. Energy 2011, 6, 8936–8942. [Google Scholar] [CrossRef]
- Neklyudov, I.K.; Bortz, B.V.; Polevich, O.W.; Tkachenko, V.I.; Shilyaev, B.A. Alternative hydrogen sulfide energetics in the Black Sea. Part I. State of the art, problems and perspectives (in Russian). Int. Sci. J. Altern. Energy Ecol. 2006, 12, 23–30. [Google Scholar]
- Fornes, J.P.; Bisang, J.M. Cathode depassivation using ultrasound for the production of colloidal sulphur by reduction of sulphur dioxide. Electrochim. Acta 2016, 213, 186–193. [Google Scholar] [CrossRef]
- Dutta, P.K.; Rabaey, K.; Yuan, Z.; Keller, J. Spontaneous electrochemical removal of aqueous sulfide. Water Res. 2008, 42, 4965–4975. [Google Scholar] [CrossRef] [PubMed]
- Saad, E.G.; Zewail, T.M.; Zatout, A.A.; El-Ashtoukhy, E.-S.Z.; Abdel-Aziz, M.H. Electrochemical removal of sulfide ions and recovery of sulfur from sulfide ions containing wastes. J. Industr. Eng. Chem. 2021, 94, 390–396. [Google Scholar] [CrossRef]
- Zhai, L.-F.; Song, W.; Tong, Z.-H.; Sun, M. A fuel-cell-assisted iron redox process for simultaneous sulfur recovery and electricity production from synthetic sulfide wastewater. J. Hazard. Mater. 2012, 243, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, J.; Liu, Y.; Hao, C.; Tian, C.; Feng, C.; Lei, Z.; Huang, W.; Zhang, Z. Identification of removal principles and involved bacteria in microbial fuel cells for sulfide removal and electricity generation. Int. J. Hydrog. Energy 2013, 38, 14348–14355. [Google Scholar] [CrossRef] [Green Version]
- Uzun, D.; Razkazova-Velkova, E.; Petrov, K.; Beschkov, V. H2S/O2 fuel cells using hydrogen sulfide from Black Sea waters. J. Appl. Electrochem. 2016, 46, 943–949. [Google Scholar] [CrossRef]
- Beschkov, V.; Razkazova-Velkova, E.; Martinov, M.; Stefanov, S. Electricity production from marine water by sulfide-driven fuel cell. Appl. Sci. 2018, 8, 1926. [Google Scholar] [CrossRef] [Green Version]
- Midilli, A.; Ay, M.; Kale, A.; Nejat Veziroglu, T. A parametric investigation of hydrogen energy potential based on H2S in Black Sea deep waters. Int. J. Hydrog. Energy 2007, 32, 117–124. [Google Scholar] [CrossRef]
- Demirbas, A. Hydrogen sulfide from the black sea for hydrogen production. Energy Sources Part A Recovery Util. Environ. Eff. 2009, 31, 1866–1872. [Google Scholar] [CrossRef]
- Suhotin, A.M. (Ed.) Guidebook on Electrochemistry (In Russian); Himia: Leningrad, Russia, 1981. [Google Scholar]
- Sanli, A.E.; Canan, B.; Aytaç, A. Use of the Black Sea water containing hydrogen sulfide (H2S) as a Fuel Cell Fuel. ECS Transactions 2015, 65, 51–58. [Google Scholar] [CrossRef]
- Uzun, D.; Razkazova-Velkova, E.; Petrov, K.; Beschkov, V. Electrochemical method for energy production from hydrogen sulfide in the Black Sea waters in sulfide-driven fuel cell. Bulg. Chem. Commun. 2015, 47, 859–866. [Google Scholar]
- Beschkov, V.; Razkazova-Velkova, E.; Martinov, M.; Stefanov, S. Energy production from black sea water by sulfide-driven fuel cell. In Proceedings of the 15th International Multidisciplinary Scientific Conference SGEM, Albena, Bulgaria, 18–24 June 2015; Energy and Clean Technologies. pp. 207–212. [Google Scholar]
- Kim, K.; Han, J.-I. Performance of direct alkaline sulfide fuel cell without sulfur deposition of anode. Int. J. Hydrog. Energy 2014, 39, 7142–7146. [Google Scholar] [CrossRef]
- Yang, H.-C.; Park, S.-K. Oxygen transfer characteristics of an ejector aeration system. Int. J. Fluid Mach. Syst. 2012, 5, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, L.; Xia, Z.; Roy, A.; Chang, D.W.; Baek, J.-B.; Dai, L. BCN graphene as efficient metal-free electrocatalyst for the oxygen reduction reaction. Angew. Chem. 2012, 51, 4209–4212. [Google Scholar] [CrossRef] [PubMed]
- Ljutzkanov, L.; Atanasov, A. Method for Treatment of Carbon-Containing Materials. BG Patent 63594/26.06.2002, 28 June 2002. [Google Scholar]
- Dermendzhieva, N.D.; Razkazova-Velkova, E.N.; Beschkov, V.N. Kinetics of oxidation of sulfide ions in model solutions of sea water. Bulg. Chem. Commun. 2015, 47, 766–770. [Google Scholar]
- Rees, T.D.; Gyllenpetz, A.B.; Docherty, A.C. The determination of trace amounts of sulphide in condensed steam with N-diethyl-P-phenylenediamine. Analyst 1971, 96, 201–208. [Google Scholar] [CrossRef]





| No. | Reversible Redox Anode Reactions (Short Excerpt) | Number of Exchanged Electrons | Standard Electrode Potential (V), 25 °C |
|---|---|---|---|
| 1 | SO42− + H2O+ 2e = SO32− + 2OH− | 2 | −0.93 |
| 2 | SO32− + 3H2O + 6e = S2− + 6OH− | 6 | −0.66 |
| 3 | S22− + 2e = 2S2− | 2 | −0.524 |
| 4 | S + 2e = S2− | 2 | −0.33 |
| 5 | 2SO42− + 4H++ 2e = S2O62− + 2H2O | 2 | −0.22 |
| 6 | 2H2SO3(aq) +H++2e = HS2O4− +2H2O | 2 | −0.082 |
| 7 | S52− + 5H+ + 8e = 5HS− | 2 | 0.003 |
| 8 | S2O32− + 6H+ +8e = 2S2− + 3H2O | 4 | −0.006 |
| 9 | HSO3− + 5H+ + 4e = S +3H2O | 4 | 0 |
| 10 | S42− + 4H+ + 6e = 4HS | 6 | 0.033 |
| 11 | S32− + 3H+ + 4e = 3HS− | 4 | 0.090 |
| 12 | SO42− + 8H+ + 8e = S2− + 4H2O | 8 | 0.149 |
| 13 | SO32− + 6H++ 6e = S2− + 3H2O | 6 | 0.231 |
| Temperature, °C | Equilibrium Electrode Potential, V/S.H.E. | Transfer Coefficient, α (-) | Exchange Current i0, mA |
|---|---|---|---|
| 8 | 0.031 | 0.28 | 0.29 |
| −0.225 | 0.26 | 0.14 | |
| 14 | 0.03 | 0.26 | 9.2 |
| −0.235 | 0.26 | 1.5 | |
| 20 | 0.041 | 0.11 | 3.2 |
| −0.242 | 0.33 | 3.0 |
| No Catalyst | Cobalt Spinel Catalyst | |||||
|---|---|---|---|---|---|---|
| T (°C) | Equilibrium Electrode Potential (V/S.H.E.) | Transfer Coefficient α (-) | Exchange Current i0 (mA) | Equilibrium Electrode Potential (V/S.H.E.) | Transfer Coefficient α (-) | Exchange Current i0 (mA) |
| 6 | - | - | - | 0.0057 | 0.1 | 3.7 |
| - | - | - | −0.29 | 0.18 | 2.7 | |
| 10 | 0.200 | 0.084 | 0.032 | −0.014 | 0.070 | 1.9 |
| 0.235 | 0.18 | 0.071 | −0.093 | 0.11 | 0.014 | |
| 23 | −0.107 | 0.17 | 1.44 | - | - | - |
| −0.127 | 0.26 | 0.25 | - | - | - | |
| 24 | −0.086 | 0.13 | 3.6 | 0.092 | 0.11 | 3.45 |
| 0.218 | 0.17 | 2.9 | −0.193 | 0.14 | 0.51 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beschkov, V.N.; Razkazova-Velkova, E.N.; Martinov, M.S.; Stefanov, S.M. Performance of Sulfide-Driven Fuel Cell Aerated by Venturi Tube Ejector. Catalysts 2021, 11, 694. https://doi.org/10.3390/catal11060694
Beschkov VN, Razkazova-Velkova EN, Martinov MS, Stefanov SM. Performance of Sulfide-Driven Fuel Cell Aerated by Venturi Tube Ejector. Catalysts. 2021; 11(6):694. https://doi.org/10.3390/catal11060694
Chicago/Turabian StyleBeschkov, Venko N., Elena N. Razkazova-Velkova, Martin S. Martinov, and Stefan M. Stefanov. 2021. "Performance of Sulfide-Driven Fuel Cell Aerated by Venturi Tube Ejector" Catalysts 11, no. 6: 694. https://doi.org/10.3390/catal11060694






