The Effect of Catalyst Calcination Temperature on Catalytic Decomposition of HFC-134a over γ-Al2O3
Abstract
:1. Introduction
2. Results and Discussion
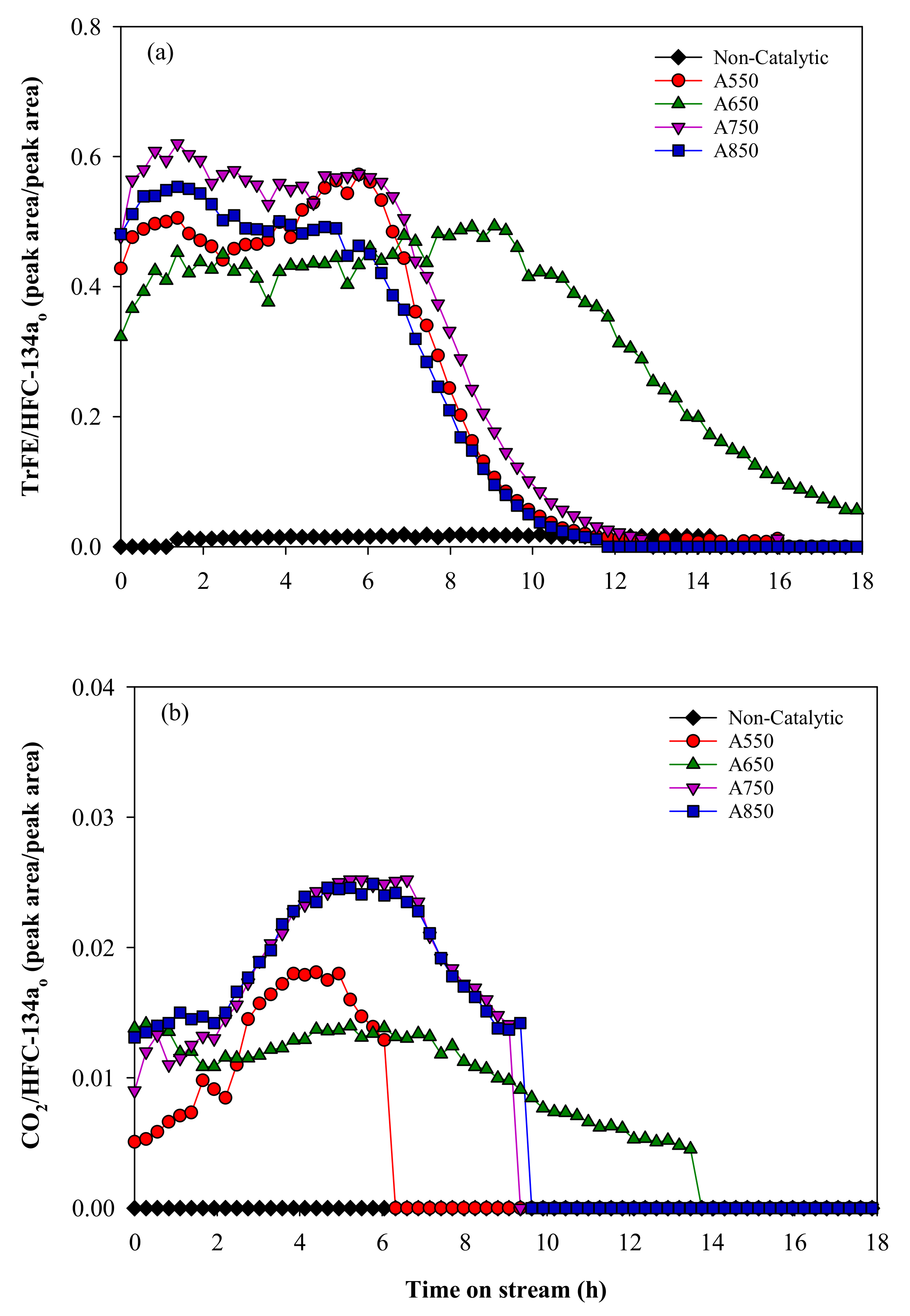
2.1. Catalytic Pyrolysis of HFC-134a
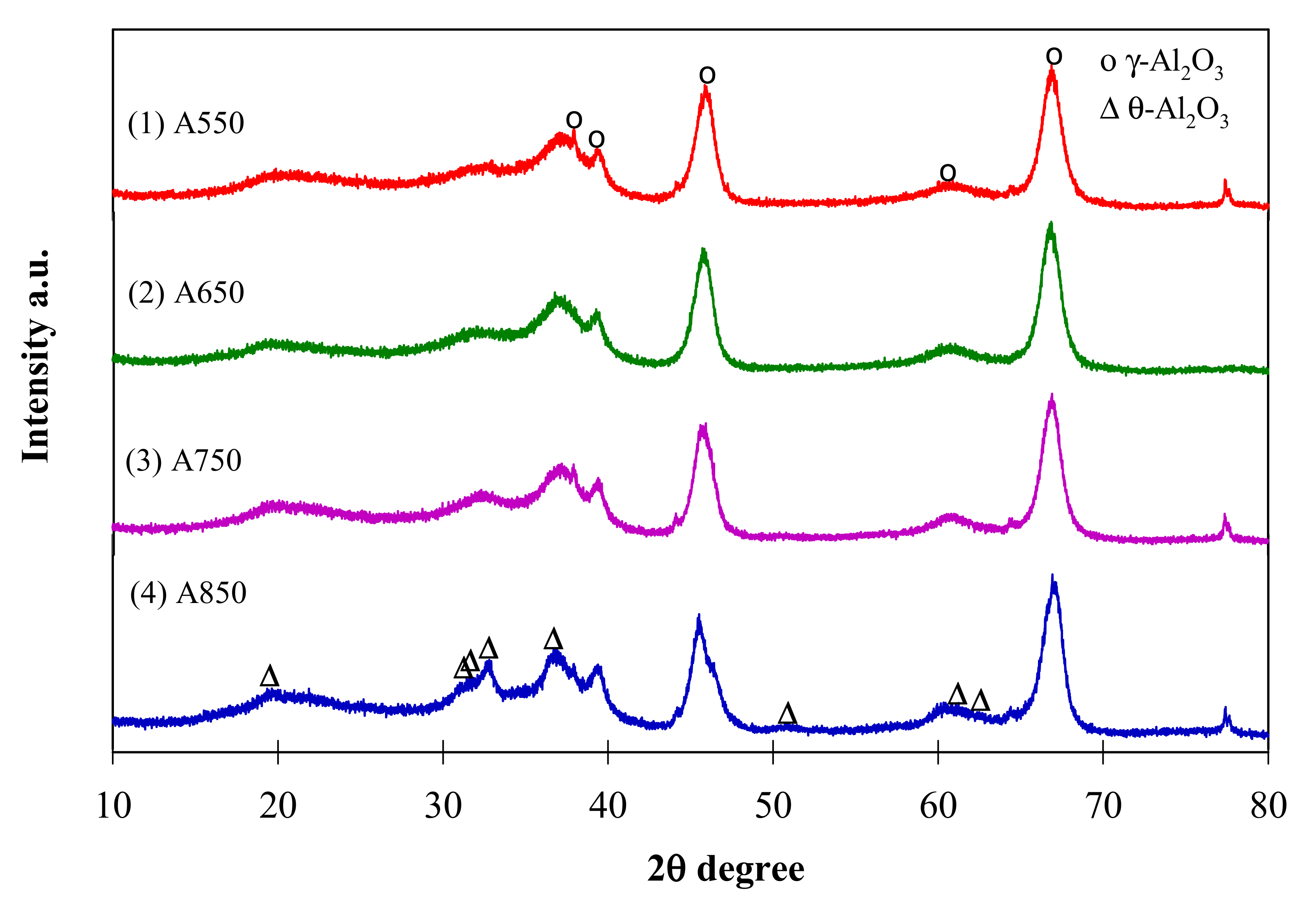
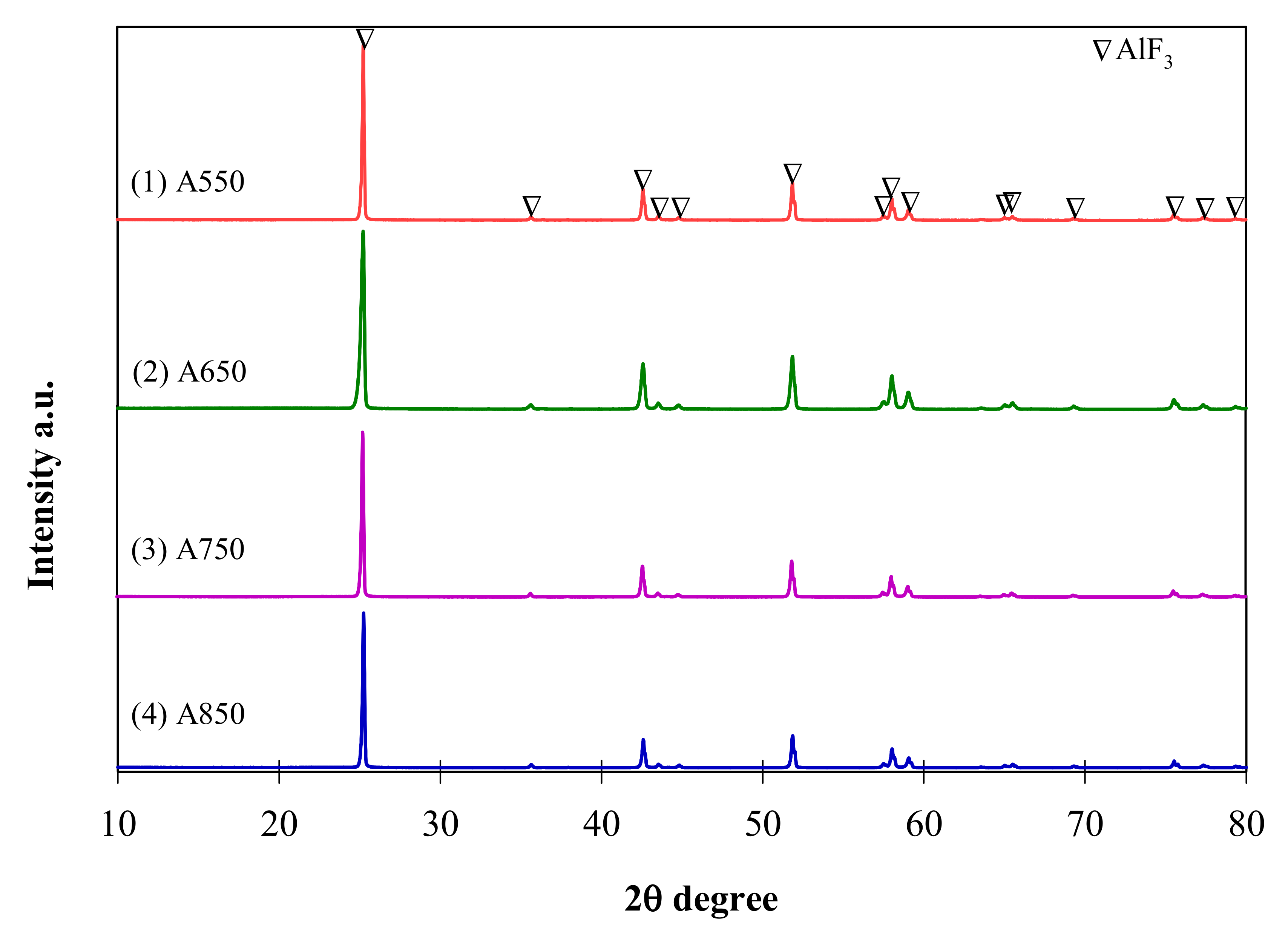
2.2. Physico-Chemical Properties of Catalysts
3. Experimental Section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Temperature in 2020. Available online: https://mailchi.mp/caa/global-temperature-in-2020?e=670df34344 (accessed on 22 April 2021).
- Srinivasan, J. Climate change, greenhouse gases and aerosols. Resonance 2008, 13, 1146–1155. [Google Scholar] [CrossRef]
- Meyer, L. IPCC Fifth Assessment Report Synthesis Report. Bogazici Univ. 2015, 10, 2015. [Google Scholar]
- de Richter, R.; Ming, T.; Davies, P.; Liu, W.; Caillol, S. Removal of non-CO2 greenhouse gases by large-scale atmospheric solar photocatalysis. Prog. Energy Combust. Sci. 2017, 60, 68–96. [Google Scholar] [CrossRef]
- Velders, G.J.M.; Andersen, S.O.; Daniel, J.S.; Fahey, D.W.; McFarland, M. The importance of the Montreal Protocol in protecting climate. Proc. Natl. Acad. Sci. USA 2007, 104, 4814–4819. [Google Scholar] [CrossRef] [Green Version]
- French, D. Kyoto Protocol to the United Nations Framework Convention on Climate Change. J. Environ. Law 1998, 10, 215–224. [Google Scholar] [CrossRef]
- Purohit, P.; Höglund-Isaksson, L.; Dulac, J.; Shah, N.; Wei, M.; Rafaj, P.; Schöpp, W. Electricity savings and greenhouse gas emission reductions from global phase-down of hydrofluorocarbons. Atmos. Chem. Phys. 2020, 20, 11305–11327. [Google Scholar] [CrossRef]
- Hayashi, D.; Michaelowa, A. Lessons from submission and approval process of large-scale energy efficiency CDM methodologies. HWWI Res. Pap. 2007, 4–11. Available online: https://www.econstor.eu/handle/10419/48262 (accessed on 23 August 2021).
- He, X.; Han, J.; Mi, T.; Qin, L. Investigation of HFC-134a decomposition by combustion and its kinetic characteristics in a laboratory scale reactor. Environ. Prot. Eng. 2015, 41, 143–150. [Google Scholar] [CrossRef]
- Han, W.; Li, Y.; Tang, H.; Liu, H. Treatment of the potent greenhouse gas, CHF 3—An overview. J. Fluor. Chem. 2012, 140, 7–16. [Google Scholar] [CrossRef]
- Shin, M.; Jang, D.; Lee, Y.; Kim, Y.; Kim, E. Comprehensive modeling of HFC-23 incineration in a CDM incinerator. J. Mater. Cycles Waste Manag. 2017, 19, 754–762. [Google Scholar] [CrossRef]
- Ohno, M.; Ozawa, Y.; Ono, T. Decomposition of HFC134a Using Arc Plasma. Int. J. Plasma Environ. Sci. Technol. 2007, 1, 159–165. [Google Scholar]
- Mok, Y.S.; Demidyuk, V.; Whitehead, J.C. Decomposition of hydrofluorocarbons in a dielectric-packed plasma reactor. J. Phys. Chem. A 2008, 112, 6586–6591. [Google Scholar] [CrossRef]
- Jasiński, M.; Dors, M.; Mizeraczyk, J. Destruction of freon HFC-134a using a nozzleless microwave plasma source. Plasma Chem. Plasma Process. 2009, 29, 363–372. [Google Scholar] [CrossRef]
- Narengerile; Saito, H.; Watanabe, T. Decomposition of tetrafluoromethane by water plasma generated under atmospheric pressure. Thin Solid Film. 2009, 518, 929–935. [Google Scholar] [CrossRef]
- Gaikwad, V.; Kennedy, E.; Mackie, J.; Holdsworth, C.; Molloy, S.; Kundu, S.; Stockenhuber, M.; Dlugogorski, B. Reaction of dichloromethane under non-oxidative conditions in a dielectric barrier discharge reactor and characterisation of the resultant polymer. Chem. Eng. J. 2016, 290, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Iizuka, A.; Ishizaki, H.; Mizukoshi, A.; Noguchi, M.; Yamasaki, A.; Yanagisawa, Y. Simultaneous decomposition and fixation of F-gases using waste concrete. Ind. Eng. Chem. Res. 2011, 50, 11808–11814. [Google Scholar] [CrossRef]
- Takita, Y.; Tanabe, T.; Ito, M.; Ogura, M.; Muraya, T.; Yasuda, S.; Nishiguchi, H.; Ishihara, T. Decomposition of CH2FCF3 (134a) over metal phosphate catalysts. Ind. Eng. Chem. Res. 2002, 41, 2585–2590. [Google Scholar] [CrossRef]
- Jia, W.; Wu, Q.; Lang, X.; Hu, C.; Zhao, G.; Li, J.; Zhu, Z. Influence of Lewis Acidity on Catalytic Activity of the Porous Alumina for Dehydrofluorination of 1,1,1,2-Tetrafluoroethane to Trifluoroethylene. Catal. Lett. 2015, 145, 654–661. [Google Scholar] [CrossRef]
- Jia, W.; Liu, M.; Lang, X.; Hu, C.; Li, J.; Zhu, Z. Catalytic dehydrofluorination of 1,1,1,2-tetrafluoroethane to synthesize trifluoroethylene over a modified NiO/Al2O3 catalyst. Catal. Sci. Technol. 2015, 5, 3103–3107. [Google Scholar] [CrossRef]
- Han, T.U.; Yoo, B.S.; Kim, Y.M.; Hwang, B.A.; Sudibya, G.L.; Park, Y.K.; Kim, S. Catalytic conversion of 1,1,1,2-tetrafluoroethane (HFC-134a). Korean J. Chem. Eng. 2018, 35, 1611–1619. [Google Scholar] [CrossRef]
- Swamidoss, C.M.A.; Sheraz, M.; Anus, A.; Jeong, S.; Park, Y.K.; Kim, Y.M.; Kim, S. Effect of Mg/Al2O3 and calcination temperature on the catalytic decomposition of HFC-134a. Catalysts 2019, 9, 270. [Google Scholar] [CrossRef] [Green Version]
- Saiki, T.; Sumida, M.; Nakano, S.; Murakami, K. Method for Producing Trifluoroethylene. U.S. Patent 5,283,379, 1 February 1994. [Google Scholar]
- Ito, M.; Kurokawa, N.; Dang, C.; Hihara, E. Disproportionation reaction of HFO-1123 refrigerant. In Proceedings of the Refrigeration Science and Technology, Purdue, IN, USA, 9–12 July 2018. [Google Scholar]
- Mori, T.; Yasuoka, T.; Morikawa, Y. Hydrodechlorination of 1,1,2-trichloro-1,2,2-trifluoroethane (CFC-113) over supported ruthenium and other noble metal catalysts. Catal. Today 2004, 88, 111–120. [Google Scholar] [CrossRef]
- Ohnishi, R.; Wang, W.L.; Ichikawa, M. Selective hydrodechlorination of CFC-113 on Bi- and Tl-modified palladium catalysts. Appl. Catal. AGen. 1994, 113, 29–41. [Google Scholar] [CrossRef]
- Wahyuni, S.; Kunarti, E.S.; Swasono, R.T.; Kartini, I. Characterization and Photocatalytic Activity of TiO2(rod)-SiO2-Polyaniline Nanocomposite. Indones. J. Chem. 2018, 18, 321. [Google Scholar] [CrossRef] [Green Version]
- Weckhuysen, B.M.; Wachs, I.E. Chapter 11—CATALYSIS BY SUPPORTED METAL OXIDES. Handb. Surf. Interfaces Mater. 2001, 1, 613–648. [Google Scholar] [CrossRef] [Green Version]
- Inmanee, T.; Pinthong, P.; Jongsomjit, B. Effect of calcination temperatures and mo modification on nanocrystalline (γ-χ)-Al2O3 catalysts for catalytic ethanol dehydration. J. Nanomater. 2017, 2017, 5018384. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Zhao, C.; Li, Y.; Ma, Z.; Lv, X. Effect of calcination temperature on the structure and catalytic performance of the cu-mcm-41 catalysts for the synthesis of dimethyl carbonate. Quim. Nova 2018, 41, 1156–1161. [Google Scholar] [CrossRef]
- Jin, M.H.; Oh, D.; Park, J.H.; Lee, C.B.; Lee, S.W.; Park, J.S.; Lee, K.Y.; Lee, D.W. Mesoporous silica supported Pd-MnOx catalysts with excellent catalytic activity in room-temperature formic acid decomposition. Sci. Rep. 2016, 6, 33502. [Google Scholar] [CrossRef]
- Iwamoto, M.; Tanaka, Y.; Sawamura, N.; Namba, S. Remarkable Effect of Pore Size on the Catalytic Activity of Mesoporous Silica for the Acetalization of Cyclohexanone with Methanol. J. Am. Chem. Soc. 2003, 125, 13032–13033. [Google Scholar] [CrossRef]
- Hongmanorom, P.; Luengnaruemitchai, A.; Chollacoop, N.; Yoshimura, Y. Effect of the Pd/MCM-41 Pore Size on the Catalytic Activity and cis–trans Selectivity for Partial Hydrogenation of Canola Biodiesel. Energy Fuels 2017, 31, 8202–8209. [Google Scholar] [CrossRef]



| Catalyst | Amount of Acidic Sites (mmol-NH3/g-Catalyst) | Coke (%) | ||||
|---|---|---|---|---|---|---|
| Weak | Moderate | Strong | Total | (Weak + Moderate)/Strong | ||
| A550 | 0.246 | 0.256 | 0.445 | 0.947 | 1.127 | 12.40 |
| A650 | 0.217 | 0.297 | 0.343 | 0.857 | 1.498 | 11.78 |
| A750 | 0.280 | 0.282 | 0.300 | 0.862 | 1.878 | 11.47 |
| A850 | 0.255 | 0.263 | 0.453 | 0.971 | 1.144 | 13.97 |
| Catalyst | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) |
|---|---|---|
| A550 | 141.2 ± 0.3 | 0.821 |
| A650 | 140.8 ± 0.3 | 0.847 |
| A750 | 130.5 ± 0.3 | 0.758 |
| A850 | 119.8 ± 0.3 | 0.817 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheraz, M.; Anus, A.; Thi Le, V.C.; Andrew Swamidoss, C.M.; Kim, S. The Effect of Catalyst Calcination Temperature on Catalytic Decomposition of HFC-134a over γ-Al2O3. Catalysts 2021, 11, 1021. https://doi.org/10.3390/catal11091021
Sheraz M, Anus A, Thi Le VC, Andrew Swamidoss CM, Kim S. The Effect of Catalyst Calcination Temperature on Catalytic Decomposition of HFC-134a over γ-Al2O3. Catalysts. 2021; 11(9):1021. https://doi.org/10.3390/catal11091021
Chicago/Turabian StyleSheraz, Mahshab, Ali Anus, Van Cam Thi Le, Caroline Mercy Andrew Swamidoss, and Seungdo Kim. 2021. "The Effect of Catalyst Calcination Temperature on Catalytic Decomposition of HFC-134a over γ-Al2O3" Catalysts 11, no. 9: 1021. https://doi.org/10.3390/catal11091021







