Tailoring g-C3N4 with Lanthanum and Cobalt Oxides for Enhanced Photoelectrochemical and Photocatalytic Activity
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.2. Synthesis of g-C3N4
2.3. Synthesis of La2O3–g-C3N4, CoO–g-C3N4, and La2O3–CoO–g-C3N4 Nanocomposites
- (i)
- 50% by weight each of lanthanum (III) nitrate hexahydrate with g-C3N4 nanoflakes,
- (ii)
- 50% by weight each of cobalt (II) nitrate hexahydrate and g-C3N4 nanoflakes, and
- (iii)
- 25% by weight, each of lanthanum (III) nitrate hexahydrate and cobalt (II) nitrate hexahydrate solutions and 50% by weight of g-C3N4 nanoflakes solutions were prepared in deionized water.
2.4. Characterization
2.5. Photoelectrochemical Measurements and Setup
2.6. Photocatlytic Dye Degradation Studies
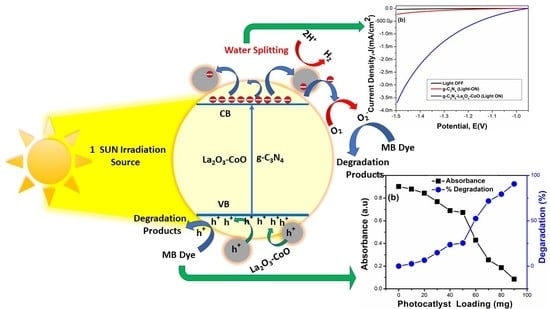
3. Results and Discussion
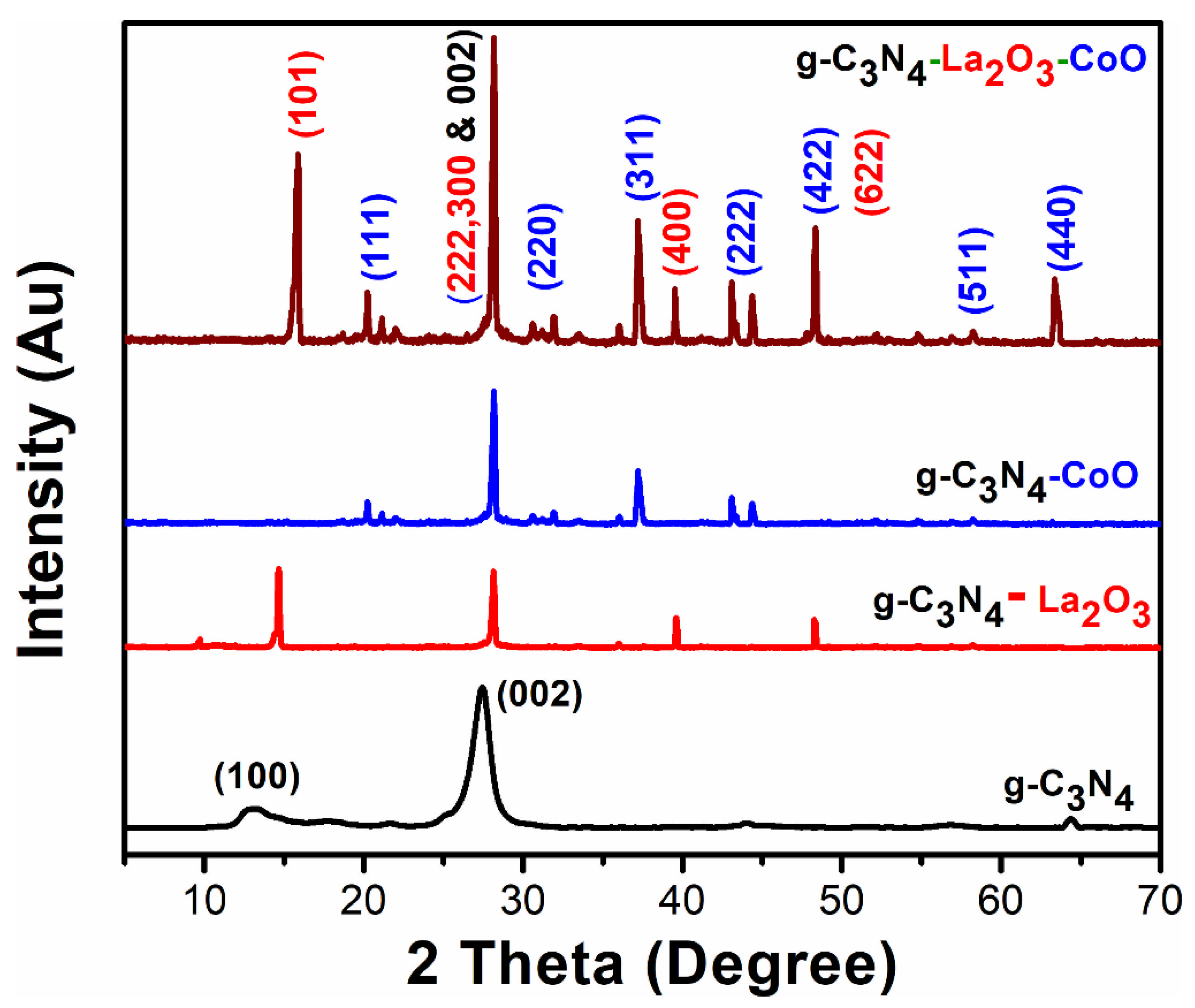
3.1. X-ray Diffraction (XRD) Analysis
3.2. Surface Morphology and EDX Analysis
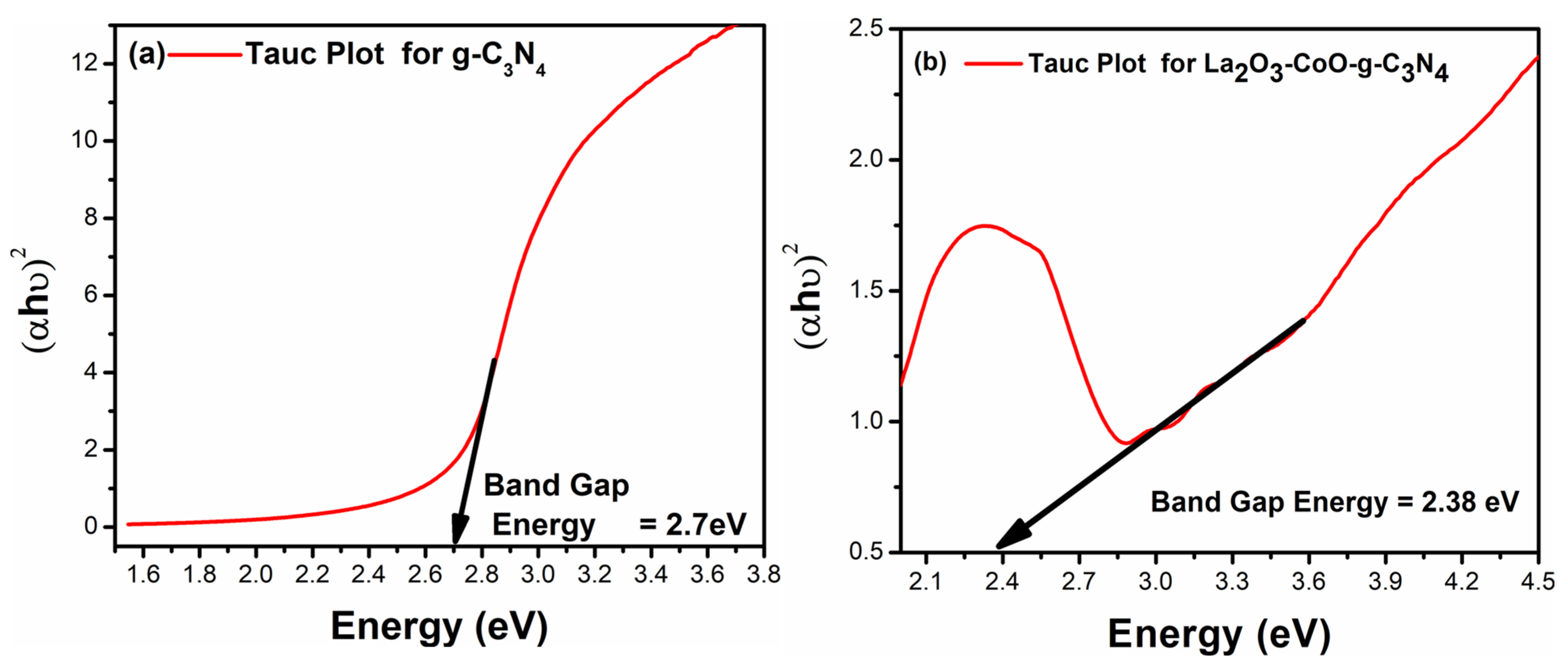
3.3. UV–Visible Spectroscopy
3.4. FTIR Spectroscopy
3.5. Photoelectrochemical Measurements
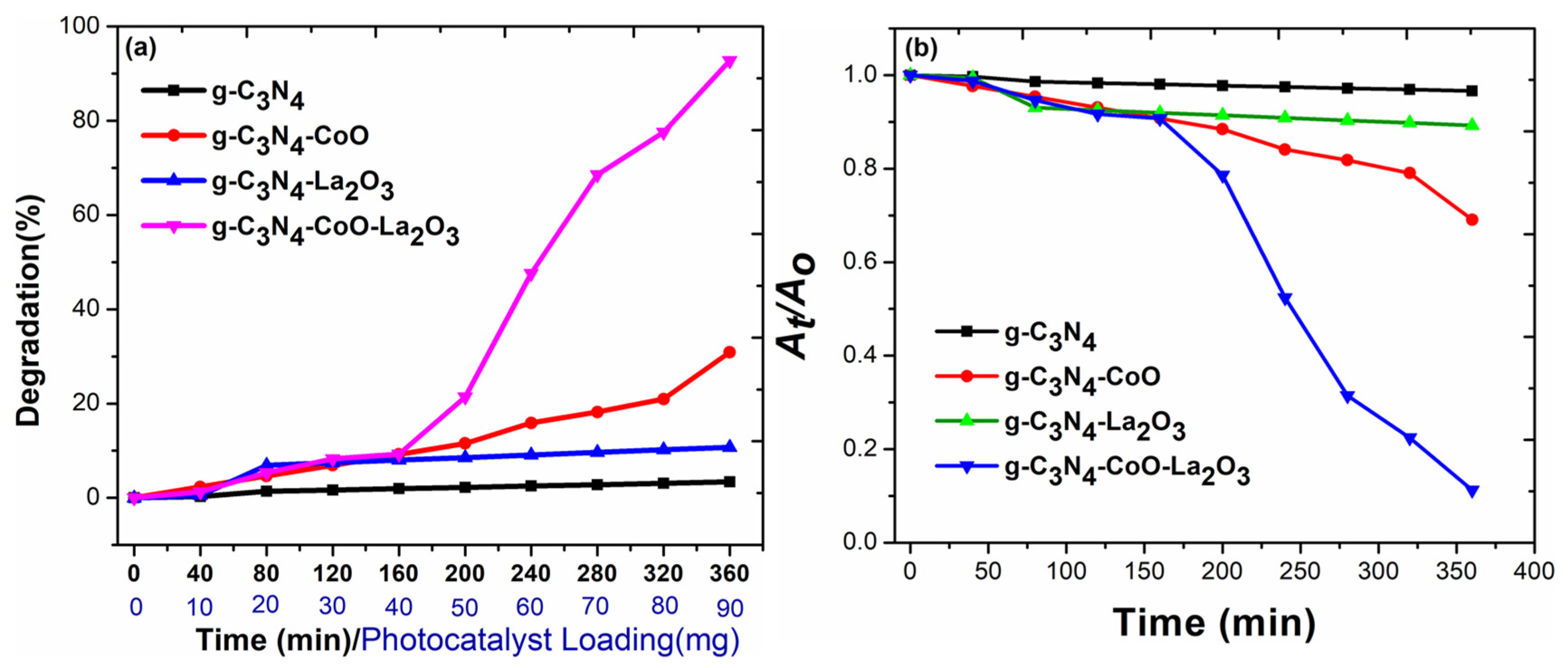
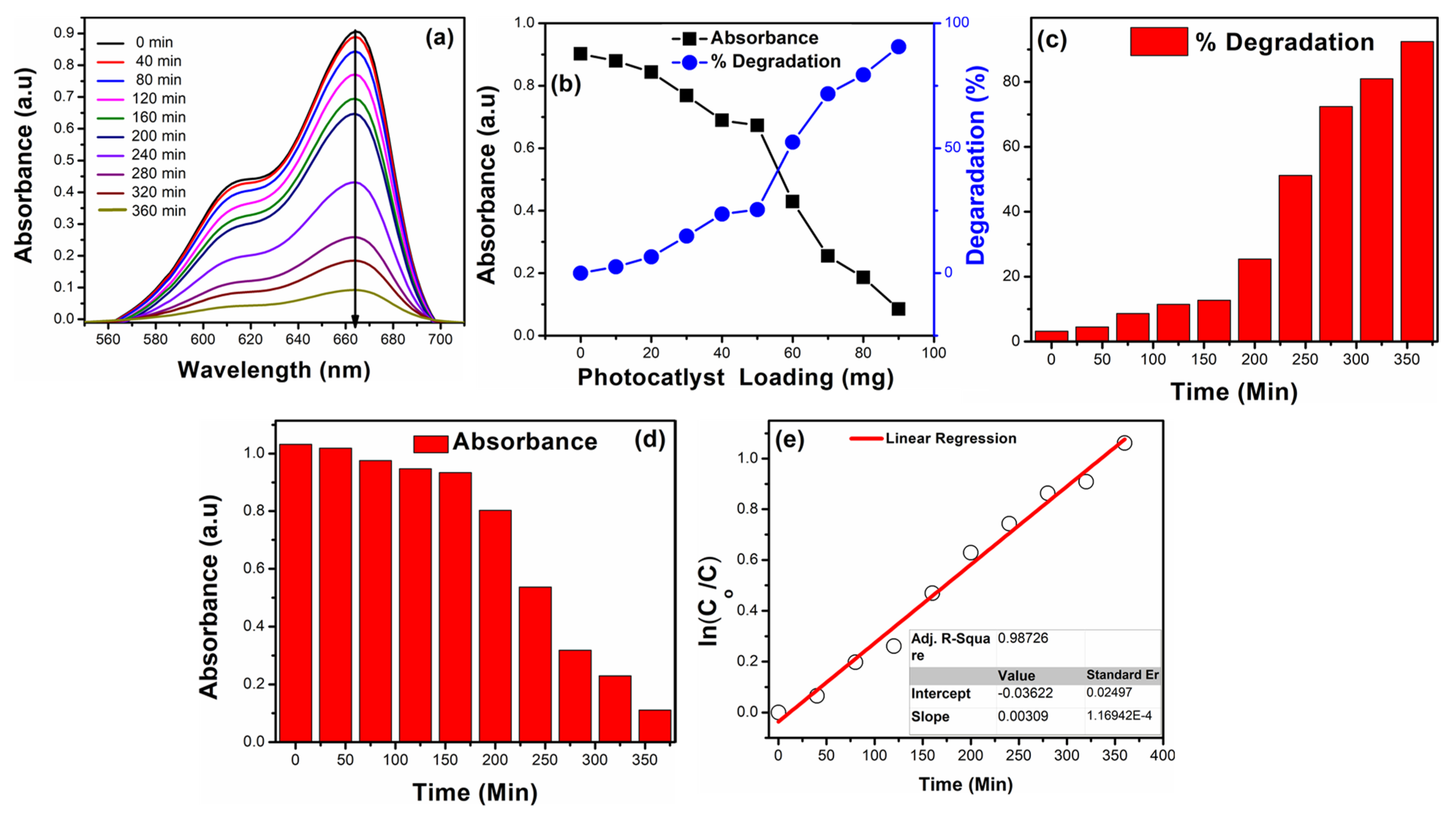
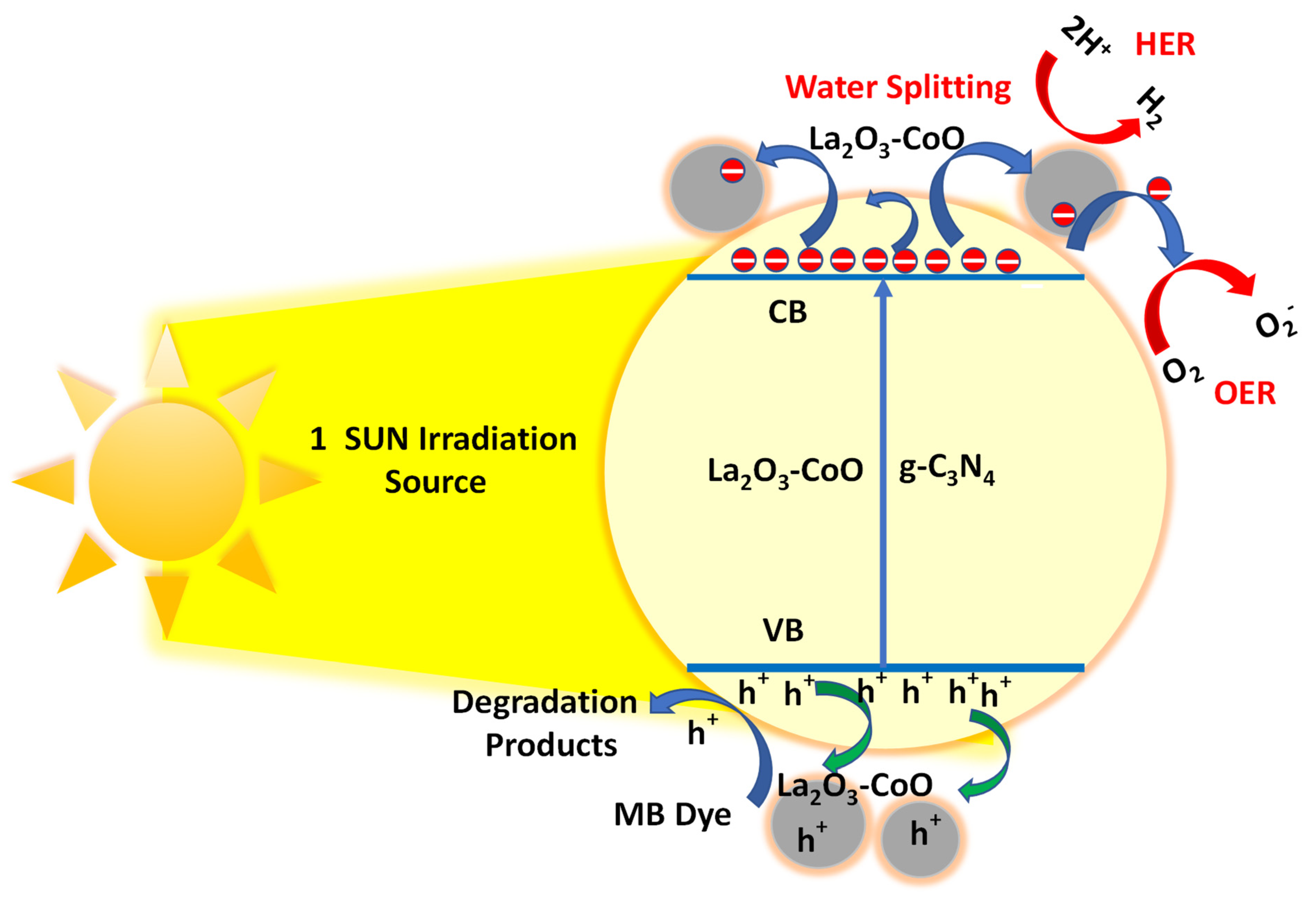
3.6. Methylene Blue Photodegradation Studies
- Photon’s absorption:(hν ≥ band energy = 2.38 eV for La2O3-CoO-g-C3N4)La2O3-CoO-g-C3N4 + hν → La +3/Co+2/g-C3N4 [CBe− + VBh+]
- Ionosorption of oxygen to :
- Neutralization of OH− groups and OH• radical formation by photoholes(H2O ⇌ H+ + OH−)ads + VBh+ + H+ + OH●
- neutralization of by protons:
- Dismutation of oxygen with transient hydrogen peroxide formation:
- The disintegration of H2O2 and subsequent oxygen reduction:
- Oxidation of the organic reactant of MB by radicals:
- Direct oxidation by reaction with holes to generate degradation products
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Che, H.; Ngaw, C.K.; Hu, P.; Wang, J.; Li, Y.; Wang, X.; Teng, W. Fabrication of molybdenum doped carbon nitride nanosheets for efficiently photocatalytic water splitting. J. Alloy. Compd. 2020, 849, 156440. [Google Scholar] [CrossRef]
- Guo, C.; Tian, K.; Wang, L.; Liang, F.; Wang, F.; Chen, D.; Ning, J.; Zhong, Y.; Hu, Y. Approach of fermi level and electron-trap level in cadmium sulfide nanorods via molybdenum doping with enhanced carrier separation for boosted photocatalytic hydrogen production. J. Colloid Interface Sci. 2021, 583, 661–671. [Google Scholar] [CrossRef]
- Guo, C.; Li, L.; Chen, F.; Ning, J.; Zhong, Y.; Hu, Y. One-step phosphorization preparation of gradient-P-doped CdS/CoP hybrid nanorods having multiple channel charge separation for photocatalytic reduction of water. J. Colloid Interface Sci. 2021, 596, 431–441. [Google Scholar] [CrossRef]
- Navarro Yerga, R.M.; Alvarez-Galván, M.C.; Vaquero, F.; Arenales, J.; Fierro, J.L.G. Chapter 3-Hydrogen Production from Water Splitting Using Photo-Semiconductor Catalysts. In Renewable Hydrogen Technologies; Gandía, L.M., Arzamendi, G., Diéguez, P.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 43–61. [Google Scholar]
- Sivula, K.; van de Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Han, H.S.; Park, W.; Sivanantham, A.; Hwang, S.W.; Surendran, S.; Sim, U.; Cho, I.S. Facile fabrication of nanotubular heterostructure for enhanced photoelectrochemical performance. Ceram. Int. 2021, 47, 3972–3977. [Google Scholar] [CrossRef]
- Sim, Y.; John, J.; Surendran, S.; Moon, B.; Sim, U. Efficient Photoelectrochemical Water Splitting Reaction using Electrodeposited Co3Se4 Catalyst. Appl. Sci. 2018, 9, 16. [Google Scholar] [CrossRef] [Green Version]
- Stroyuk, O.; Raievska, O.; Zahn, D.R.T. Graphitic carbon nitride nanotubes: A new material for emerging applications. RSC Adv. 2020, 10, 34059–34087. [Google Scholar] [CrossRef]
- de Oliveira, D.C.; Silva, W.O.; Chatenet, M.; Lima, F.H.B. NiOx-Pt/C nanocomposites: Highly active electrocatalysts for the electrochemical oxidation of hydrazine. Appl. Catal. B Environ. 2017, 201, 22–28. [Google Scholar] [CrossRef]
- Ledendecker, M.; Calderón, S.K.; Papp, C.; Steinrück, H.-P.; Antonietti, M.; Shalom, M. The Synthesis of Nanostructured Ni5P4 Films and their Use as a Non-Noble Bifunctional Electrocatalyst for Full Water Splitting. Angew. Chem. Int. Ed. 2015, 54, 12361–12365. [Google Scholar] [CrossRef]
- Han, H.S.; Park, W.; Hwang, S.W.; Kim, H.; Sim, Y.; Surendran, S.; Sim, U.; Cho, I.S. (0 2 0)-Textured tungsten trioxide nanostructure with enhanced photoelectrochemical activity. J. Catal. 2020, 389, 328–336. [Google Scholar] [CrossRef]
- Chen, R.-R.; Ren, Q.-F.; Liu, Y.-X.; Ding, Y.; Zhu, H.-T.; Xiong, C.-Y.; Jin, Z.; Oh, W.-C. Synthesis of g-C3N4/diatomite/MnO2 composites and their enhanced photo-catalytic activity driven by visible light. J. Korean Ceram. Soc. 2021, 58, 548–558. [Google Scholar] [CrossRef]
- Huang, Y.; Ning, L.; Feng, Z.; Ma, G.; Yang, S.; Su, Y.; Hong, Y.; Wang, H.; Peng, L.; Li, J. Graphitic carbon nitride nanosheets with low ON1-doping content as efficient photocatalysts for organic pollutant degradation. Environ. Sci. Nano 2021, 8, 460–469. [Google Scholar] [CrossRef]
- Cai, W.-Q.; Zhang, D.-F.; Zhang, F.-J.; Oh, W.-C. Preparation and photocatalytic activity of a novel BiOCl/g-C3N4 thin film prepared via spin coating. J. Korean Ceram. Soc. 2020, 57, 331–337. [Google Scholar] [CrossRef]
- Huang, C.; Wen, Y.; Ma, J.; Dong, D.; Shen, Y.; Liu, S.; Ma, H.; Zhang, Y. Unraveling fundamental active units in carbon nitride for photocatalytic oxidation reactions. Nat. Commun. 2021, 12, 320. [Google Scholar] [CrossRef]
- Wojtyła, S.; Śpiewak, K.; Baran, T. Synthesis, characterization and activity of doped graphitic carbon nitride materials towards photocatalytic oxidation of volatile organic pollutants emitted from 3D printer. J. Photochem. Photobiol. A Chem. 2020, 391, 112355. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- He, F.; Chen, G.; Zhou, Y.; Yu, Y.; Li, L.; Hao, S.; Liu, B. ZIF-8 derived carbon (C-ZIF) as a bifunctional electron acceptor and HER cocatalyst for g-C3N4: Construction of a metal-free, all carbon-based photocatalytic system for efficient hydrogen evolution. J. Mater. Chem. A 2016, 4, 3822–3827. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, I.; Yamani, Z.H.; Qurashi, A. Sonochemical Assisted Solvothermal Synthesis of Gallium Oxynitride Nanosheets and their Solar-Driven Photoelectrochemical Water-Splitting Applications. Sci. Rep. 2016, 6, 32319. [Google Scholar] [CrossRef] [Green Version]
- Sonya, K.; Yuriy, P.; Ivan, T.; Kazuma, M.; Jin, U.; Yutaka, K.; Kikuo, M.; Takeyoshi, S.; Takuya, M.; Daisuke, F.; et al. Tandem photovoltaic–photoelectrochemical GaAs/InGaAsP–WO3/BiVO4 device for solar hydrogen generation. Jpn. J. Appl. Phys. 2016, 55, 04ES01. [Google Scholar]
- Bellardita, M.; García-López, E.I.; Marcì, G.; Krivtsov, I.; García, J.R.; Palmisano, L. Selective photocatalytic oxidation of aromatic alcohols in water by using P-doped g-C3N4. Appl. Catal. B Environ. 2018, 220, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Salcedo, M.; Monge, M.; Tena, M.T. Photocatalytic degradation of ibuprofen in water using TiO2/UV and g-C3N4/visible light: Study of intermediate degradation products by liquid chromatography coupled to high-resolution mass spectrometry. Chemosphere 2019, 215, 605–618. [Google Scholar] [CrossRef]
- Navarro-Aguilar, A.I.; Obregón, S.; Sanchez-Martinez, D.; Hernández-Uresti, D.B. An efficient and stable WO3/g-C3N4 photocatalyst for ciprofloxacin and orange G degradation. J. Photochem. Photobiol. A Chem. 2019, 384, 112010. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.; Qian, X.; Dong, Y.; Gao, J.; Qian, G.; Yao, J. Direct Synthesis of Porous Nanorod-Type Graphitic Carbon Nitride/CuO Composite from Cu-Melamine Supramolecular Framework towards Enhanced Photocatalytic Performance. Chem. Asian J. 2015, 10, 1276–1280. [Google Scholar] [CrossRef]
- Rahman, M.; Davey, K.; Mullins, C.B. Tuning the Intrinsic Properties of Carbon Nitride for High Quantum Yield Photocatalytic Hydrogen Production. Adv. Sci. 2018, 5, 1800820. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.; Zhao, S.; Li, Z.; Lin, Z. Noble metal–metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Niu, P.; Yang, Y.; Yu, J.C.; Liu, G.; Cheng, H.-M. Switching the selectivity of the photoreduction reaction of carbon dioxide by controlling the band structure of a g-C3N4photocatalyst. Chem. Commun. 2014, 50, 10837–10840. [Google Scholar] [CrossRef]
- Yuan, L.; Xu, Y.-J. Photocatalytic conversion of CO2 into value-added and renewable fuels. Appl. Surf. Sci. 2015, 342, 154–167. [Google Scholar] [CrossRef]
- Liu, H.; Wu, P.; Li, H.; Chen, Z.; Wang, L.; Zeng, X.; Zhu, Y.; Jiang, Y.; Liao, X.; Haynes, B.S.; et al. Unravelling the effects of layered supports on Ru nanoparticles for enhancing N2 reduction in photocatalytic ammonia synthesis. Appl. Catal. B Environ. 2019, 259, 118026. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X. Two dimensional conjugated polymers with enhanced optical absorption and charge separation for photocatalytic hydrogen evolution. Energy Environ. Sci. 2014, 7, 1902–1906. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Wang, Y.; Qin, H.; Li, X.; Zuo, Y.; Kang, S.; Cui, L. Synthesis of Mo-doped graphitic carbon nitride catalysts and their photocatalytic activity in the reduction of CO2 with H2O. Catal. Commun. 2016, 74, 75–79. [Google Scholar] [CrossRef]
- Su, F.; Mathew, S.C.; Lipner, G.; Fu, X.; Antonietti, M.; Blechert, S.; Wang, X. mpg-C3N4-Catalyzed Selective Oxidation of Alcohols Using O2 and Visible Light. J. Am. Chem. Soc. 2010, 132, 16299–16301. [Google Scholar] [CrossRef] [PubMed]
- Butchosa, C.; Guiglion, P.; Zwijnenburg, M.A. Carbon Nitride Photocatalysts for Water Splitting: A Computational Perspective. J. Phys. Chem. C 2014, 118, 24833–24842. [Google Scholar] [CrossRef]
- Martin, D.J.; Qiu, K.; Shevlin, S.A.; Handoko, A.D.; Chen, X.; Guo, Z.; Tang, J. Highly Efficient Photocatalytic H2 Evolution from Water using Visible Light and Structure-Controlled Graphitic Carbon Nitride. Angew. Chem. Int. Ed. 2014, 53, 9240–9245. [Google Scholar] [CrossRef] [Green Version]
- Habisreutinger, S.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic Reduction of CO2on TiO2and Other Semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- Jorge, A.B.; Martin, D.J.; Dhanoa, M.T.S.; Rahman, A.S.; Makwana, N.; Tang, J.; Sella, A.; Corà, F.; Firth, S.; Darr, J.A.; et al. H2 and O2 Evolution from Water Half-Splitting Reactions by Graphitic Carbon Nitride Materials. J. Phys. Chem. C 2013, 117, 7178–7185. [Google Scholar] [CrossRef]
- Guan, M.; Wang, Q.; Zhang, X.; Bao, J.; Gong, X.; Liu, Y. Two-Dimensional Transition Metal Oxide and Hydroxide-Based Hierarchical Architectures for Advanced Supercapacitor Materials. Front. Chem. 2020, 8, 390. [Google Scholar] [CrossRef]
- Fang, S.; Bresser, D.; Passerini, S. Transition Metal Oxide Anodes for Electrochemical Energy Storage in Lithium- and Sodium-Ion Batteries. Adv. Energy Mater. 2019, 10, 1902485. [Google Scholar] [CrossRef]
- Xu, J.; Yu, H.; Guo, H. Synthesis and behaviors of g-C3N4 coupled with LaxCo3-xO4 nanocomposite for improved photocatalytic activeity and stability under visible light. Mater. Res. Bull. 2018, 105, 342–348. [Google Scholar] [CrossRef]
- Modak, B.; Ghosh, S.K. Exploring the Role of La Codoping beyond Charge Compensation for Enhanced Hydrogen Evolution by Rh–SrTiO3. J. Phys. Chem. B 2015, 119, 11089–11098. [Google Scholar] [CrossRef]
- Jin, L.; Zhou, X.; Ning, X.; Zhan, L.; Kong, M.; Tan, K.; Li, J.; Lin, Z. Boosting visible light photocatalytic performance of g-C3N4 nanosheets by combining with LaFeO3 nanoparticles. Mater. Res. Bull. 2018, 102, 353–361. [Google Scholar] [CrossRef]
- Li, N.; Jayaraman, S.; Tee, S.Y.; Kumar, P.S.; Lee, C.J.J.; Liew, S.L.; Chi, D.; Hor, T.S.A.; Ramakrishna, S.; Luo, H.-K. Effect of La-Doping on optical bandgap and photoelectrochemical performance of hematite nanostructures. J. Mater. Chem. A 2014, 2, 19290–19297. [Google Scholar] [CrossRef]
- Arabi, A.; Fazli, M.; Ehsani, M. Tuning the morphology and photocatalytic activity of La0.7Ca0.3MnO3 nanorods via different mineralizer-assisted hydrothermal syntheses. Mater. Res. Bull. 2017, 90, 205–211. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, K.; Yan, L.; Dai, Y.; Guo, H.; Huo, M.; Guo, Y. Fabrication of carbon nitride nanotubes by a simple water-induced morphological transformation process and their efficient visible-light photocatalytic activity. RSC Adv. 2014, 4, 59513–59518. [Google Scholar] [CrossRef]
- Rehman, A.; Ehsan, M.A.; Afzal, A.; Ali, A.; Iqbal, N. Aerosol-assisted nanostructuring of nickel/cobalt oxide thin films for viable electrochemical hydrazine sensing. Analyst 2021, 146, 3317–3327. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, M.A.; Aziz, A.; Rehman, A.; Hakeem, A.S.; Qasem, M.A.A.; Saadi, O.W. Facile Synthesis of Gold-Supported Thin Film of Cobalt Oxide via AACVD for Enhanced Electrocatalytic Activity in Oxygen Evolution Reaction. ECS J. Solid State Sci. Technol. 2018, 7, P711–P718. [Google Scholar] [CrossRef]
- Pathan, A.A.; Desai, K.R.; Bhasin, C. Synthesis of La2O3 Nanoparticles using Glutaric acid and Propylene glycol for Future CMOS Applications. Int. J. Nanomater. Chem. 2017, 3, 21–25. [Google Scholar] [CrossRef]
- Iqbal, N.; Afzal, A.; Khan, I.; Khan, M.S.; Qurashi, A. Molybdenum impregnated g-C3N4 nanotubes as potentially active photocatalyst for renewable energy applications. Sci. Rep. 2021, 11, 16886. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, I.; Ali, A.; Qurashi, A. A sustainable molybdenum oxysulphide-cobalt phosphate photocatalyst for effectual solar-driven water splitting. J. Adv. Res. 2021. [Google Scholar] [CrossRef]
- Han, C.; Ge, L.; Chen, C.; Li, Y.; Xiao, X.; Zhang, Y.; Guo, L. Novel visible light induced Co3O4-g-C3N4 heterojunction photocatalysts for efficient degradation of methyl orange. Appl. Catal. B Environ. 2014, 147, 546–553. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Wen, Z.; Cui, S.; Yuan, C.; Chen, J. Co3O4 nanoparticles embedded in nitrogen-doped porous carbon dodecahedrons with enhanced electrochemical properties for lithium storage and water splitting. Nano Energy 2015, 12, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.-D.; Liu, X.-Y.; Cui, S.-C.; Liu, J.-G. Loading of Co3O4 onto Pt-modified nitrogen-doped TiO2 nanocomposites promotes photocatalytic hydrogen production. RSC Adv. 2017, 7, 25650–25656. [Google Scholar] [CrossRef] [Green Version]
- Goodall, J.B.M.; Kellici, S.; Illsley, D.; Lines, R.; Knowles, J.C.; Darr, J.A. Optical and photocatalytic behaviours of nanoparticles in the Ti–Zn–O binary system. RSC Adv. 2014, 4, 31799–31809. [Google Scholar] [CrossRef]
- Schevciw, O.; White, W.B. The optical absorption edge of rare earth sesquisulfides and alkaline earth-rare earth sulfides. Mater. Res. Bull. 1983, 18, 1059–1068. [Google Scholar] [CrossRef]
- Köferstein, R.; Jäger, L.; Ebbinghaus, S. Magnetic and optical investigations on LaFeO3 powders with different particle sizes and corresponding ceramics. Solid State Ionics 2013, 249–250, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Hao, H.; Liu, Y.; Zhu, Y.; Zhang, X. Rapid Screening of Graphitic Carbon Nitrides for Photocatalytic Cofactor Regeneration Using a Drop Reactor. Micromachines 2017, 8, 175. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Rodil, S.E.; Robertson, J. Interpretation of infrared and Raman spectra of amorphous carbon nitrides. Phys. Rev. B 2003, 67, 155306. [Google Scholar] [CrossRef] [Green Version]
- Jürgens, B.; Irran, E.; Senker, J.; Kroll, P.; Müller, H.; Schnick, W. Melem (2,5,8-Triamino-tri-s-triazine), an Important Intermediate during Condensation of Melamine Rings to Graphitic Carbon Nitride: Synthesis, Structure Determination by X-ray Powder Diffractometry, Solid-State NMR, and Theoretical Studies. J. Am. Chem. Soc. 2003, 125, 10288–10300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, M.; Sharma, M.; Mahajan, S.; Sheikh, H.N.; Kalsotra, B.L. Synthesis and Characterization of Group-6 Metal Carbonyl Complexes of Aroyl Hydrazone Derivatives. E-J. Chem. 2012, 9, 807–817. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Zeng, M.; Li, J.; Xu, J.; Wang, X. Bandgap Engineering and Mechanism Study of Nonmetal and Metal Ion Codoped Carbon Nitride: C+Fe as an Example. Chem. Eur. J. 2014, 20, 9805–9812. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Li, Q.; Lu, W.; Ning, J.; Zhong, Y.; Zhang, Z.; Hu, Y. Molecule-assisted modulation of the high-valence Co3+ in 3D honeycomb-like CoxSy networks for high-performance solid-state asymmetric supercapacitors. Sci. China Mater. 2021, 64, 840–851. [Google Scholar] [CrossRef]
- Niu, H.; Liu, Y.; Mao, B.; Xin, N.; Jia, H.; Shi, W. In-situ embedding MOFs-derived copper sulfide polyhedrons in carbon nanotube networks for hybrid supercapacitor with superior energy density. Electrochim. Acta 2020, 329, 135130. [Google Scholar] [CrossRef]
- Li, L.; Guo, C.; Shen, J.; Ning, J.; Zhong, Y.; Hu, Y. Construction of sugar-gourd-shaped CdS/Co1-xS hollow hetero-nanostructure as an efficient Z-scheme photocatalyst for hydrogen generation. Chem. Eng. J. 2020, 400, 125925. [Google Scholar] [CrossRef]
- Alkaykh, S.; Mbarek, A.; Ali-Shattle, E.E. Photocatalytic degradation of methylene blue dye in aqueous solution by MnTiO3 nanoparticles under sunlight irradiation. Heliyon 2020, 6, e03663. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.W. Purification of water with near—u.v. illuminated suspensions of titanium dioxide. Water Res. 1990, 24, 653–660. [Google Scholar] [CrossRef]
- He, H.Y.; Dong, W.X.; Zhang, G.H. Photodegradation of aqueous methyl orange on MnTiO3 powder at different initial pH. Res. Chem. Intermed. 2010, 36, 995–1001. [Google Scholar] [CrossRef]
- Azeez, F.; Al-Hetlani, E.; Arafa, M.; Abdelmonem, Y.; Nazeer, A.A.; Amin, M.O.; Madkour, M. The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep. 2018, 8, 7104. [Google Scholar] [CrossRef] [PubMed]
- Kuila, S.K.; Sarkar, R.; Kumbhakar, P.; Kumbhakar, P.; Tiwary, C.S.; Kundu, T.K. Photocatalytic dye degradation under sunlight irradiation using cerium ion adsorbed two-dimensional graphitic carbon nitride. J. Environ. Chem. Eng. 2020, 8, 103942. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, N. Tailoring g-C3N4 with Lanthanum and Cobalt Oxides for Enhanced Photoelectrochemical and Photocatalytic Activity. Catalysts 2022, 12, 15. https://doi.org/10.3390/catal12010015
Iqbal N. Tailoring g-C3N4 with Lanthanum and Cobalt Oxides for Enhanced Photoelectrochemical and Photocatalytic Activity. Catalysts. 2022; 12(1):15. https://doi.org/10.3390/catal12010015
Chicago/Turabian StyleIqbal, Naseer. 2022. "Tailoring g-C3N4 with Lanthanum and Cobalt Oxides for Enhanced Photoelectrochemical and Photocatalytic Activity" Catalysts 12, no. 1: 15. https://doi.org/10.3390/catal12010015