1. Introduction
Despite the active development of renewable energy sources, the resource of crude oil remains still a major contributor to the energy sector. According to forecasts, the average daily oil consumption by 2040 will increase to 109.4 million barrels. The depletion of the reserves of light and medium oils is forcing oil refineries to switch to hydrocarbon raw materials such as heavy and extra heavy oil [
1,
2,
3,
4,
5]. However, their processing according to the classical schemes of thermal and catalytic cracking has proven to be unprofitable due to the high content of resin–asphaltene substances (RASs) [
6,
7,
8]. The presence of these components in the structure, primarily asphaltenes, S, N, and O heteroatoms, among others, causes their tendency to condense on the active sites of catalysts [
7,
8]. This prevents their transformation into liquid products, and, accordingly, leads to a decrease in the yield of valuable fractions. In addition, polycondensation products accumulate on the pieces of the equipment, which ultimately leads to a decrease in its service life. Hence, there is an urgent need to develop catalytic systems for heavy oil feedstock processing, the main task of which will be to reduce the content of asphaltenes and increase the yield of light fractions [
9].
The large production of marketable oil products from heavy hydrocarbon feedstock is impossible since it is characterized by a low H/C ratio. Since the use of widely accepted hydrotreatment with the purpose to increase this ratio is economically unviable, the catalytic aquathermolysis of heavy oil seems to be a promising method. In this process, water acts as a hydrogen donor and also a hydrocarbon solvent [
10]. Active hydrogen is formed due to the water–gas shift (WGS) reaction [
10], and water dissociation on the catalyst surface [
11]. However, the efficiency of converting water into hydrogen is low [
2]. Despite the fact that, after catalytic aquathermolysis, the viscosity of heavy oils decreases, secondary condensation processes can occur with the formation of resins and asphaltenes and an increase in viscosity. Various hydrogen donors can close the active chains formed during the catalytic aquathermolysis of heavy oil [
12,
13,
14,
15,
16].
In general, the use of hydrogen donors makes it possible not only to increase the yield of light fractions but also to suppress the processes of coke formation [
17,
18]. Not only water but also formic acid [
19], methane [
20], isopropanol [
21,
22], cyclohexane [
23], tetralin [
24], decalin [
25], and other substances [
26] can be used as donors. The efficiency of their use depends on a number of factors, such as the nature of the oil [
27], the catalyst, and the conditions for upgrading [
24,
28]. In a number of works, hydrogen donors (tetralin and decalin) and gaseous hydrogen were compared during the cracking of heavy oil in the presence of nickel–molybdenum catalysts [
24,
29]. The results suggest the efficiency of using hydrogen donors to upgrade oil with a low coke yield. This is due to the fact that atomic hydrogen, unlike molecular hydrogen, arrests the polymerization of free radicals with the formation of stable and low molecular weight molecules.
To achieve the maximum effect from upgrading heavy oil in the presence of a hydrogen donor, it is worth taking into account the H-donor index and the hydrogen transfer index. The effect of the use of naphthalene derivatives on the composition of products of the vacuum residue cracking was described by [
30]. It was shown that the efficiency of these compounds as hydrogen donors increased in the following order: 1-methylnaphthalene < decalin < naphthalene < tetralin. The obtained data correlated with the values of the H-donor index, where it was the highest for tetralin. Jia-Kai Bai et al. found a relationship between the activity of hydrogen donors and the structure of their molecules [
31]. An analysis of the diagram of the highest occupied molecular orbitals (HOMOs) of tetralin showed the presence of hyperconjugation between the C
α-H bond and the π bond of the benzene ring, which resulted in the delocalization of the σ electrons in the C
α-H bond to the benzene ring and the weakening of the C
α-H bond. The fact that tetralin has the highest hydrogen transfer rate among all naphthalene derivatives was also confirmed by the results obtained by Sung-Ho Kim et al. [
30]. The reactions of hydrogen formation from tetralin and decalin can be expressed as follows:
Generally, the dehydrogenation of decalin (
Scheme 1) includes a few simultaneous, reversible reaction pathways: tetralin formation from decalin and the conversion of tetralin to naphthalene.
Ameen A. Al-Muntaser et al. [
32] demonstrated the effect of aquathermolysis conditions on the composition of heavy oil-cracking products. It was found that an increase in the heat treatment temperature led to a decrease in the viscosity and density of oil due to the deep destruction of the resin–asphaltene components. In this case, sample losses due to gas and coke formation were found to be high. The use of dispersed catalysts and water-soluble catalyst precursors can significantly improve the quality of heavy oil aquathermolysis products; however, their removal and subsequent reuse are fundamentally difficult [
33]. The prospects of using the compounds of transition group metals (Ni, Co, Fe, Mo, and others) for these purposes are described in [
6,
33,
34,
35,
36,
37,
38]. Nickel compounds are of particular interest because of their high activity in the degradation of resin–asphaltene components and the absence of problems with diffusion, which in turn leads to efficient contact between the reagents and the catalyst [
39,
40,
41]. Alfiya Lakhova et al. [
9] demonstrated the efficiency of using nickel nanoparticles in the cracking of Athabasca oil. The researchers suggested that the use of a nanosized nickel catalyst in the cracking of super heavy oil accelerated the destruction of asphaltenes, which resulted in a significant decrease in its viscosity [
34]. However, it was shown that the use of these catalysts also resulted in an increase in the yield of not only light fractions but also by-products, in particular, coke [
18]. Aquathermolysis in the presence of a nickel catalyst (nanocompounds with the Keggin structure) made it possible to suppress polymerization reactions, thereby reducing the viscosity of heavy oil by 96% [
42].
The aim of this work was to determine the yield of light fractions, the transformation of oil components, and the nature of structural-group changes in asphaltene molecules in the case of the in situ catalytic aquathermolysis of heavy oil in the presence of hydrogen donors.
2. Results and Discussion
It was revealed that the introduction of additives of tetralin (T) and decalin (D) in both the thermal cracking (C) and catalytic aquathermolysis (CA) of heavy oil resulted in an increase in the yield of gaseous products (
Table 1). In the CA, CA+T, and C+T experiments, the yield of condensation products was reduced by a factor of 2–4, compared with conventional cracking. It should be noted that the use of tetralin in aquathermolysis (CA+T) made it possible not only to slow down the coke formation but also to increase the destruction of asphaltenes with the formation of mainly resins. The obtained results once again confirmed the effectiveness of using this additive as an H-donor, which prevented the interaction of radicals with each other and, accordingly, the formation of molecules with a large molecular weight [
43]. By contrast, the addition of decalin to the cracked system accelerated the condensation reactions, so the coke content increased by 1.5–2 wt.% in the cases of both aquathermolysis and cracking (C+D and CA+D). Additionally, in both cases, significant sample losses were observed, since the total yield of by-products is about 13%.
The maximum depth of the desulfurization of liquid products was observed in the CA+T experiment. This result was probably due to the fact that the active hydrogen formed according to
Scheme 2 promoted the hydrodesulfurization reaction, where sulfur-containing gases were among its products. Indirectly, this assumption confirmed the increase in their yield by a factor of three, compared with experiment C, and two times, compared with CA. Thus, the simultaneous presence of a nickel catalyst and water and tetralin acting as hydrogen donors in the system had the most effective effect on sulfur removal.
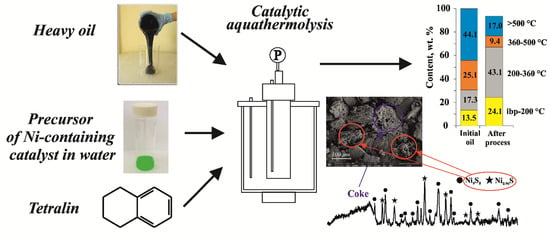
According to the data presented in
Figure 1, the fractional composition of oil after thermal cracking changed as follows: the content of fractions boiling away in the temperature range of ibp–200 °C increased from 13.5 to 22.0 wt.%, while the content of 200–360 °C distillate increased from 17.3 to 28.6 wt.%. Both additives considered in this work (C+T and C+D) affected the yield of light fractions insignificantly. After CA, the yield of light fractions increased to 59.3 wt.%. The residual fraction (IBP > 500 °C) decreased by 12.9% compared with thermal cracking and by 27.2% compared with the initial oil. This suggested that the high molecular weight components of the feedstock underwent deep degradation in the case of catalytic aquathermolysis. The use of tetralin in this process (CA+T) made it possible to increase the yield of fractions boiling up to 360 °C to 67.2 wt.%. This was probably due to the active hydrogenation of radicals formed upon breaking the C–C, C–S, and C–O bonds of resin and asphaltene molecules. Smaller amounts of distillate components evaporating in the ranges IBP–200 and 200–360 °C were observed in the CA+D experiment. Hence, the content of distillate increased only by 1.3 and 4.7 wt.%, respectively, which was presumably due to the low values of the H-donor index of decalin [
43].
The results of determining the elemental composition of asphaltenes indicated that the conditions of cracking (the presence or absence of water in the system), as well as the use of additives, insignificantly affected the distribution of C, H, and N atoms in their structure [
44]. Probably, during thermal and catalytic cracking, alkyl substituents were removed, and the aromatization of naphthenic cycles of molecules occurred, which caused a decrease in their H/C ratio (
Table 2).
Table 3 presents the calculated structural-group parameters of the initial asphaltenes and the products of C, CA, CA+T, and C+T cracking, on the basis of which the spatial structures of their molecules were constructed (
Figure 2).
The asphaltenes isolated from the initial oil were a mixture of hetero-organic molecules mainly consisting of four blocks with an average composition of C
128H
146.5N
2.5S
3.3O
6 and a molecular mass of 1920 a.m.u. (
Table 3). The structural block of such a mean molecule consists of 33–34 carbon atoms combined into 6 naphthenic-aromatic cycles. It was revealed that the percentage of carbon atoms enclosed in aromatic structures was almost half of their total number, being equal to 48.5%. In particular, there were two or three naphthenic cycles for each aromatic core. The total number of carbon atoms in the alkyl fragments of one average block (C
S*) was 8.8, and the alkyl chain length (n) did not exceed 3.56. The initial asphaltenes also contained from two to three nitrogen atoms, three sulfur atoms, and up to six oxygen atoms. It followed from this that each of their structural blocks conventionally accounted for 0.65 N* atoms, 0.87 S* atoms, and 1.57 O* atoms. Presumably, such a distribution of heteroatoms indicated that the asphaltenes under consideration contained a significant amount of the functional groups of carboxylic acids and ester bridges connecting the structural blocks of molecules.
The asphaltenes obtained after the thermal cracking of oil significantly differed in their characteristics. Their mean molecules were heteroorganic two-block structures with an average composition of C
51.8H
45.2N
1.3S
1.3O
3.4 and an average molecular mass of 782 a.m.u. In this case, there were about 27 carbon atoms per structural block, i.e., 6–7 less than for the asphaltenes of the initial oil. Due to aromatization reactions, the average number of cycles in one block increased from 6.5 to 6.9 (the amount of R*
Ar increased, while R*
N decreased), respectively, while the aromaticity factor increased to 66.1%. The number of carbon atoms in aliphatic fragments and their average length decreased from 33.6 to 5.2 and from 3.56 to 2.49, respectively, which suggested the active dealkylation and cyclization reactions. According to the data in
Table 3, the total content of N and O in the mean molecule of the asphaltenes of C products decreased by two times, while the content of S by three times. Thus, each structural block contained N* and S* by 0.69 and O* by 1.80. The larger values of N* and O* indicated that the formation of asphaltenes proceeded, among other things, due to the condensation of high-molecular nitrogen- and oxygen-containing compounds.
After catalytic aquathermolysis, the asphaltenes became single-block structures with an average composition of C56.6H48.9N1.3S1.9O2.7 and MM of 851 a.m.u. Their structural blocks were enlarged due to condensation and aromatization reactions. Hence, the total number of rings was 14.9, 11.3 rings of which were aromatic. The number of carbon atoms in them also naturally increased (by four in comparison with C asphaltenes). In the course of CA, the reactions of dealkylation proceeded less intensively than during thermal cracking, which is indicated by the larger values of CS and n (7.5 and 2.79, respectively). The number of heteroatoms per average structural block of the asphaltene molecule also significantly increased.
The use of tetralin in the CA process resulted in the production of asphaltenes with similar MM values (851 and 863 a.m.u.). However, these asphaltenes were significantly different in their structure. They were composed of heteroorganic monoblock and diblock molecules with an average composition of C58.6H52.6N1.4S1.9O1.6. Their average structural block consisted of 10 cycles, with R*Ar equal to 7 and R*N equal to 3. An increase in the content of naphthenic rings together with a decrease in aromatic rings suggested, on the one hand, the occurrence of hydrogenation reactions, and, on the other hand, a slowdown in aromatization reactions. The similar values of CS and n for the asphaltenes obtained after CA+T and C suggested that the dealkylation of alkyl fragments in these processes proceeded at approximately the same rate. It should be noted that the addition of tetralin did not affect the distribution of N and S atoms (compared with CA asphaltenes), while the oxygen content decreased by one atom (from 2.7 to 1.6).
The analysis of the diffraction patterns (
Figure 3) of solid products after CA and CAT showed the presence of Ni
0.96S and Ni
9S
8 nickel sulfide phases. During CA, the nickel-containing catalyst was sulfided by the sulfur components of the oil to form Ni
0.96S (PDF 00-050-1791). The reflections of weak intensity at 2Θ = 31.2, 50.6, and 73.1° suggested the presence of the Ni
9S
8 phase (PDF 00-022-1193). The average CSR value, estimated from the most pronounced reflection, was 15.89 nm and 16.72 nm for Ni
0.96S and Ni
9S
8, respectively. Catalytic aquathermolysis in the presence of tetralin was accompanied by the formation of well-crystallized Ni
0.96S (PDF 00-050-1791) and Ni
9S
8 (PDF 00-022-1193) phases, the CSR values of which were found to be 24.00 nm and 11.98 nm, respectively.
Probably, at the first stage of the cracking process, nickel oxide interacted with oil sulfur compounds. The resulting Ni
9S
8 disproportionated into Ni
0.96S and Ni
x−0.96S
6. Then, the Ni
x−0.96S
6 phase was sulfided by sulfur-containing components to Ni
9S
8 [
45]. With the accumulation of the Ni
0.96S phase, which was the end product of the sulfidation of the Ni-containing catalyst (
Scheme 3), the desulfurization of oil slowed down. Consequently, the depth of oil desulfurization was affected by the ratio of the Ni
0.96S and Ni
9S
8 phases, as well as the amount of hydrogen sulfide and “free” hydrogen in the reaction medium [
46].
Figure 4 and
Figure 5 show the SEM images of a coke-containing catalyst. In the course of CA and CA+T, the condensation of asphaltene molecules resulted in the formation of sponge-like coke [
47,
48]. This was presumably due to the occurrence of decarboxylation and dehydrogenation reactions accompanied by the evolution of gaseous products. A decrease in the intensity of reflection at 2Θ = 25.4° of the CA+T condensation products suggested that the presence of tetralin in the reaction medium promoted the formation of coke, which was more hydrogen-enriched than coke in the case of CA (
Figure 3). In addition, when using T, spherical nickel particles were formed, which, according to the literature data, is due to the processes of hydrogen redistribution in the system [
49].
The mapping of the surface of the coke-containing catalyst isolated after CA showed an inhomogeneous distribution of the sulfur and nickel, the formation of which required the ratio S:Ni ≈ 1 (
Figure 5a). After CA+T, the distribution of sulfur on the surface was more uniform, which could contribute to the formation of the two phases of nickel sulfides (
Figure 5b). This was probably due to the presence of a larger amount of hydrogen in the reaction medium.
XPS analysis was carried out to study the chemical state of Ni and S after CA and CA+T coke-containing catalysts (
Figure 6). As shown in
Figure 6, for both samples, the Ni 2p spectra showed two spin-orbit doublets accompanied by two shake-up satellites (“Sat”). The analysis of the spectra showed that two photoelectron peaks for Ni 2p
3/2 and Ni 2p
1/2 at 856 eV and 872.7 eV corresponded to the state of Ni
2+ [
50]. These values of binding energy (BE) indicated that Ni
xS
y on the surface was in an oxidized state due to its contact with moisture and oxygen in the air. In the S2p spectra, in addition to the peaks associated with sulfur–metal bonds (163.3 eV and 164.25 eV), spectra related to sulfur–oxygen bonds (168.5 eV) [
51] were also observed as a result of their interaction with air [
52].
3. Research Methods
For this research, we selected the oil of Zyuzeevskoye field (Tatarstan Republic), with the following physicochemical properties (
Table 4): this oil is heavy (class I—ρ = 934–966 kg/m
3), with a low content of light fractions, with especially high sulfur (> to 3.5 wt.% according to GOST 51858-2002).
The laboratory modeling of the aquathermolysis process was carried out in an autoclave with a volume of 12 cm
3 (
Figure 7). The weight of the heavy oil loaded into the reactor was 7 g, and those of the water additives were up to 2 wt.%. The nickel catalyst precursor and the hydrogen donor were introduced at 0.4 wt.% and 5.0 wt.%, respectively [
37]. The experiments were carried out at a temperature of 450 °C and duration of 80 min.
The physicochemical properties of the feedstock and the products of cracking were determined in accordance with the ASTM standards. The kinematic viscosity was determined using a rotational viscometer. The content of saturated, aromatic hydrocarbons, resins, and asphaltenes (SARA components) was determined by the method described in ASTM D4124. The sulfur content was measured according to ASTM-D4294, and distillation curves were obtained according to ASTM-D2887.
The elemental analysis of the samples was performed using a “Vario EL Cube” CHNS analyzer at a temperature of 1150 °C. The CHNS analyzer’s operating principle is based on the classical technique of burning the sample in a quartz tube in an oxygen medium, where O analysis is a method of recovery in the atmosphere He/H
2. The analysis was carried out on the emitted gases on a katharometer using the method of displacement chromatography, and the separation of gases on a chromatographic column. The absolute error of the analyzer did not exceed ±0.1 % for each element being determined. The oxygen content was evaluated from the difference between 100 % and the sum of the C, H, N, and S elements. The molecular mass (MM) was measured via cryoscopy in naphthalene. The
1H NMR spectra were recorded using a Bruker AVANCE AV 300 NMR–Fourier spectrometer at 300 MHz. The asphaltenes samples were dissolved in deuterated chloroform (CDCl
3). The obtained
1H-NMR spectra were divided into four zones (H
Ar, H
α, H
β, and H
γ) in accordance with the chemical shifts in hydrogen atoms presented in
Table 5 [
53].
The average structural parameters were calculated according to the Brown–Ladner empirical formulas based on the
1H-NMR and elemental analysis data and the MM values (
Table 6) described in the work of Sun et al. [
54].
The phase composition of the catalytic additive was investigated via X-ray phase analysis (a Bruker D8 XRD powder diffractometer, a Lynx Eye detector, and monochrome CuKα radiation). The morphology of the samples was investigated using a Thermo Fisher Scientific Apreo S LoVac scanning electron microscope with the EDS+EDBS analysis system at an accelerating voltage of 30 kV. The elemental composition of the sample surface was analyzed using the Octane Elect EDS system and APEX software.
X-ray photoelectron spectroscopy (XPS) was applied for a study of the oxidation state of nickel and sulfur using a Thermo Fisher Scientific XPS NEXSA spectrometer with a monochromated Al K-α X-ray source (1486.6 eV) and equipped with a flood gun for the charge compensation. The survey spectra were recorded with a pass energy rate of 200 eV and a step size of 1 eV, while a pass energy rate of 50 eV and a step size of 0.1 eV were applied for the high-resolution spectra. The analyzed area was 200 μm
2. The charging shifts were referenced against adventitious carbon at the binding energy (BE) of 285 eV. It should be noted that XPS provides information about the electronic/chemical state of elements only in the near-surface layer (5–15 nm), the composition of which can change during the reaction and upon contact with the environment. The CASA XPS software (version 2.3.15, CASA Software Ltd., Teignmouth, UK,
http://www.casaxps.com/, (accessed on 1 September 2022)) was used for processing the spectra.