Efficient Removal of Eriochrome Black T (EBT) Dye and Chromium (Cr) by Hydrotalcite-Derived Mg-Ca-Al Mixed Metal Oxide Composite
Abstract
:1. Introduction
2. Results and Discussion
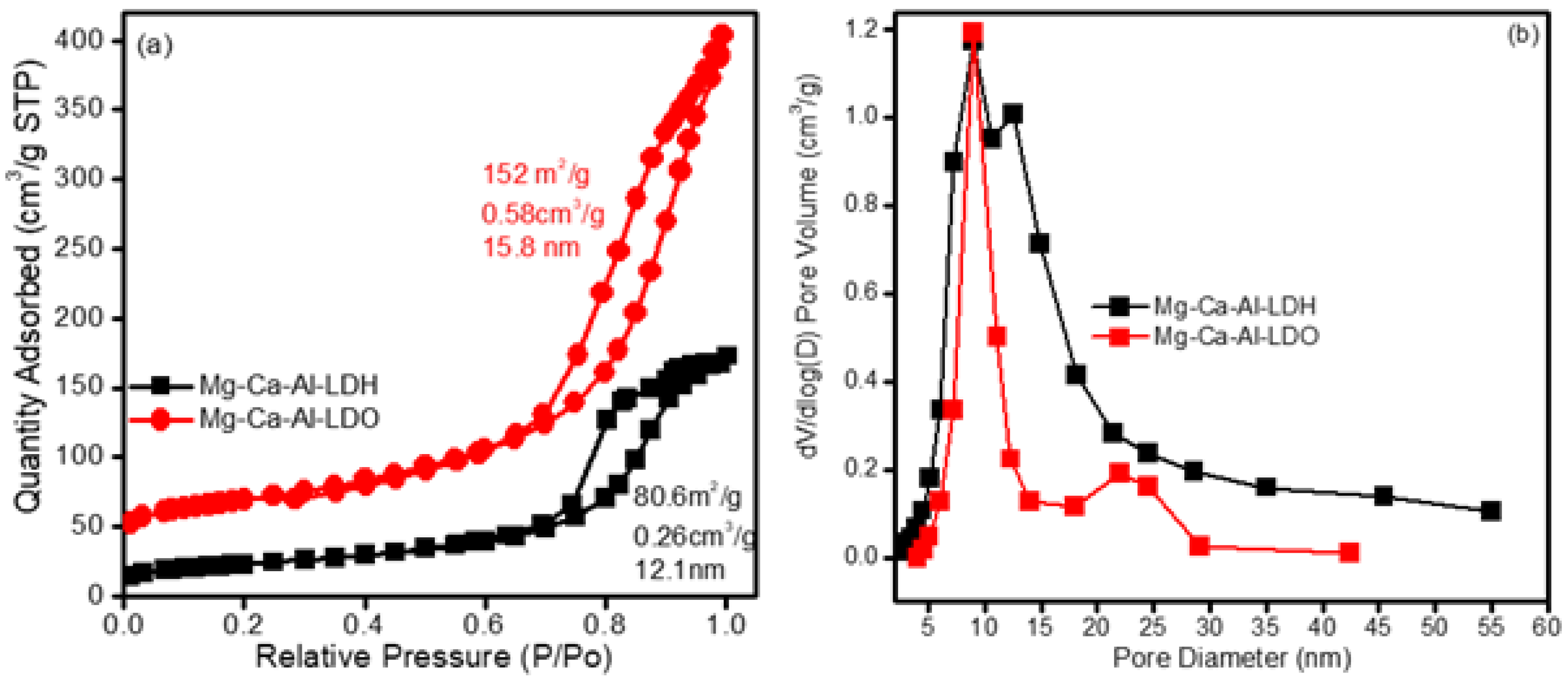
2.1. Textural Properties
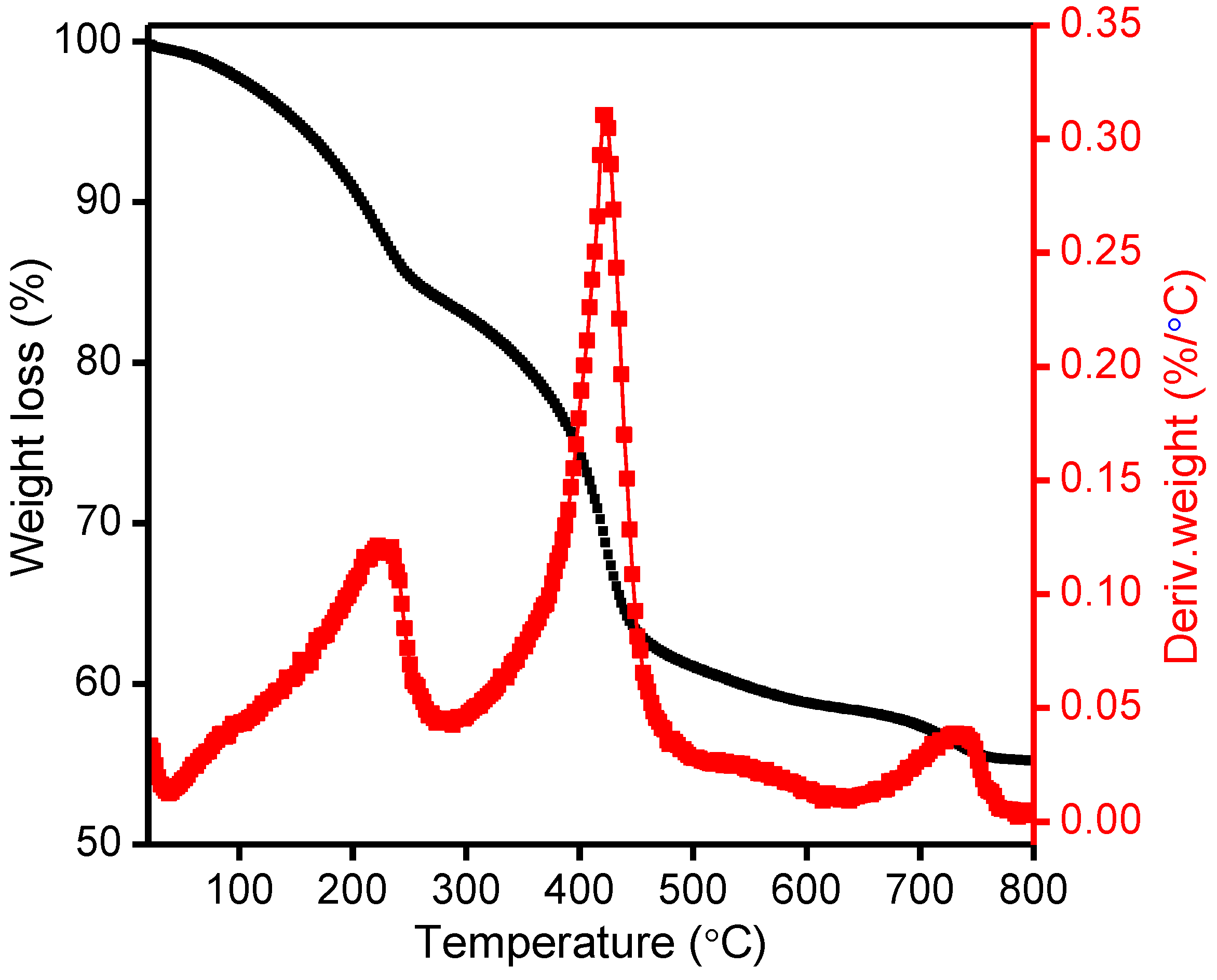
2.2. Thermo-Gravimetric Analysis (TGA-DTG)
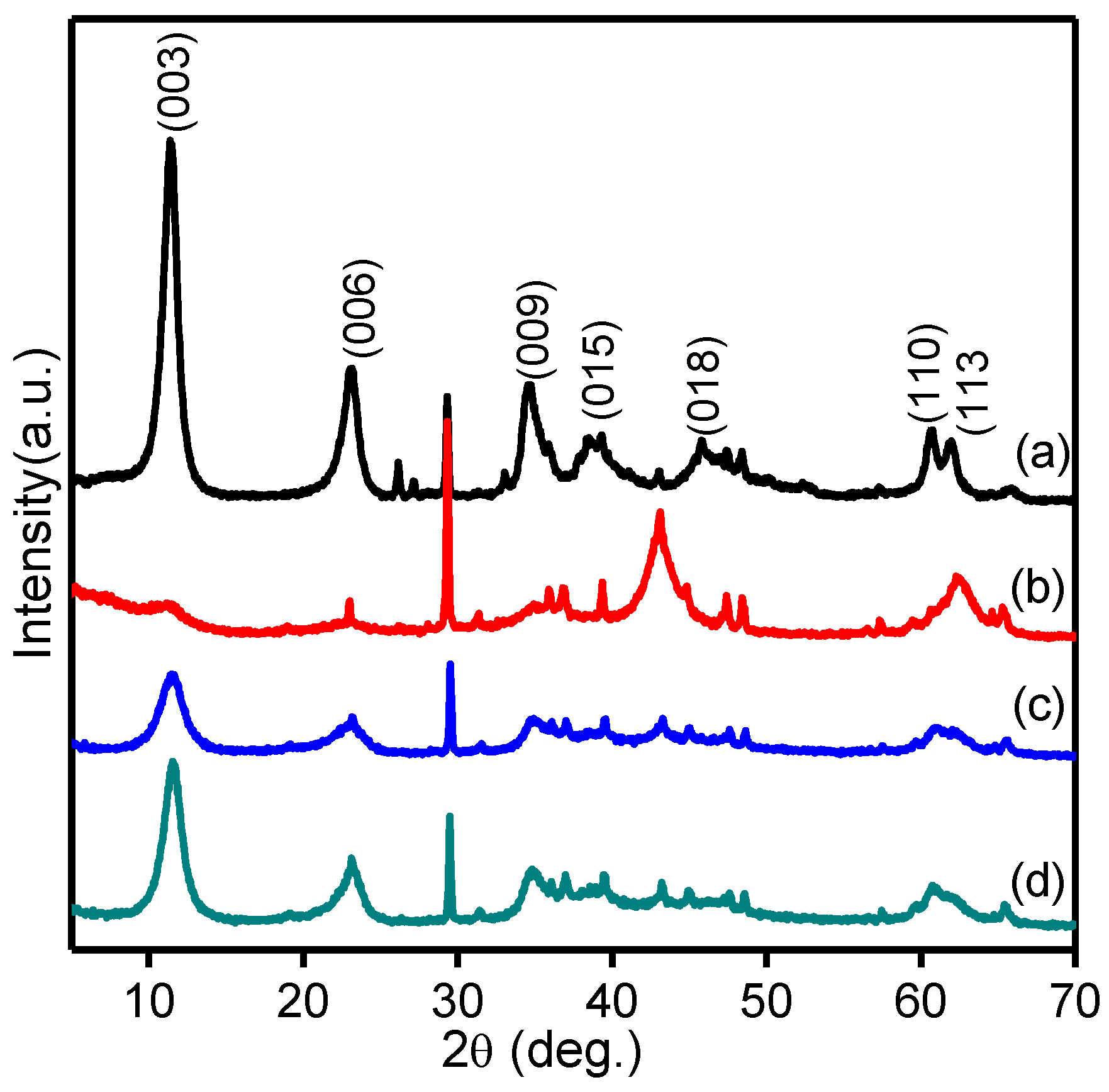
2.3. X-ray Diffraction Analysis (XRD)
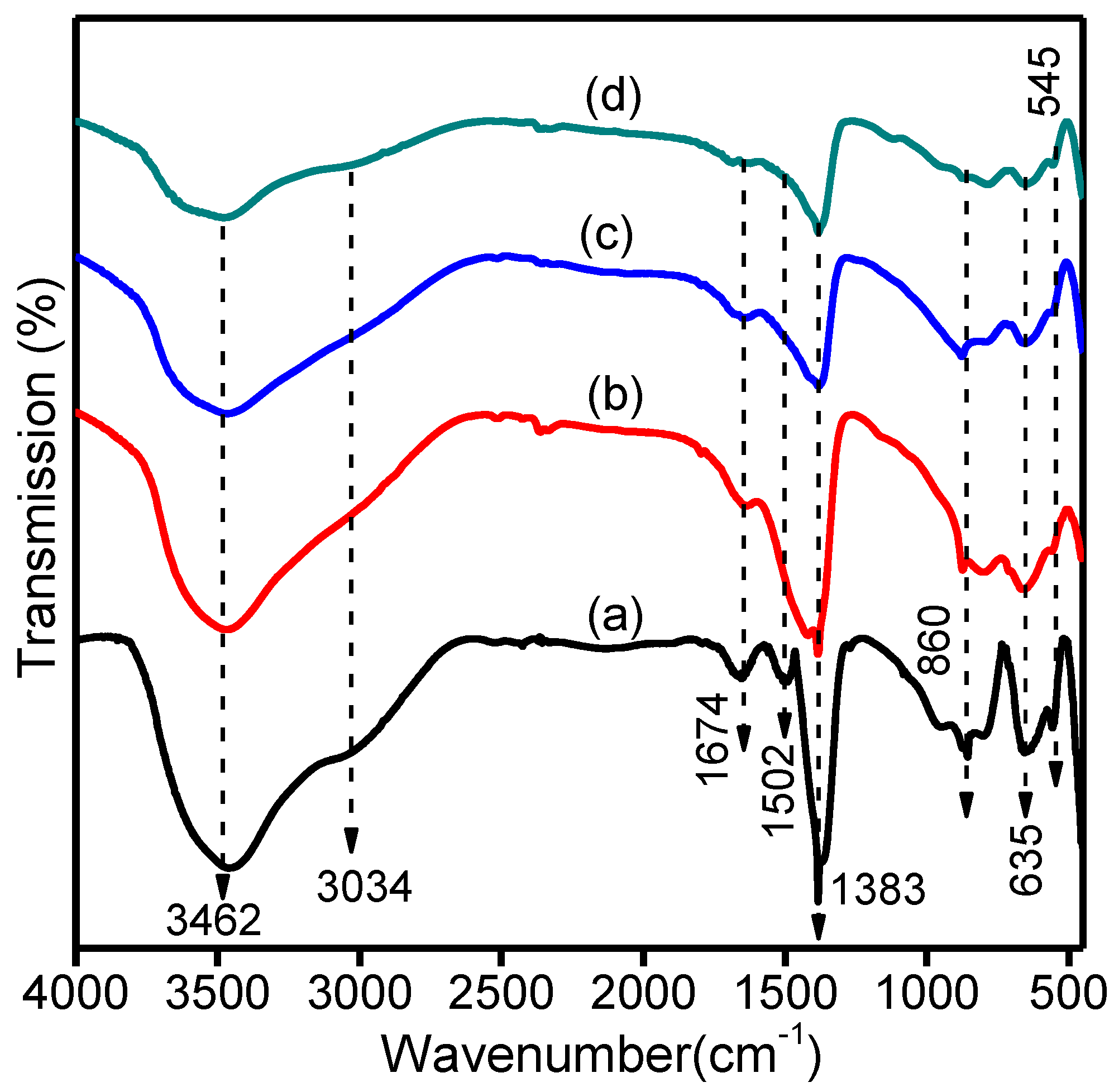
2.4. Fourier Transformation Infrared Spectroscopy (FT-IR) Analysis
2.5. FESEM-EDS Analysis
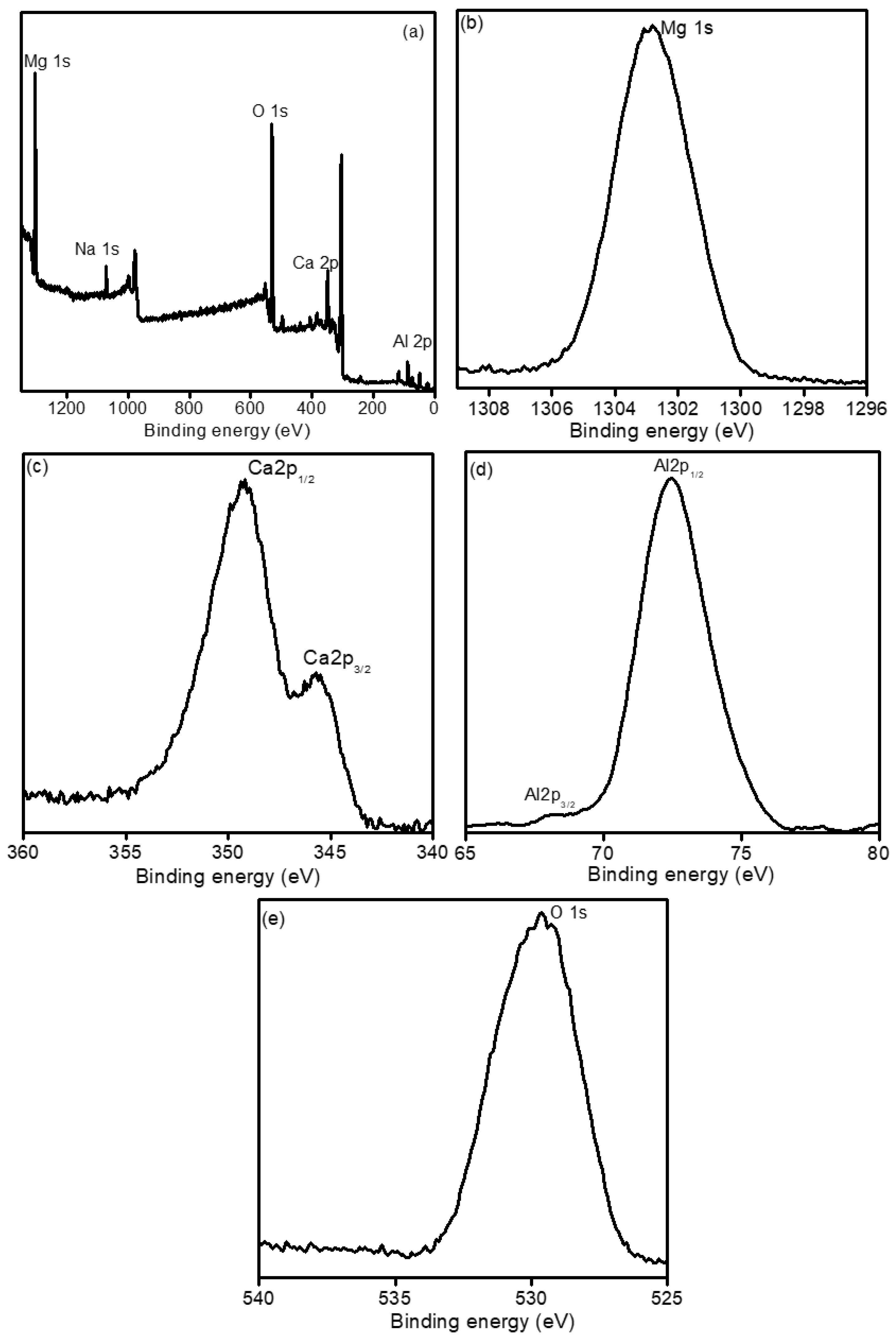
2.6. X-ray Photo Electronic Spectroscopy (XPS)
3. Adsorption Performance Evaluation over Mg-Ca-Al-LDO Composite
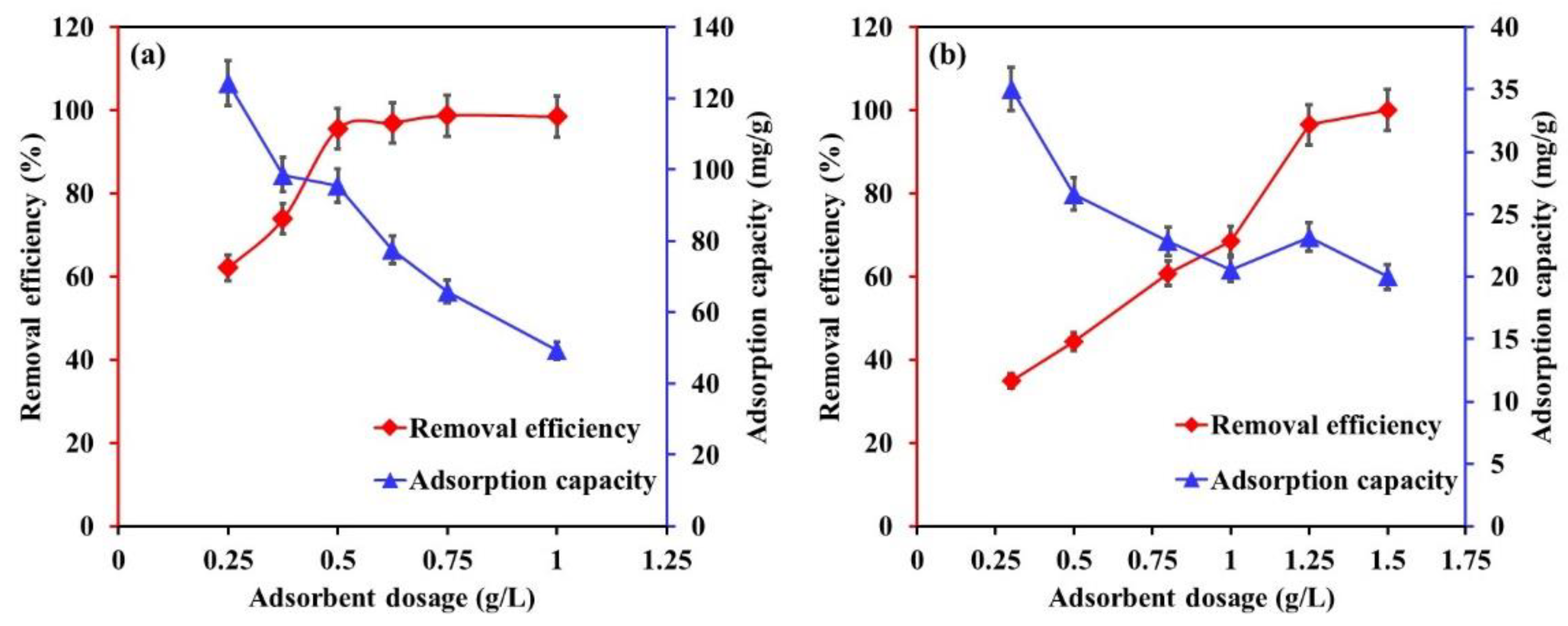
3.1. Influence of Mg-Ca-Al LDO Dosage
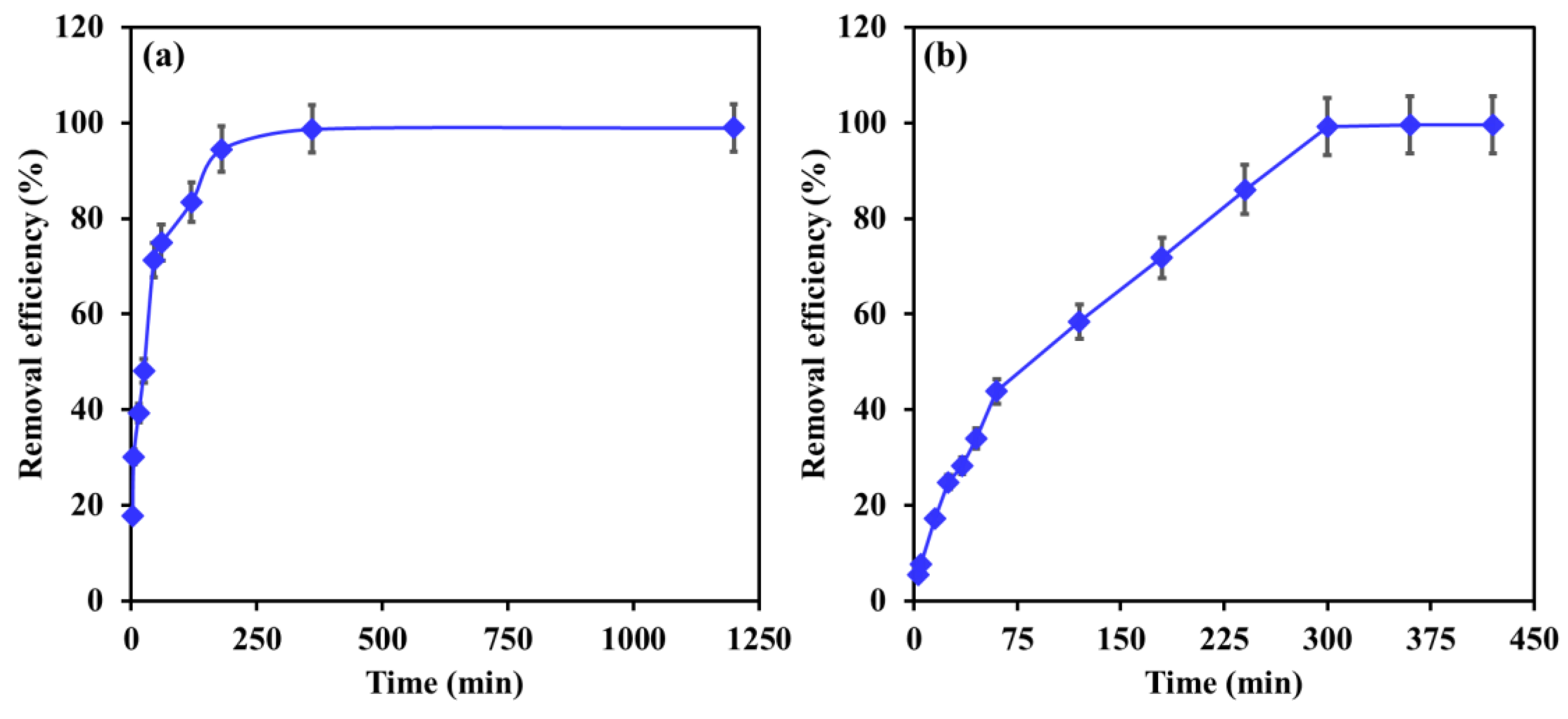
3.2. Influence of Contact Time
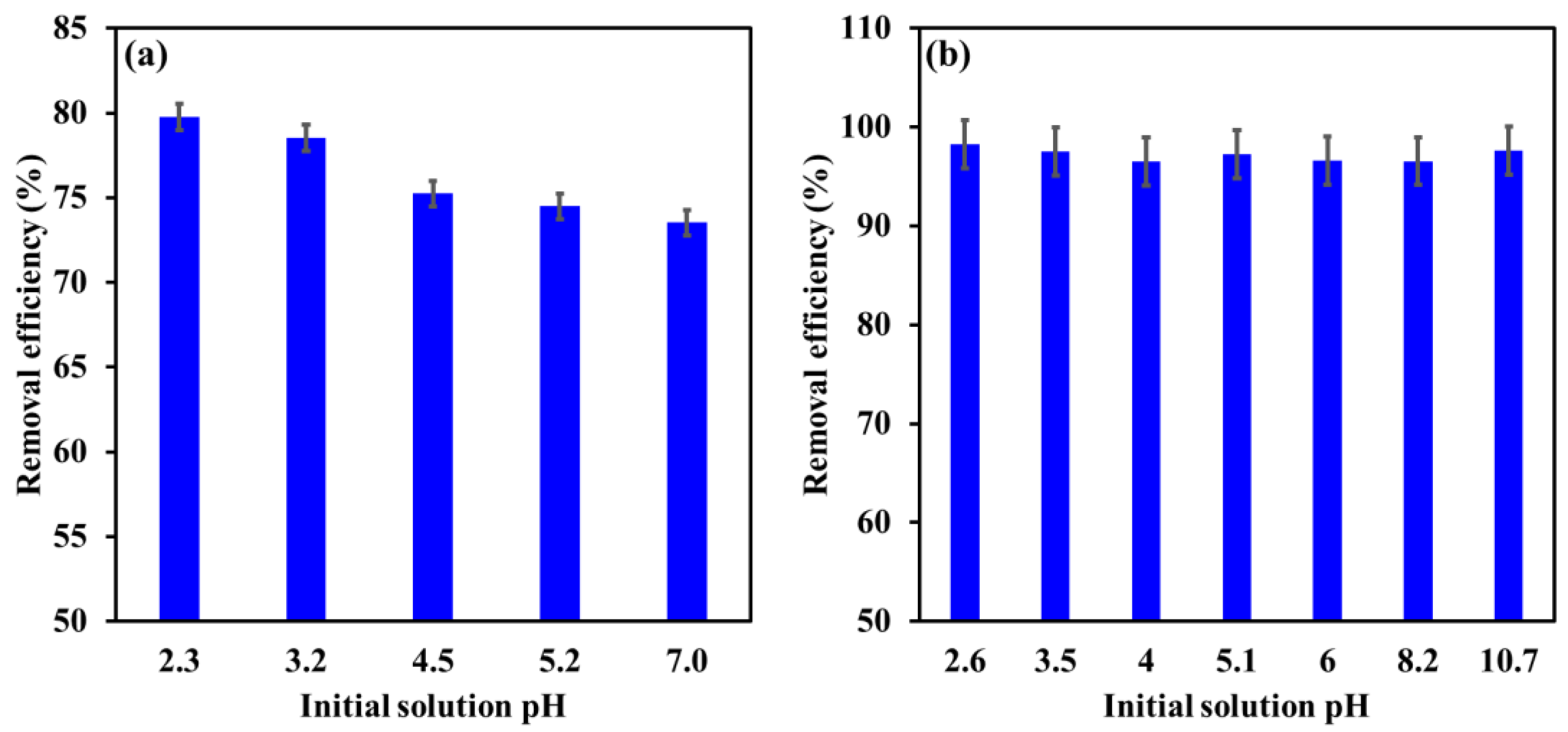
3.3. Influence of Initial Solution pH
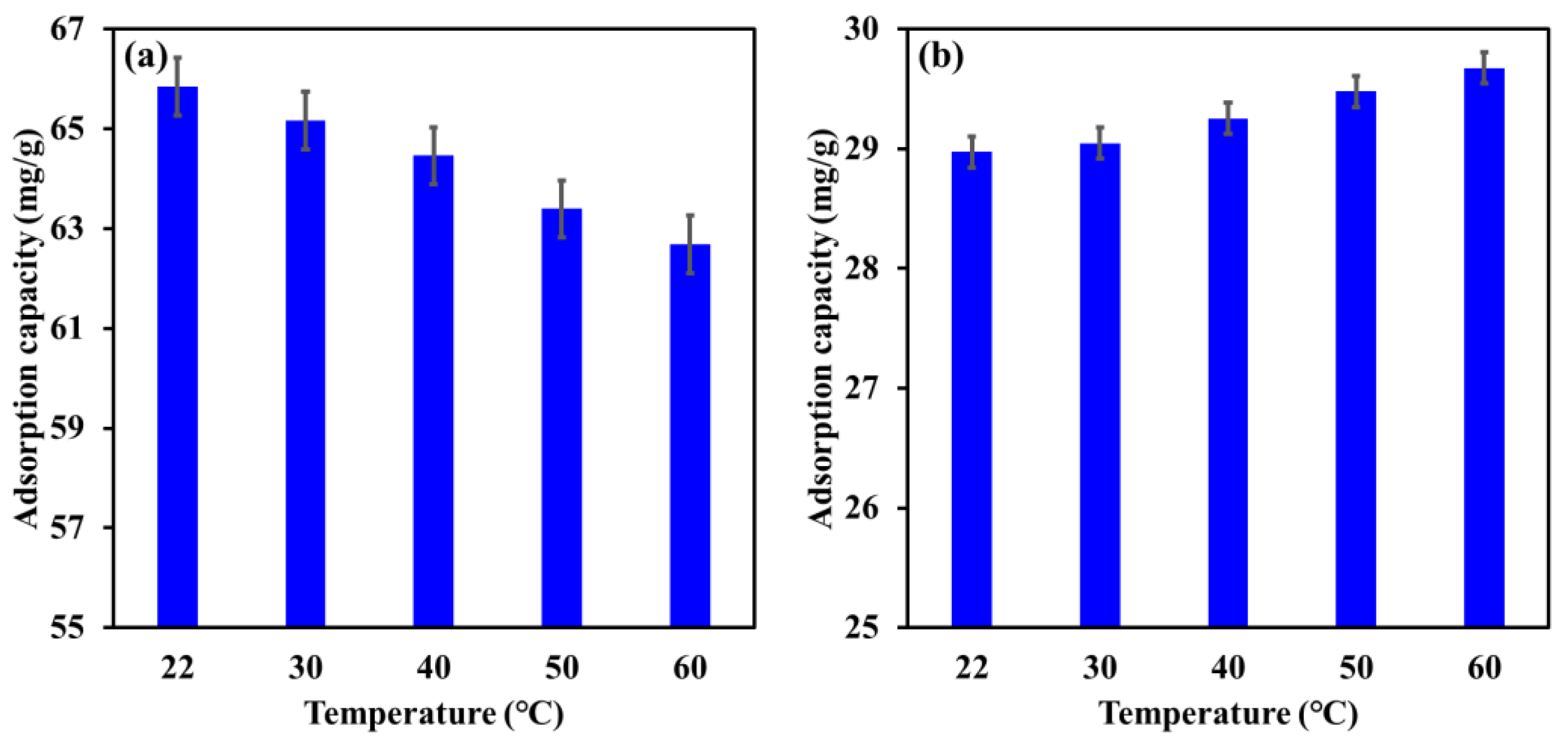
3.4. Influence of Temperature
3.5. Kinetics Modeling
3.6. Isotherm Modeling
3.7. Thermodynamics Investigation
3.8. Regeneration
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Mg-Ca-Al-LDH
4.3. Adsorbent Characterization
4.4. Adsorption and Desorption Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatla, A.; Almanassra, I.W.; Jaber, L.; Kochkodan, V.; Laoui, T.; Alawadhi, H.; Atieh, M.A. Influence of calcination atmosphere on Fe doped activated carbon for the application of lead removal from water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129928. [Google Scholar] [CrossRef]
- Arslan, H.; Eskikaya, O.; Bilici, Z.; Dizge, N.; Balakrishnan, D. Comparison of Cr(VI) adsorption and photocatalytic reduction efficiency using leonardite powder. Chemosphere 2022, 300, 134492. [Google Scholar] [CrossRef]
- Pakade, V.E.; Tavengwa, N.T.; Madikizela, L.M. Recent advances in hexavalent chromium removal from aqueous solutions by adsorptive methods. RSC Adv. 2019, 9, 26142–26164. [Google Scholar] [CrossRef] [Green Version]
- Almanassra, I.W.; Al-ansari, T.; Ihsanullah, I.; Kochkodan, V.; Anjaneyulu, C.; Muataz Ali, A.; Shanableh, A.; Tahar, L. Carbide-derived carbon as an extraordinary material for the removal of chromium from an aqueous solution. Chemosphere 2022, 307, 135953. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.S.A.; Mubarak, N.M.; Tan, Y.H.; Khalid, M.; Karri, R.R.; Walvekar, R.; Abdullah, E.C.; Nizamuddin, S.; Mazari, S.A. A comprehensive review on magnetic carbon nanotubes and carbon nanotube-based buckypaper for removal of heavy metals and dyes. J. Hazard. Mater. 2021, 413, 125375. [Google Scholar] [CrossRef]
- Isik, Z.; Saleh, M.; M’barek, I.; Yabalak, E.; Dizge, N.; Deepanraj, B. Investigation of the adsorption performance of cationic and anionic dyes using hydrochared waste human hair. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Sharma, V.T.; Halanur, M.M.; Kamath, S.V.; Mondal, D.; Sanna Kotrappanavar, N. Fe–Al based nanocomposite reinforced hydrothermal carbon: Efficient and robust absorbent for anionic dyes. Chemosphere 2020, 259, 127421. [Google Scholar] [CrossRef]
- Boudouaia, N.; Bengharez, Z.; Jellali, S. Preparation and characterization of chitosan extracted from shrimp shells waste and chitosan film: Application for Eriochrome black T removal from aqueous solutions. Appl. Water Sci. 2019, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Kaur, Y.; Jasrotia, T.; Kumar, R.; Chaudhary, G.R.; Chaudhary, S. Adsorptive removal of eriochrome black T (EBT) dye by using surface active low cost zinc oxide nanoparticles: A comparative overview. Chemosphere 2021, 278, 130366. [Google Scholar] [CrossRef] [PubMed]
- Khayet, M.; Zahrim, A.Y.; Hilal, N. Modelling and optimization of coagulation of highly concentrated industrial grade leather dye by response surface methodology. Chem. Eng. J. 2011, 167, 77–83. [Google Scholar] [CrossRef]
- Senguttuvan, S.; Senthilkumar, P.; Janaki, V.; Kamala-kannan, S. Significance of conducting polyaniline based composites for the removal of dyes and heavy metals from aqueous solution and wastewaters—A review. Chemosphere 2021, 267, 129201. [Google Scholar] [CrossRef]
- Cai, L.; Ying, D.; Liang, X.; Zhu, M.; Lin, X.; Xu, Q.; Cai, Z.; Xu, X.; Zhang, L. A novel cationic polyelectrolyte microsphere for ultrafast and ultra-efficient removal of heavy metal ions and dyes. Chem. Eng. J. 2021, 410, 128404. [Google Scholar] [CrossRef]
- Wijannarong, S.; Aroonsrimorakot, S.; Thavipoke, P. Removal of Reactive Dyes from Textile Dyeing Industrial Effluent by Ozonation Process. APCBEE Procedia 2013, 5, 279–282. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Li, Y.; Chen, H.; Lu, J.; Yu, G.; Möslang, M. Superior adsorption capacity of functionalised straw adsorbent for dyes and heavy-metal ions. J. Hazard. Mater. 2020, 382, 121040. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Bimeghdar, S. Synthesis of mesoporous NiO nanoparticles and their application in the adsorption of Cr(VI). Chem. Eng. J. 2014, 239, 105–113. [Google Scholar] [CrossRef]
- Ding, Y.; Wu, J.; Wang, J.; Wang, J.; Ye, J.; Liu, F. Superhydrophilic carbonaceous-silver nanofibrous membrane for complex oil/water separation and removal of heavy metal ions, organic dyes and bacteria. J. Memb. Sci. 2020, 614, 118491. [Google Scholar] [CrossRef]
- Zhang, P.; Gong, J.; Zeng, G.; Deng, C.; Yang, H.; Liu, H. Cross-linking to prepare composite graphene oxide-framework membranes with high-flux for dyes and heavy metal ions removal. Chem. Eng. J. 2017, 322, 657–666. [Google Scholar] [CrossRef]
- Zeng, G.; He, Y.; Zhan, Y.; Zhang, L.; Pan, Y. Novel polyvinylidene fluoride nanofiltration membrane blended with functionalized halloysite nanotubes for dye and heavy metal ions removal. J. Hazard. Mater. 2016, 317, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Eskikaya, O.; Gun, M.; Bouchareb, R.; Bilici, Z.; Dizge, N.; Ramaraj, R.; Balakrishnan, D. Photocatalytic activity of calcined chicken eggshells for Safranin and Reactive Red 180 decolorization. Chemosphere 2022, 304, 135210. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Ji, L.; Li, C.; Hu, C.; Wu, K. Rapid, efficient and economic removal of organic dyes and heavy metals from wastewater by zinc-induced in-situ reduction and precipitation of graphene oxide. J. Taiwan Inst. Chem. Eng. 2018, 88, 137–145. [Google Scholar] [CrossRef]
- Pohl, A. Removal of Heavy Metal Ions from Water and Wastewaters by Sulfur-Containing Precipitation Agents. Water Air Soil Pollut. 2020, 231, 503. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Kochkodan, V.; Mckay, G.; Atieh, M.A.; Al-Ansari, T. Kinetic and thermodynamic investigations of surfactants adsorption from water by carbide-derived carbon. J. Environ. Sci. Health Part A 2021, 56, 1206–1220. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.K.A.; Dweiri, F.; Almanassra, I.W.; Chatla, A.; Atieh, M.A. Mg-Al Layered Double Hydroxide Doped Activated Carbon Composites for Phosphate Removal from Synthetic Water: Adsorption and Thermodynamics Studies. Sustainability 2022, 14, 6991. [Google Scholar] [CrossRef]
- Abushawish, A.; Almanassra, I.W.; Namboorimadathil, S.; Jaber, L.; Khalil, A.K.A.; Ali, M.; Taha, E.; Alawadhi, H.; Shanableh, A.; Ali, M. High-efficiency removal of hexavalent chromium from contaminated water using nitrogen-doped activated carbon : Kinetics and isotherm study. Mater. Chem. Phys. 2022, 291, 126758. [Google Scholar] [CrossRef]
- Jaber, L.; Ihsanullah, I.; Almanassra, I.W.; Backer, S.N.; Abushawish, A.; Khalil, A.K.A.; Alawadhi, H.; Shanableh, A.; Atieh, M.A. Adsorptive Removal of Lead and Chromate Ions from Water by Using Iron-Doped Granular Activated Carbon Obtained from Coconut Shells. Sustainability 2022, 14, 10877. [Google Scholar] [CrossRef]
- Gao, H.; Zhao, S.; Cheng, X.; Wang, X.; Zheng, L. Removal of anionic azo dyes from aqueous solution using magnetic polymer multi-wall carbon nanotube nanocomposite as adsorbent. Chem. Eng. J. 2013, 223, 84–90. [Google Scholar] [CrossRef]
- Sahraei, R.; Pour, Z.S.; Ghaemy, M. Novel magnetic bio-sorbent hydrogel beads based on modi fi ed gum tragacanth/graphene oxide : Removal of heavy metals and dyes from water. J. Clean. Prod. 2017, 142, 2984. [Google Scholar] [CrossRef]
- Fang, Q.; Ye, S.; Yang, H.; Yang, K.; Zhou, J.; Gao, Y.; Lin, Q.; Tan, X.; Yang, Z. Application of layered double hydroxide-biochar composites in wastewater treatment: Recent trends, modification strategies, and outlook. J. Hazard. Mater. 2021, 420, 126569. [Google Scholar] [CrossRef] [PubMed]
- Daud, M.; Hai, A.; Banat, F.; Wazir, M.B.; Habib, M.; Bharath, G.; Al-Harthi, M.A. A review on the recent advances, challenges and future aspect of layered double hydroxides (LDH)—Containing hybrids as promising adsorbents for dyes removal. J. Mol. Liq. 2019, 288, 110989. [Google Scholar] [CrossRef]
- Zubair, M.; Jarrah, N.; Manzar, M.S.; Al-Harthi, M.; Daud, M.; Mu’azu, N.D.; Haladu, S.A. Adsorption of eriochrome black T from aqueous phase on MgAl-, CoAl- and NiFe-calcined layered double hydroxides: Kinetic, equilibrium and thermodynamic studies. J. Mol. Liq. 2017, 230, 344–352. [Google Scholar] [CrossRef]
- De Sá, F.P.; Cunha, B.N.; Nunes, L.M. Effect of pH on the adsorption of Sunset Yellow FCF food dye into a layered double hydroxide (CaAl-LDH-NO3). Chem. Eng. J. 2013, 215–216, 122–127. [Google Scholar] [CrossRef]
- Lyu, P.; Li, L.; Huang, X.; Wang, G.; Zhu, C. Pre-magnetic bamboo biochar cross-linked Ca–Mg–Al layered double-hydroxide composite: High-efficiency removal of As(III) and Cd(II) from aqueous solutions and insight into the mechanism of simultaneous purification. Sci. Total Environ. 2022, 823, 153743. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, X.; Chen, Z.; Wang, J.; Wang, S.; Hayat, T.; Wang, X. Layered double hydroxide intercalated with aromatic acid anions for the efficient capture of aniline from aqueous solution. J. Hazard. Mater. 2017, 321, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ouyang, T.; Chen, J.; Wang, Z.; Liao, S.; Li, X.; Liu, Z.Q. Synthesis of nickel–iron layered double hydroxide via topochemical approach: Enhanced surface charge density for rapid hexavalent chromium removal. J. Colloid Interface Sci. 2022, 605, 602–612. [Google Scholar] [CrossRef]
- Hu, H.; Liu, J.; Xu, Z.; Zhang, L.; Cheng, B.; Ho, W. Hierarchical porous Ni/Co-LDH hollow dodecahedron with excellent adsorption property for Congo red and Cr(VI) ions. Appl. Surf. Sci. 2019, 478, 981–990. [Google Scholar] [CrossRef]
- Jabkhiro, H.; El Hassani, K.; Chems, M.; Anouar, A. Simultaneous removal of anionic dyes onto Mg(Al)O mixed metal oxides from ternary aqueous mixture: Derivative spectrophotometry and Density Functional Theory study. Colloids Interface Sci. Commun. 2021, 45, 100549. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Zhang, J.; Li, W.; Zhou, J.; Shao, L.; Qian, G. Efficient removal of dyes by a novel magnetic Fe3O4/ZnCr-layered double hydroxide adsorbent from heavy metal wastewater. J. Hazard. Mater. 2012, 243, 152–160. [Google Scholar] [CrossRef]
- Chao, H.P.; Wang, Y.C.; Tran, H.N. Removal of hexavalent chromium from groundwater by Mg/Al-layered double hydroxides using characteristics of in-situ synthesis. Environ. Pollut. 2018, 243, 620–629. [Google Scholar] [CrossRef]
- Zhu, K.; Gao, Y.; Tan, X.; Chen, C. Polyaniline-Modified Mg/Al Layered Double Hydroxide Composites and Their Application in Efficient Removal of Cr(VI). ACS Sustain. Chem. Eng. 2016, 4, 4361–4369. [Google Scholar] [CrossRef]
- Sahu, S.; Kar, P.; Bishoyi, N.; Mallik, L.; Patel, R.K. Synthesis of Polypyrrole-Modified Layered Double Hydroxides for Efficient Removal of Cr(VI). J. Chem. Eng. Data 2019, 64, 4357–4368. [Google Scholar] [CrossRef]
- Zubair, M.; Aziz, H.A.; Ihsanullah, I.; Ahmad, M.A.; Al-Harthi, M.A. Enhanced removal of Eriochrome Black T from water using biochar/layered double hydroxide/chitosan hybrid composite: Performance evaluation and optimization using BBD-RSM approach. Environ. Res. 2022, 209, 112861. [Google Scholar] [CrossRef] [PubMed]
- Mu’azu, N.D.; Jarrah, N.; Kazeem, T.S.; Zubair, M.; Al-Harthi, M. Bentonite-layered double hydroxide composite for enhanced aqueous adsorption of Eriochrome Black, T. Appl. Clay Sci. 2018, 161, 23–34. [Google Scholar] [CrossRef]
- Lei, C.; Zhu, X.; Zhu, B.; Jiang, C.; Le, Y.; Yu, J. Superb adsorption capacity of hierarchical calcined Ni/Mg/Al layered double hydroxides for Congo red and Cr(VI) ions. J. Hazard. Mater. 2017, 321, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Zaghouane-Boudiaf, H.; Boutahala, M.; Arab, L. Removal of methyl orange from aqueous solution by uncalcined and calcined MgNiAl layered double hydroxides (LDHs). Chem. Eng. J. 2012, 187, 142–149. [Google Scholar] [CrossRef]
- Kowalik, P.; Konkol, M.; Kondracka, M.; Próchniak, W.; Bicki, R.; Wiercioch, P. Memory effect of the CuZnAl-LDH derived catalyst precursor—In situ XRD studies. Appl. Catal. A Gen. 2013, 464–465, 339–347. [Google Scholar] [CrossRef]
- Lv, T.; Ma, W.; Xin, G.; Wang, R.; Xu, J.; Liu, D.; Liu, F.; Pan, D. Physicochemical characterization and sorption behavior of Mg-Ca-Al (NO3) hydrotalcite-like compounds toward removal of fluoride from protein solutions. J. Hazard. Mater. 2012, 237–238, 121–132. [Google Scholar] [CrossRef]
- Chagas, L.H.; De Carvalho, G.S.G.; Do Carmo, W.R.; San Gil, R.A.S.; Chiaro, S.S.X.; Leitão, A.A.; Diniz, R.; De Sena, L.A.; Achete, C.A. MgCoAl and NiCoAl LDHs synthesized by the hydrothermal urea hydrolysis method: Structural characterization and thermal decomposition. Mater. Res. Bull. 2015, 64, 207–215. [Google Scholar] [CrossRef]
- Yao, W.; Yu, S.; Wang, J.; Zou, Y.; Lu, S.; Ai, Y.; Alharbi, N.S.; Alsaedi, A.; Hayat, T.; Wang, X. Enhanced removal of methyl orange on calcined glycerol-modified nanocrystallined Mg/Al layered double hydroxides. Chem. Eng. J. 2017, 307, 476–486. [Google Scholar] [CrossRef]
- Othman, M.R.; Rasid, N.M.; Fernando, W.J.N. Mg–Al hydrotalcite coating on zeolites for improved carbon dioxide adsorption. Chem. Eng. Sci. 2006, 61, 1555–1560. [Google Scholar] [CrossRef]
- Wang, X.P.; Yu, J.U.N.J.I.E. High-Temperature Adsorption of Carbon Dioxide on Mixed Oxides Derived from Hydrotalcite-Like Compounds. Environ. Sci. Technol 2008, 42, 614–618. [Google Scholar] [CrossRef]
- Rozov, K.; Berner, U.; Taviot-gueho, C.; Leroux, F.; Renaudin, G.; Kulik, D.; Diamond, L.W. Cement and Concrete Research Synthesis and characterization of the LDH hydrotalcite–pyroaurite solid-solution series. Cement Concrete Res. 2010, 40, 1248–1254. [Google Scholar] [CrossRef]
- Vágvölgyi, V.; Palmer, S.J.; Kristóf, J.; Frost, R.L.; Horváth, E. Mechanism for hydrotalcite decomposition : A controlled rate thermal analysis study. J. Colloid Interface Sci. 2008, 318, 302–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatla, A.; Abu-rub, F.; Prakash, A.V.; Ibrahim, G.; Elbashir, O. Highly stable and coke-resistant Zn-modified Ni-Mg-Al hydrotalcite derived catalyst for dry reforming of methane : Synergistic effect of Ni and Zn. Fuel 2022, 308, 122042. [Google Scholar] [CrossRef]
- Çakırca, E.E.; Akın, N. Study on heterogeneous catalysts from calcined Ca riched hydrotalcite like compounds for biodiesel production. Sustain. Chem. Pharm. 2021, 20, 100378. [Google Scholar] [CrossRef]
- Ma, W.; Lv, T.; Song, X.; Cheng, Z.; Duan, S.; Xin, G. Characteristics of selective fluoride adsorption by biocarbon-Mg/Al layered double hydroxides composites from protein solutions : Kinetics and equilibrium isotherms study. J. Hazard. Mater. 2014, 268, 166–176. [Google Scholar] [CrossRef]
- Taei, M.; Havakeshian, E.; Hasanpour, F.; Abedi, F.; Movahedi, M. Mg–Ca–Fe layered double hydroxide–gold nanoparticles as an efficient electrocatalyst for ethanol oxidation. J. Taiwan Inst. Chem. Eng. 2016, 67, 184–190. [Google Scholar] [CrossRef]
- Mittal, V.K.; Bera, S.; Nithya, R.; Srinivasan, M.P.; Velmurugan, S.; Narasimhan, S. V Solid state synthesis of Mg–Ni ferrite and characterization by XRD and XPS. J. Nucl. Mater. 2004, 335, 302–310. [Google Scholar] [CrossRef]
- Korin, E.; Froumin, N.; Cohen, S. Surface Analysis of Nanocomplexes by X-ray Photoelectron Spectroscopy (XPS). ACS Biomater. Sci. Eng. 2017, 3, 882–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almanassra, I.W.; Zakaria, Y.; Kochkodan, V.; Mroue, K.; Zekri, A.; Atieh, M.A.; Al-Ansari, T. XPS and material properties of raw and oxidized carbide-derived carbon and their application in antifreeze thermal fluids/nanofluids. J. Therm. Anal. Calorim. 2022, 147, 11787–11803. [Google Scholar] [CrossRef]
- Bibi, I.; Khan Niazi, N.; Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Dumat, C.; Imtiaz Rashid, M. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef]
- He, X.; Zhong, P.; Qiu, X. Remediation of hexavalent chromium in contaminated soil by Fe(II)-Al layered double hydroxide. Chemosphere 2018, 210, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Zubair, M.; Aziz, H.A.; Ahmad, M.A.; Ihsanullah, I.; Al-Harthi, M.A. Adsorption and reusability performance of M-Fe (M = Co, Cu, Zn and Ni) layered double hydroxides for the removal of hazardous Eriochrome Black T dye from different water streams. J. Water Process Eng. 2021, 42, 102060. [Google Scholar] [CrossRef]
- Bharali, D.; Deka, R.C. Preferential adsorption of various anionic and cationic dyes from aqueous solution over ternary CuMgAl layered double hydroxide. Colloids Surf. A Physicochem. Eng. Asp. 2017, 525, 64–76. [Google Scholar] [CrossRef]
- Tran, H.N. Comments on “Fast and efficient removal of Cr(VI) to ppb level together with Cr(III) sequestration in water using layered double hydroxide interclated with diethyldithiocarbamate”. Sci. Total Environ. 2020, 746, 139854. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Yan, T.; Zhu, R.; Yan, L.; Pei, Z. Adsorption and photocatalytic reduction of aqueous Cr(VI) by Fe3O4-ZnAl-layered double hydroxide/TiO2 composites. J. Colloid Interface Sci. 2020, 562, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liao, X.; Zhang, X.; Peng, G.; Gao, J.; Chen, L. Enhanced adsorption of hexavalent chromium and the microbial effect on quartz sand modified with Al-layered double hydroxides. Sci. Total Environ. 2021, 762, 143094. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Aziz, H.A.; Ihsanullah, I.; Ahmad, M.A.; Al-Harthi, M.A. Biochar supported CuFe layered double hydroxide composite as a sustainable adsorbent for efficient removal of anionic azo dye from water. Environ. Technol. Innov. 2021, 23, 101614. [Google Scholar] [CrossRef]
- Long, F.; Niu, C.; Tang, N.; Guo, H.; Li, Z.; Yang, Y.; Lin, L. Highly efficient removal of hexavalent chromium from aqueous solution by calcined Mg/Al-layered double hydroxides/polyaniline composites. Chem. Eng. J. 2021, 404, 127084. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, Y.; Wang, J.; Zhou, C.; Tang, Q.; Rao, X. Calcined graphene/MgAl-layered double hydroxides for enhanced Cr(VI) removal. Chem. Eng. J. 2013, 221, 204–213. [Google Scholar] [CrossRef]


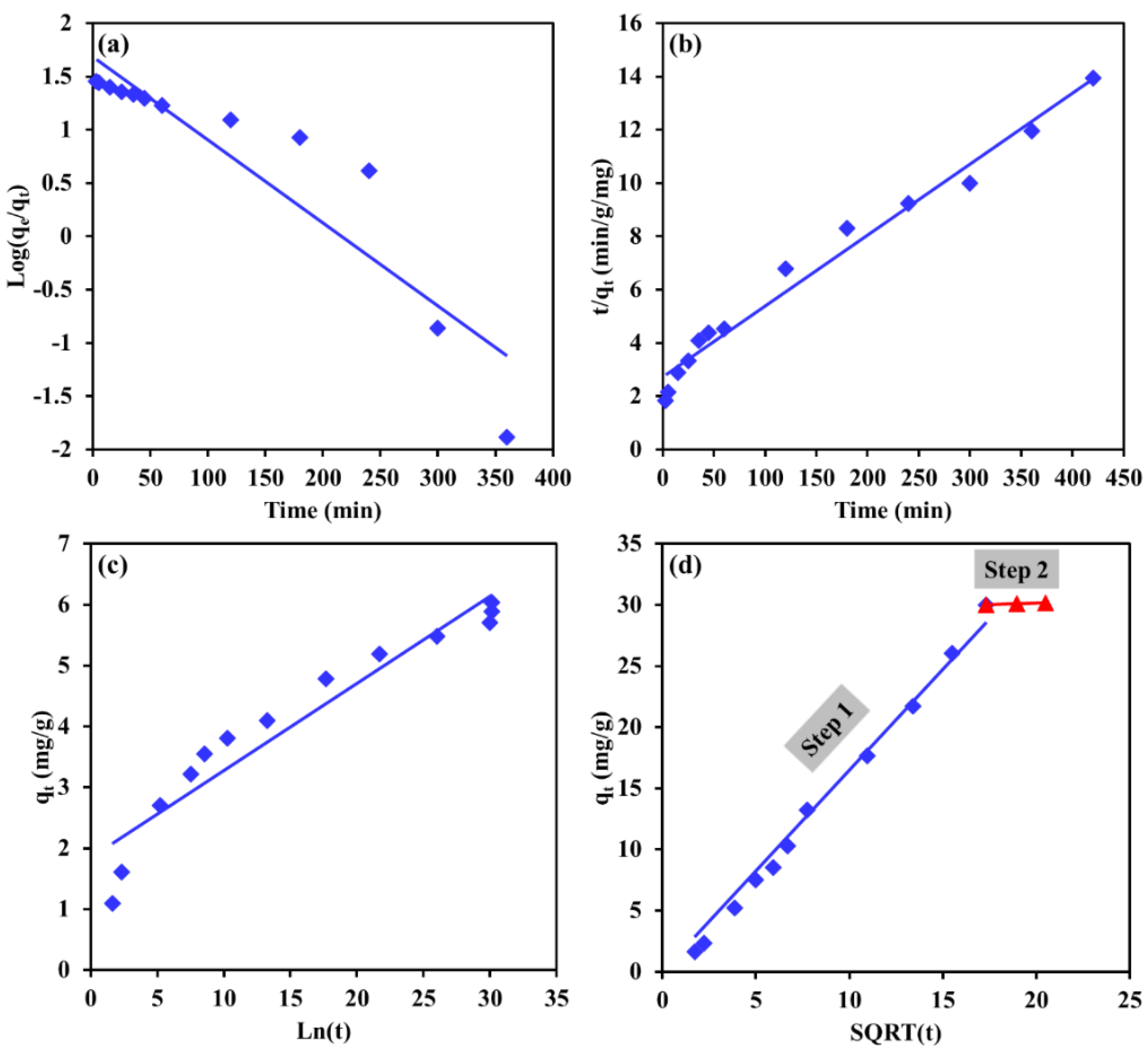
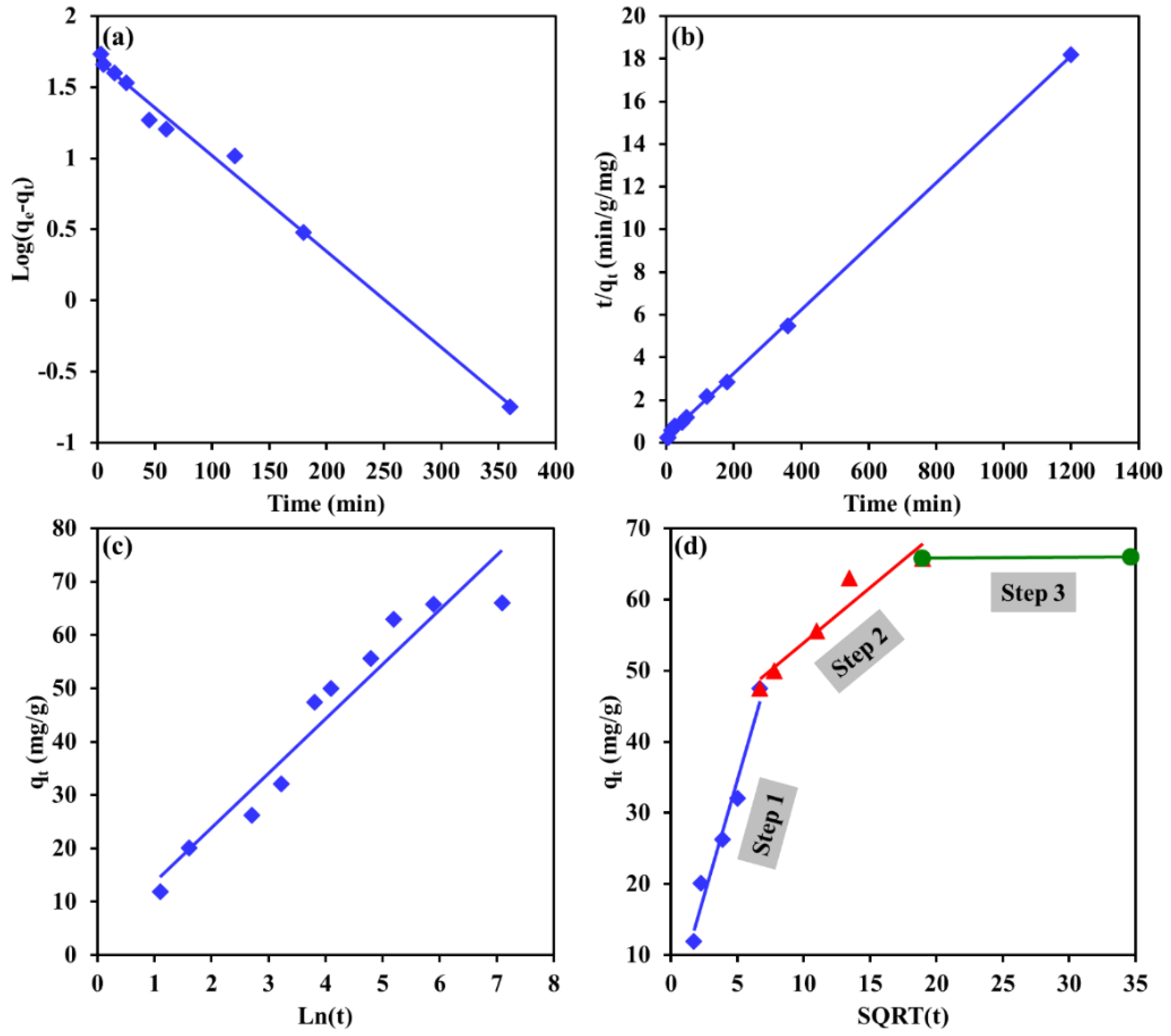


| Kinetic Model | Linearized Form |
|---|---|
| Lagergren PFO | |
| Ho-McKay PSO | |
| Elovich | |
| IPD |
| EBT | Cr | ||
|---|---|---|---|
| qe (experimental, mg/g) | 66.0 | 30.1 | |
| Pseudo 1st order | qe (model) | 49.0 | 48.4 |
| k1 | 0.015 | 0.018 | |
| R2 | 0.991 | 0.844 | |
| Pseudo 2nd order | qe (model) | 67.1 | 37.6 |
| k2 | 0.0008 | 0.0003 | |
| t1/2 | 18.1 | 102 | |
| R2 | 0.999 | 0.979 | |
| Elovich | 14.3 | 53,509 | |
| 0.10 | 6.98 | ||
| R2 | 0.929 | 0.920 | |
| Intraparticle diffusion | kip (1) | 6.48 | 1.649 |
| c (1) | 2.23 | 0 | |
| R2 (1) | 0.966 | 0.989 | |
| kip (2) | 1.55 | 0.044 | |
| c (2) | 38.4 | 29.26 | |
| R2 (2) | 0.920 | 0.838 | |
| kip (3) | 0.01 | - | |
| c (3) | 65.6 | - | |
| R2 (3) | 1 | - | |
| Isotherm | Model |
|---|---|
| Langmuir | |
| Freundlich | |
| Temkin | |
| Sips | |
| Model | R2 | SSE | Parameters | ||
|---|---|---|---|---|---|
| Langmuir | EBT | 0.902 | 4391 | 150.3 | |
| 0.07 | |||||
| 0.03–0.22 | |||||
| Cr | 0.851 | 597 | 65.5 | ||
| 0.04 | |||||
| 0.04–0.44 | |||||
| Freundlich | EBT | 0.960 | 238 | 75.8 | |
| 8.9 | |||||
| Cr | 0.965 | 71 | 17.4 | ||
| 4.4 | |||||
| Temkin | EBT | 0.911 | 525 | 2722 | |
| 237 | |||||
| Cr | 0.866 | 261 | 6.7 | ||
| 313 | |||||
| Sips | EBT | 0.959 | 245 | 3375 | |
| 0.02 | |||||
| 0.12 | |||||
| Cr | 0.964 | 72 | 15,366 | ||
| 0.001 | |||||
| 0.23 | |||||
| Adsorbent | Pollutant | Initial Concentration of Cr or EBT (mg/L) | Adsorbent Dosage (g/L) | Maximum Adsorption Capacity (mg/g) | Ref. |
|---|---|---|---|---|---|
| Mg-Ca-Al-LDO | EBT | 30–500 | 0.75 | 150.3 | Current study |
| CoFe LDH | EBT | 20–100 | - | 137 | [63] |
| CuFe LDH | EBT | 20–100 | - | 250 | [63] |
| ZnFe LDH | EBT | 20–100 | - | 123.6 | [63] |
| NiFe LDH | EBT | 20–100 | - | 123.4 | [63] |
| CuMgAl LDH | EBT | 10–70 | 0.25 | 90.5 | [64] |
| Mg-Ca-Al-LDO | Cr | 30–500 | 1.25 | 65.5 | Current study |
| NiFe LDH | Cr | 10–200 | 0.75 | 14.2 | [34] |
| Divalent iron doped NiFe LDH | Cr | 10–200 | 0.75 | 35.9 | [34] |
| MgAl LDH intercalated with diethyldithiocarbamate | Cr | 0–160 | 0.5 | 52.0 | [65] |
| TiO2 modified Fe3O4-ZnAl-LDH | Cr | 5–300 | - | 47.7 | [66] |
| Quartz sand coated by ZnAl-LDHs | Cr | 0.5–32 | 10 | 14.3 | [67] |
| Adsorbent | |||||||
|---|---|---|---|---|---|---|---|
| 22 °C | 30 °C | 40 °C | 50 °C | 60 °C | |||
| EBT | –34.5 | –79.2 | –11.5 | –10.2 | –9.5 | –8.7 | –8.4 |
| Cr | 25.2 | 112.0 | –8.2 | –8.6 | –9.6 | –10.8 | –12.5 |
| Parameter | Investigated Range | Other Experimental conditions |
|---|---|---|
| Influence of Mg-Ca-Al LDO composite dosage | EBT: 0.25–1.25 g/L Cr: 0.3–1.5 | [EBT]i 50 mg/L; [Cr]i 30 mg/L; temperature, 22 °C; contact time, 24 h; [pH]i, EBT 5.2, Cr 6. |
| Influence of contact time | 1–1250 min | [EBT]i 50 mg/L; [Cr]i 30 mg/L; temperature, 22 °C; dosage, EBT 0.75 g/L, Cr 1 g/L; [pH]i, EBT 5.2, Cr 6. |
| Influence of [pH]i | EBT: 2.3–7.0 Cr: 2.6–10.7 | [EBT]i 100 mg/L; [Cr]i 30 mg/L; temperature, 22 °C; contact time, 24 h; dosage, EBT 0.75 g/L, Cr 1.25 g/L. |
| Influence of temperature | 22–60 °C | [EBT]i 50 mg/L; [Cr]i 30 mg/L; contact time, 24 h; dosage, EBT 0.75 g/L, Cr 1.25 g/L; [pH]i, EBT 5.2, Cr 6. |
| Influence of initial concentration of [EBT]i and [Cr]i | 30–500 mg/L | Temperature, 22 °C; contact time, 24 h; dosage, EBT 0.75 g/L, Cr 1.25 g/L; [pH]i, EBT 5.2, Cr 6. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatla, A.; Almanassra, I.W.; Kochkodan, V.; Laoui, T.; Alawadhi, H.; Atieh, M.A. Efficient Removal of Eriochrome Black T (EBT) Dye and Chromium (Cr) by Hydrotalcite-Derived Mg-Ca-Al Mixed Metal Oxide Composite. Catalysts 2022, 12, 1247. https://doi.org/10.3390/catal12101247
Chatla A, Almanassra IW, Kochkodan V, Laoui T, Alawadhi H, Atieh MA. Efficient Removal of Eriochrome Black T (EBT) Dye and Chromium (Cr) by Hydrotalcite-Derived Mg-Ca-Al Mixed Metal Oxide Composite. Catalysts. 2022; 12(10):1247. https://doi.org/10.3390/catal12101247
Chicago/Turabian StyleChatla, Anjaneyulu, Ismail W. Almanassra, Viktor Kochkodan, Tahar Laoui, Hussain Alawadhi, and Muataz Ali Atieh. 2022. "Efficient Removal of Eriochrome Black T (EBT) Dye and Chromium (Cr) by Hydrotalcite-Derived Mg-Ca-Al Mixed Metal Oxide Composite" Catalysts 12, no. 10: 1247. https://doi.org/10.3390/catal12101247







