A Review on Cement-Based Composites for Removal of Organic/Heavy Metal Contaminants from Water
Abstract
:1. Introduction
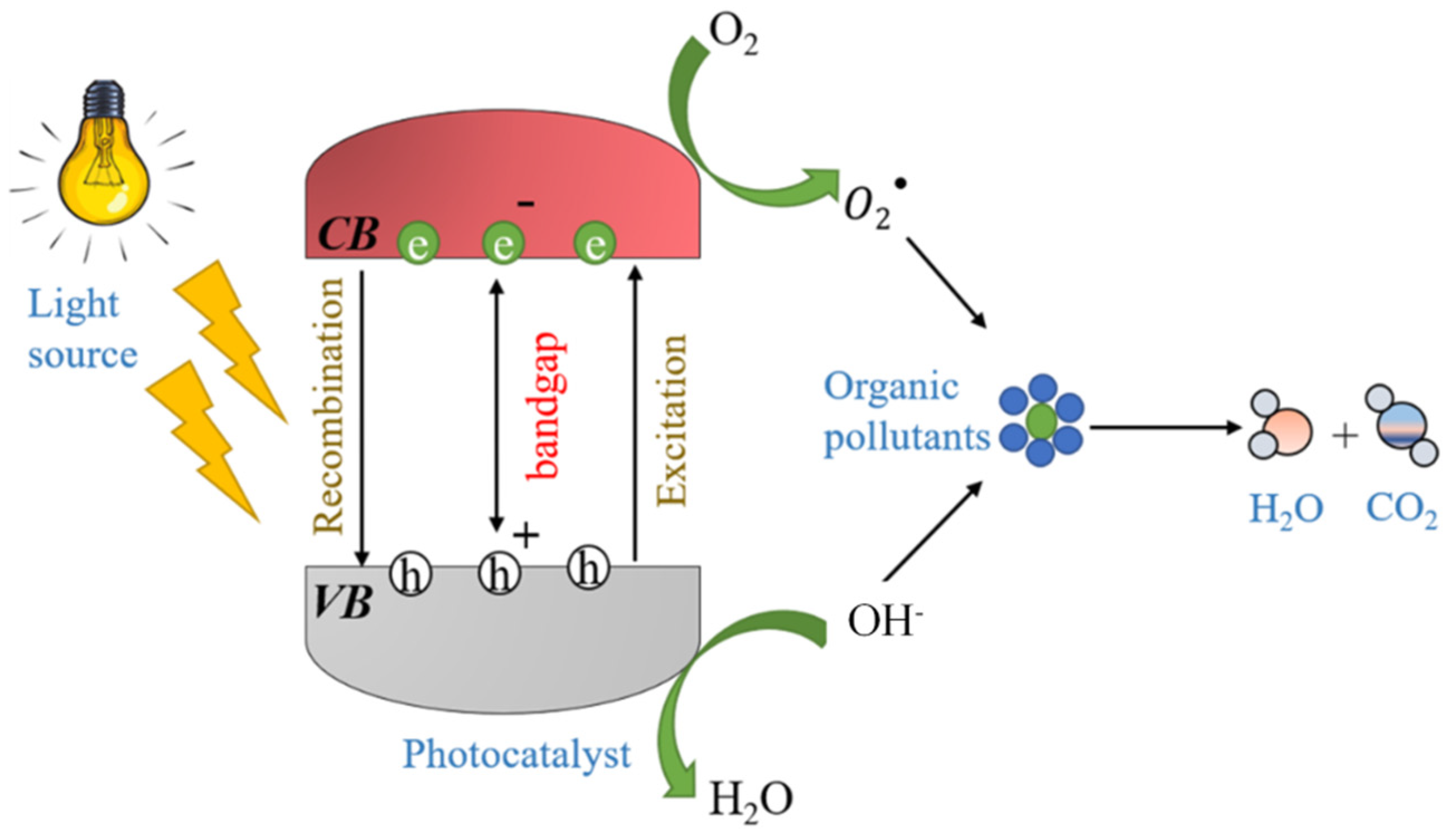
2. Photocatalysis and Photocatalytic Materials
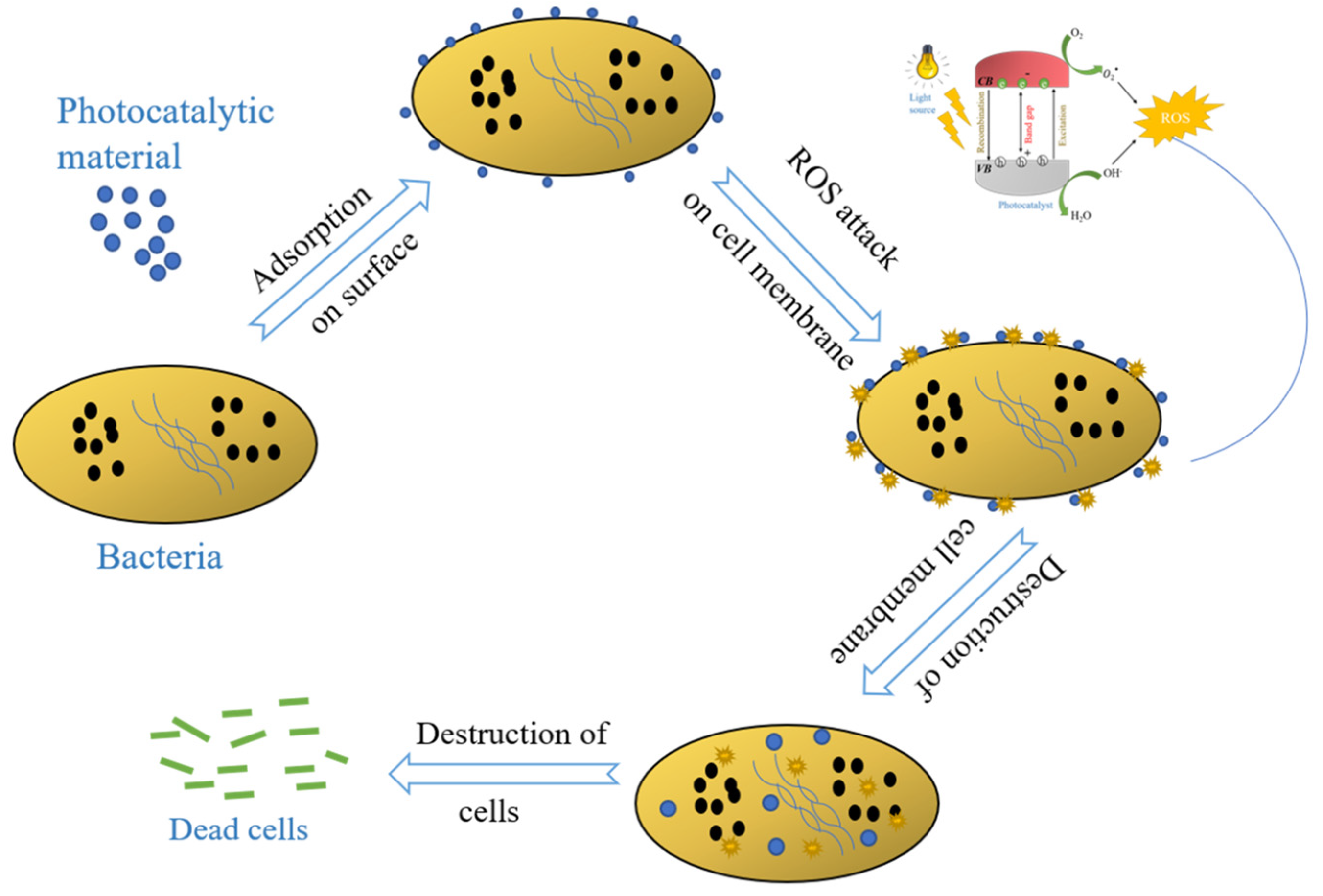
2.1. Photocatalysis-Based Water Detoxification Using Building Material Composites
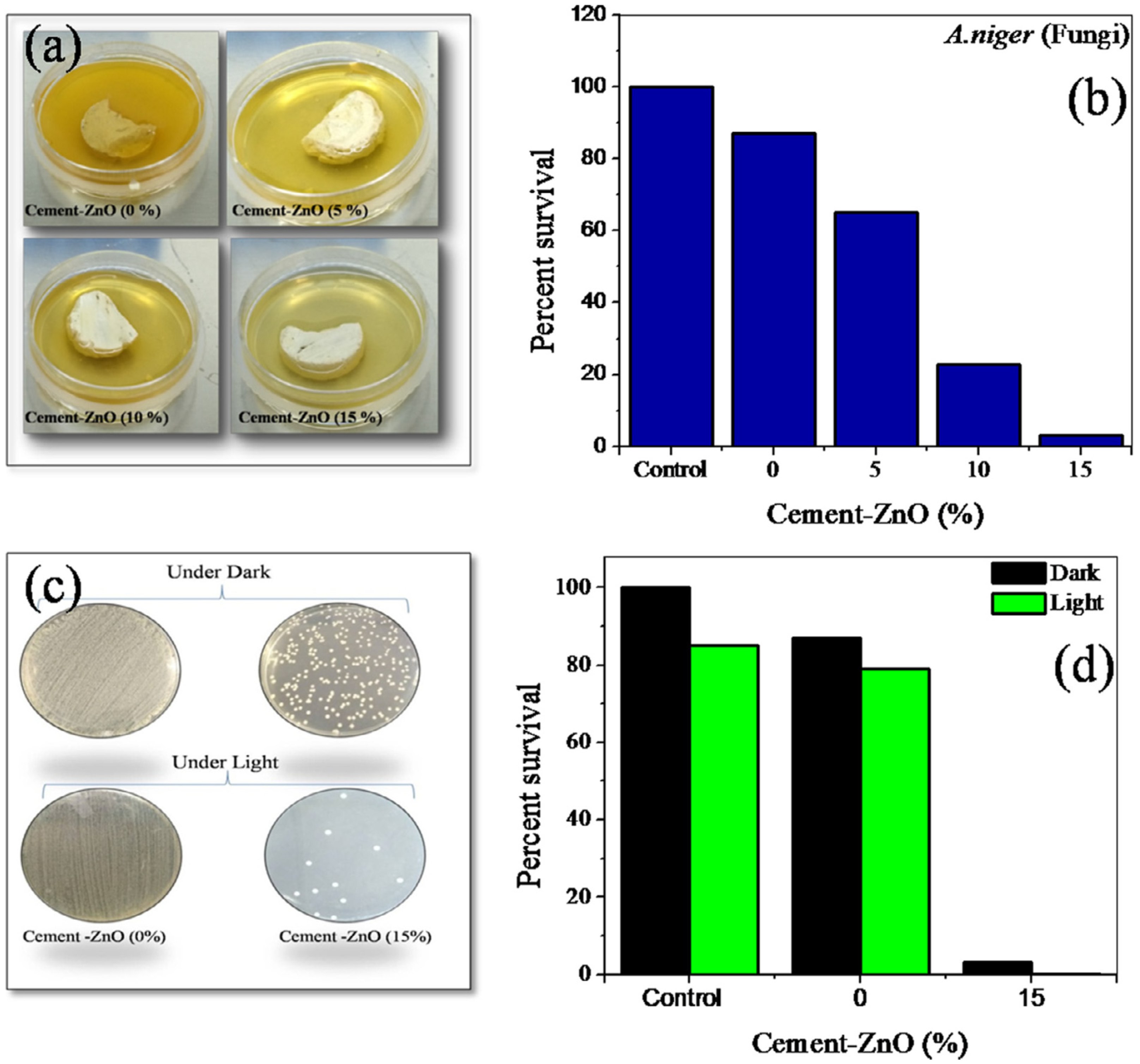
2.2. Antifungal and Antimicrobial Cement-Based Water Detoxification
3. Adsorption-Based Water Detoxification
4. Discussion of Limitations and Future Scope
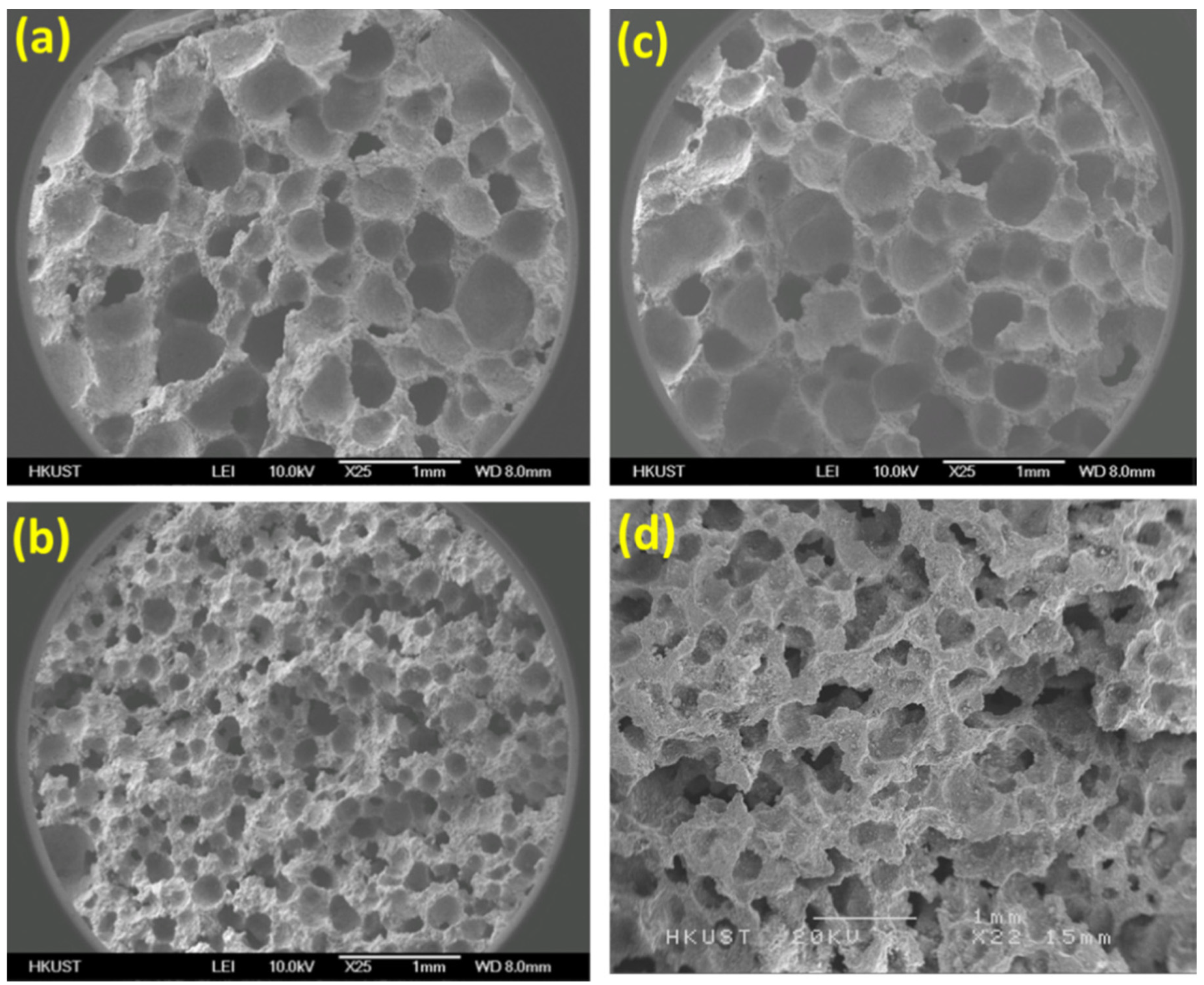
- Photocatalysis is the most exploited technique for water treatment using functional building materials with TiO2 and ZnO being primary photocatalyst. Both catalysts mostly require UV light irradiation for their effective usage. However, solar irradiation only contains 4% UV irradiation, which significantly restricts photocatalytic-based functional building material applications for storage tanks and canals, etc. One way to circumvent this issue is the use of doping, structural orientation, formation of hybrid composites, and photosensitization of surfaces for bandgap tuning. The visible-light-active catalyst can be prepared using these methods, and visible-light-active photocatalytic building materials can be fabricated for practical applications. However, that does not address the aspect that the efficiency of the photocatalyst depends on the intensity of the light reaching the surface of the catalyst. In most reports, the catalyst was used as filler in the cement matrix, which ultimately lowers the proportion of light falling on the catalysts surface, and the efficiency of degradation is diminished. There are different solutions proposed by various researchers to encounter this problem. The porous TiO2/foam composites were fabricated by mixing foaming agent. The porous structure increases the surface area of the catalyst, and more light penetration in the cement can be achieved. Similarly, floating cement–TiO2 composites were prepared to improve light irradiation on the surface of the catalyst. Another approach studied by few researchers was to use catalyst as coatings. It maximizes the light irradiation proportion on the surface of the catalyst, and higher degradation efficiency was observed. These are strategies beneficial for an application in direct wastewater treatment. However, water with high turbidity may generally prevent the application of this approach in structures such as pillars from bridges over lakes and rivers. Thus, the self-cleaning properties or contribution to water cleaning of the structures may be inhibited.
- In case the claim is made that investigated composites are useful for self-cleaning surfaces in constructions, data should generally be provided that the mechanical properties do not suffer or could even be enhanced. The filler in the cement can alter the hardening and the mechanical properties of cement. There are few reports which have studied the influence of these fillers on aging and mechanical properties. Still, more robust and detailed studies are required to analyze the most optimum proportion of filler to obtain the best catalytic activity without much affecting its mechanical and hydration properties. Additionally, the robustness of coatings is essential to ensure longevity in extreme weather conditions.
- Very often, the merit of using cement-based composites is just the effective removal of contaminants and not necessarily the applications in buildings and constructions. This should be highlighted rather with the focus on efficient and economic wastewater treatment plants.
- Leaching of the catalytically active nanomaterials into the environment is critical, as well. Therefore, the environmental impact of the composites themselves should also be in the focus of the research. In some publications, the conclusion is drawn that coatings on cement are better than embedding the catalysts in the cement. Even though this may be true for many approaches, leaching of the nanomaterials is likely much more prominent for coatings. Furthermore, detrimental effects could occur even in the case of sufficiently bound nanomaterials. The removal and degradation of molecules and bacteria are not specific. Thus, the use in constructions and buildings could, for example, lead to an undefined impact on the microbiome of the surroundings. Additionally, harmful degradation products could also develop, which are as toxic as or even more toxic than the contaminant itself.
- Economic aspects of fabrication and the implementation of these functional materials have not been discussed in any of the reports. It is just assumed that the strategies are more economical because they are based on cement. However, no definite proof or comparison with other procedures is given. Therefore, even if these functional materials found suitable for performance background, it is vital to perform an economic study for the feasibility of practical applications.
- Adsorption-based functional building materials are more versatile than photocatalytic building materials as they can adsorb both organic and inorganic pollutants (heavy metals ions). However, the biggest disadvantage of adsorbents is nonreusability. Unlike photocatalysis, pollutants are not degraded in the adsorption process. They are transferred to the surface of the adsorbent. Therefore, the reusability of adsorbent is possible after desorption of adsorbed compounds in a suitable desorption media. It may make the process complex and uneconomical for practical applications.
- Ferroelectric materials can be explored for better catalytic efficiency due to their spontaneous polarization. Recent reports suggest that remnant polarization on the surface of ferroelectric materials can generate ROS in the water, and bacterial disinfection can be achieved. Composite of cement and ferroelectric materials can be studied for photocatalytic and piezocatalytically degrading organic contaminants and bacterial disinfection.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schweitzer, L.; Noblet, J. Water Contamination and Pollution. In Green Chemistry: An Inclusive Approach; Elsevier: Amsterdam, The Netherlands, 2018; pp. 261–290. [Google Scholar]
- Sonune, A.; Ghate, R. Developments in Wastewater Treatment Methods. Desalination 2004, 167, 55–63. [Google Scholar] [CrossRef]
- Chan, Y.J.; Chong, M.F.; Law, C.L.; Hassell, D.G. A Review on Anaerobic-Aerobic Treatment of Industrial and Municipal Wastewater. Chem. Eng. J. 2009, 155, 1–18. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.J.; Li, Q. Applications of Nanotechnology in Water and Wastewater Treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A Critical Review on Textile Wastewater Treatments: Possible Approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Mamiyev, Z. Integrated processes involving adsorption, photolysis, and photocatalysis. In Hybrid and Combined Processes for Air Pollution Control; Elsevier: Amsterdam, The Netherlands, 2022; pp. 117–153. [Google Scholar]
- Singh, V.P.; Sharma, M.; Vaish, R. Enhanced dye adsorption and rapid photocatalysis of candle soot coated BaTiO3 ceramics. Mater. Chem. Phys. 2020, 252, 123311. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A Review of ZnO Nanoparticles as Solar Photocatalysts: Synthesis, Mechanisms and Applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Zheng, N.; Dillert, R.; Bahnemann, D.W. Visible-light-mediated photocatalytic aerobic dehydrogenation of N-heterocycles by surface-grafted TiO2 and 4-amino-TEMPO. ACS Catal. 2019, 9, 10694–10704. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Folli, A.; Pade, C.; Hansen, T.B.; De Marco, T.; MacPhee, D.E. TiO2 Photocatalysis in Cementitious Systems: Insights into Self-Cleaning and Depollution Chemistry. Cem. Concr. Res. 2012, 42, 539–548. [Google Scholar] [CrossRef]
- Bahnemann, D. Photocatalytic Water Treatment: Solar Energy Applications. Sol. Energy 2004, 77, 445–459. [Google Scholar] [CrossRef]
- Oller, I.; Gernjak, W.; Maldonado, M.I.; Pérez-Estrada, L.A.; Sánchez-Pérez, J.A.; Malato, S. Solar Photocatalytic Degradation of Some Hazardous Water-Soluble Pesticides at Pilot-Plant Scale. J. Hazard. Mater. 2006, 138, 507–517. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and Disinfection of Water by Solar Photocatalysis: Recent Overview and Trends. Catalysis Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Alhaji, M.H.; Sanaullah, K.; Khan, A.; Hamza, A.; Muhammad, A.; Ishola, M.S.; Rigit, A.R.H.; Bhawani, S.A. Recent Developments in Immobilizing Titanium Dioxide on Supports for Degradation of Organic Pollutants in Wastewater—A Review. Int. J. Environ. Sci. Technol. 2017, 14, 2039–2052. [Google Scholar] [CrossRef]
- Tokarský, J.; Mamulová Kutláková, K.; Podlipná, R.; Vaněk, T. Phytotoxicity of ZnO/Kaolinite Nanocomposite—Is Anchoring the Right Way to Lower Environmental Risk? Environ. Sci. Pollut. Res. 2019, 26, 22069–22081. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium Dioxide Nanoparticles: A Review of Current Toxicological Data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Tourinho, P.S.; van Gestel, C.A.M.; Lofts, S.; Svendsen, C.; Soares, A.M.V.M.; Loureiro, S. Metal-Based Nanoparticles in Soil: Fate, Behavior, and Effects on Soil Invertebrates. Environ. Toxicol. Chem. 2012, 31, 1679–1692. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. Human Health Effects of Air Pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Balayeva, N.O.; Fleisch, M.; Bahnemann, D.W. Bahnemann. Surface-grafted WO3/TiO2 photocatalysts: Enhanced visible-light activity towards indoor air purification. Catal. Today 2018, 313, 63–71. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-Cleaning Applications of TiO2 by Photo-Induced Hydrophilicity and Photocatalysis. Appl. Catal. B Environ. 2015, 176, 396–428. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.; Haghighat, F. Photocatalytic Air Cleaners and Materials Technologies—Abilities and Limitations. Build. Environ. 2015, 91, 191–203. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, X. Photocatalytic Oxidation for Indoor Air Purification: A Literature Review. Build. Environ. 2003, 38, 645–654. [Google Scholar] [CrossRef]
- Zailan, S.N.; Mahmed, N.; Abdullah, M.M.; Sandu, A.V. Self-cleaning geopolymer concrete—A review. IOP Conf. Ser. Mater. Sci. Eng. 2016, 133, 012026. [Google Scholar] [CrossRef]
- Ali, I.; Gupta, V.K. Advances in Water Treatment by Adsorption Technology. Nat. Protoc. 2007, 1, 2661. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment Technologies for Emerging Contaminants in Water: A Review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef] [Green Version]
- Pendergast, M.M.; Hoek, E.M.V. A Review of Water Treatment Membrane Nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef] [Green Version]
- Mills, A.; Davies, R.H.; Worsley, D. Water Purification by Semiconductor Photocatalysis. Chem. Soc. Rev. 1993, 22, 417–425. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous Photocatalyst Materials for Water Splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Z.; Huang, J.; Lim, L.Y.; Li, W.; Deng, J.; Gong, D.; Tang, Y.; Lai, Y.; Chen, Z. Titanate and Titania Nanostructured Materials for Environmental and Energy Applications: A Review. RSC Adv. 2015, 5, 79479–79510. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent Developments of Zinc Oxide Based Photocatalyst in Water Treatment Technology: A Review. Water Res. 2016, 428–448. [Google Scholar] [CrossRef]
- Sikora, P.; Augustyniak, A.; Cendrowski, K.; Nawrotek, P.; Mijowska, E. Antimicrobial Activity of Al2O3, CuO, Fe3O4, and ZnO Nanoparticles in Scope of Their Further Application in Cement-Based Building Materials. Nanomaterials 2018, 8, 212. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, V.P.; Vaish, R. Waste Paper Pulp Derived Reduced Graphene Oxide for Antimicrobial Cement Composites. J. Electron. Mater. 2018, 47, 6862–6867. [Google Scholar] [CrossRef]
- Gogniat, G.; Dukan, S. TiO2 Photocatalysis Causes DNA Damage via Fenton Reaction-Generated Hydroxyl Radicals during the Recovery Period. Appl. Environ. Microbiol. 2007, 73, 7740–7743. [Google Scholar] [CrossRef] [Green Version]
- Beeldens, A. An Environmental Friendly Solution for Air Purification and Self-Cleaning Effect: The Application of TiO2 as Photocatalyst in Concrete. Proc. Transp. Res. Arena Eur. 2006, 12–16. Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=35.%09Beeldens%2C+A.+An+Environmental+Friendly+Solution+for+Air+Purification+and+Self-Cleaning+Effect%3A+The+Application+of+TIO2+as+Photocatalyst+in+Concrete.+Proc.+Transp.+Res.+Arena+Eur.+2006%2C+12%E2%80%9316&btnG= (accessed on 7 October 2022).
- Rochkind, M.; Pasternak, S.; Paz, Y. Using Dyes for Evaluating Photocatalytic Properties: A Critical Review. Molecules 2015, 20, 88–110. [Google Scholar] [CrossRef] [Green Version]
- Lackhoff, M.; Prieto, X.; Nestle, N.; Dehn, F.; Niessner, R. Photocatalytic Activity of Semiconductor-Modified Cement—Influence of Semiconductor Type and Cement Ageing. Appl. Catal. B Environ. 2003, 43, 205–216. [Google Scholar] [CrossRef]
- Neppolian, B.; Kanel, S.R.; Choi, H.C.; Shankar, M.V.; Arabindoo, B.; Murugesan, V. Photocatalytic Degradation of Reactive Yellow 17 Dye in Aqueous Solution in the Presence of TiO2 with Cement Binder. Int. J. Photoenergy 2003, 5, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Vulic, T.; Hadnadjev-Kostic, M.; Rudic, O.; Radeka, M.; Marinkovic-Neducin, R.; Ranogajec, J. Improvement of Cement-Based Mortars by Application of Photocatalytic Active Ti-Zn-Al Nanocomposites. Cem. Concr. Compos. 2013, 36, 121–127. [Google Scholar] [CrossRef]
- Cerro-Prada, E.; Manso, M.; Torres, V.; Soriano, J. Microstructural and Photocatalytic Characterization of Cement-Paste Sol-Gel Synthesized Titanium Dioxide. Front. Struct. Civ. Eng. 2016, 10, 189–197. [Google Scholar] [CrossRef]
- de Andrade, F.V.; de Lima, G.M.; Augusti, R.; da Silva, J.C.C.; Coelho, M.G.; Paniago, R.; Machado, I.R. A Novel TiO2 Autoclaved Cellular Concrete Composite: From a Precast Building Material to a New Floating Photocatalyst for Degradation of Organic Water Contaminants. J. Water Process Eng. 2015, 7, 27–35. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, Q.; Yin, R.; Chen, B.; Li, Z. A Novel TiO2/Foam Cement Composite with Enhanced Photodegradation of Methyl Blue. Constr. Build. Mater. 2016, 129, 159–162. [Google Scholar] [CrossRef]
- Jafari, H.; Afshar, S. Improved Photodegradation of Organic Contaminants Using Nano-TiO2 and TiO2-SiO2 Deposited on Portland Cement Concrete Blocks. Photochem. Photobiol. 2016, 92, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Cerro-Prada, E.; García-Salgado, S.; Quijano, M.Á.; Varela, F. Controlled Synthesis and Microstructural Properties of Sol-Gel TiO2 Nanoparticles for Photocatalytic Cement Composites. Nanomaterials 2019, 9, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, S.; Liu, F.; Fu, X.; Peng, X.; Zhu, J.; Zeng, Q.; Song, J. Photocatalytic Performances and Durability of TiO2/Cement Composites Prepared by a Smear Method for Organic Wastewater Degradation. Ceram. Int. 2019, 45, 23061–23069. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, G. Recent Advances in Synthesis and Applications of Clay-Based Photocatalysts: A Review. Phys. Chem. Chem. Phys. 2014, 16, 8178–8192. [Google Scholar] [CrossRef]
- Singh, V.P.; Sandeep, K.; Kushwaha, H.S.; Powar, S.; Vaish, R. Photocatalytic, Hydrophobic and Antimicrobial Characteristics of ZnO Nano Needle Embedded Cement Composites. Constr. Build. Mater. 2018, 158, 285–294. [Google Scholar] [CrossRef]
- Le Pivert, M.; Poupart, R.; Capochichi-Gnambodoe, M.; Martin, N.; Leprince-Wang, Y. Direct Growth of ZnO Nanowires on Civil Engineering Materials: Smart Materials for Supported Photodegradation. Microsyst. Nanoeng. 2019, 5, 57. [Google Scholar] [CrossRef] [Green Version]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent Advances in Semiconductors for Photocatalytic and Photoelectrochemical Water Splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Feng, S.; Li, F. Photocatalytic dyes degradation on suspended and cement paste immobilized TiO2/g-C3N4 under simulated solar light. J. Environ. Chem. Eng. 2021, 9, 105488. [Google Scholar] [CrossRef]
- Wu, L.; Pei, X.; Mei, M.; Li, Z.; Lu, S. Study on Photocatalytic Performance of Ag/TiO2 Modified Cement Mortar. Materials 2022, 15, 4031. [Google Scholar] [CrossRef]
- Zhou, Y.; Elchalakani, M.; Liu, H.; Briseghella, B.; Sun, C. Photocatalytic concrete for degrading organic dyes in water. Environ. Sci. Pollut. Res. 2022, 29, 39027–39040. [Google Scholar] [CrossRef]
- Lin, R.-S.; Kim, T.; Wang, X.-Y.; Du, W. Potential application of MoS2 nanoflowers as photocatalysts in cement: Strength, hydration, and dye degradation properties. J. Clean. Prod. 2022, 330, 129947. [Google Scholar] [CrossRef]
- Rastogi, M.; Vaish, R. Visible Light Induced Water Detoxification through Portland Cement Composites Reinforced with Photocatalytic Filler: A Leap Away from TiO2. Constr. Build. Mater. 2016, 120, 364–372. [Google Scholar] [CrossRef]
- Sharma, M.; Vaish, R. Vibration energy harvesting for degradation of dye and bacterial cells using cement-based Ba0. 85Ca0. 15Zr0. 1Ti0. 90O3 composites. Mater. Today Commun. 2020, 2, 101592. [Google Scholar] [CrossRef]
- Ruot, B.; Plassais, A.; Olive, F.; Guillot, L.; Bonafous, L. TiO2-Containing Cement Pastes and Mortars: Measurements of the Photocatalytic Efficiency Using a Rhodamine B-Based Colourimetric Test. Sol. Energy 2009, 83, 1794–1801. [Google Scholar] [CrossRef]
- Yuranova, T.; Sarria, V.; Jardim, W.; Rengifo, J.; Pulgarin, C.; Trabesinger, G.; Kiwi, J. Photocatalytic Discoloration of Organic Compounds on Outdoor Building Cement Panels Modified by Photoactive Coatings. J. Photochem. Photobiol. A Chem. 2007, 188, 334–341. [Google Scholar] [CrossRef]
- Rachel, A.; Subrahmanyam, M.; Boule, P. Comparison of Photocatalytic Efficiencies of TiO2 in Suspended and Immobilised Form for the Photocatalytic Degradation of Nitrobenzenesulfonic Acids. Appl. Catal. B Environ. 2002, 37, 301–308. [Google Scholar] [CrossRef]
- Wang, F.; Yang, L.; Wang, H.; Yu, H. Facile Preparation of Photocatalytic Exposed Aggregate Concrete with Highly Efficient and Stable Catalytic Performance. Chem. Eng. J. 2015, 264, 577–586. [Google Scholar] [CrossRef]
- Watts, M.J.; Cooper, A.T. Photocatalysis of 4-Chlorophenol Mediated by TiO2 Fixed to Concrete Surfaces. Sol. Energy 2008, 82, 206–211. [Google Scholar] [CrossRef]
- Venkata Subba Rao, K.; Rachel, A.; Subrahmanyam, M.; Boule, P. Immobilization of TiO2 on Pumice Stone for the Photocatalytic Degradation of Dyes and Dye Industry Pollutants. Appl. Catal. B Environ. 2003, 46, 77–85. [Google Scholar] [CrossRef]
- Senff, L.; Tobaldi, D.M.; Lemes-Rachadel, P.; Labrincha, J.A.; Hotza, D. The Influence of TiO2 and ZnO Powder Mixtures on Photocatalytic Activity and Rheological Behavior of Cement Pastes. Constr. Build. Mater. 2014, 65, 191–200. [Google Scholar] [CrossRef]
- Janus, M.; Zatorska, J.; Czyżewski, A.; Bubacz, K.; Kusiak-Nejman, E.; Morawski, A.W. Self-cleaning properties of cement plates loaded with N,C-modified TiO2 photocatalysts. Appl. Surf. Sci. 2015, 330, 200–206. [Google Scholar] [CrossRef]
- Sikora, P.; Cendrowski, K.; Markowska-Szczupak, A.; Horszczaruk, E.; Mijowska, E. The Effects of Silica/Titania Nanocomposite on the Mechanical and Bactericidal Properties of Cement Mortars. Constr. Build. Mater. 2017, 150, 738–746. [Google Scholar] [CrossRef]
- Linkous, C.A.; Carter, G.J.; Locuson, D.V.; Ouellette, A.J.; Slattery, D.K.; Smitha, L.A. Photocatalytic Inhibition of Algae Growth Using TiO2, WO3, and Cocatalyst Modifications. Environ. Sci. Technol. 2000, 34, 4754–4758. [Google Scholar] [CrossRef]
- Nath, D.; Jangid, K.; Susaniya, A.; Kumar, R.; Vaish, R. Eggshell derived CaO-Portland cement antibacterial composites. Composites 2021, 5, 100123. [Google Scholar] [CrossRef]
- Yu, C.; Qiu, J.S.; Sun, Y.F.; Li, X.H.; Chen, G.; Zhao, Z.B. Adsorption removal of thiophene and dibenzothiophene from oils with activated carbon as adsorbent: Effect of surface chemistry. J. Porous Mater. 2008, 15, 151–157. [Google Scholar] [CrossRef]
- Ali, I. New Generation Adsorbents for Water Treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef]
- Gupta, V.K.; Suhas. Application of Low-Cost Adsorbents for Dye Removal—A Review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- El Zayat, M.; Elagroudy, S.; El Haggar, S. Removal of Some Heavy Metals in Selected Wastewater Using Cement Kiln Dust. In World Environmental and Water Resources Congress 2015: Floods, Droughts, and Ecosystems—Proceedings of the 2015 World Environmental and Water Resources Congress; ASCE: Reston, VA, USA, 2015; pp. 2459–2469. Available online: https://ascelibrary.org/doi/abs/10.1061/9780784479162.241 (accessed on 7 October 2022).
- Ok, Y.S.; Yang, J.E.; Zhang, Y.S.; Kim, S.J.; Chung, D.Y. Heavy Metal Adsorption by a Formulated Zeolite-Portland Cement Mixture. J. Hazard. Mater. 2007, 147, 91–96. [Google Scholar] [CrossRef]
- Meshko, V.; Markovska, L.; Mincheva, M.; Rodrigues, A.E. Adsorption of Basic Dyes on Granular Acivated Carbon and Natural Zeolite. Water Res. 2001, 35, 3357–3366. [Google Scholar] [CrossRef]
- Kundu, S.; Gupta, A.K. Analysis and Modeling of Fixed Bed Column Operations on As(V) Removal by Adsorption onto Iron Oxide-Coated Cement (IOCC). J. Colloid Interface Sci. 2005, 290, 52–60. [Google Scholar] [CrossRef]
- Han, H.; Rafiq, M.K.; Zhou, T.; Xu, R.; Mašek, O.; Li, X. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J. Hazard. Mater. 2019, 369, 780–796. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and Adsorption Capacities of Low-Cost Sorbents for Wastewater Treatment: A Review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef] [Green Version]
- Pimraksa, K.; Chindaprasirt, P.; Huanjit, T.; Tang, C.; Sato, T. Cement Mortars Hybridized with Zeolite and Zeolite-like Materials Made of Lignite Bottom Ash for Heavy Metal Encapsulation. J. Clean. Prod. 2013, 41, 31–41. [Google Scholar] [CrossRef]
- Pratap Singh, V.; Vaish, R. Cement-Based Diesel Exhaust Emission Soot Coatings for the Removal of Organic Pollutants from Water. Constr. Build. Mater. 2020, 234, 117377. [Google Scholar] [CrossRef]
- Manjunath, S.V.; Baghel, R.S.; Kumar, M. Performance Evaluation of Cement–Carbon Composite for Adsorptive Removal of Acidic and Basic Dyes from Single and Multi-Component Systems. Environ. Technol. Innov. 2019, 16, 100478. [Google Scholar]
- El-Awady, M.H.; Sami, T.M. Removal of Heavy Metals by Cement Kiln Dust. Bull. Environ. Contam. Toxicol. 1997, 59, 603–610. [Google Scholar] [CrossRef]
- Mahmoued, E.K. Cement Kiln Dust and Coal Filters Treatment of Textile Industrial Effluents. Desalination 2010, 255, 175–178. [Google Scholar] [CrossRef]
- Magdy, Y.H.; Altaher, H. Kinetic Analysis of the Adsorption of Dyes from High Strength Wastewater on Cement Kiln Dust. J. Environ. Chem. Eng. 2018, 6, 834–841. [Google Scholar] [CrossRef]
- Çoruh, S.; Elevli, S. Optimization Study of Dye Removal by Cement Kiln Dust Using the Central Composite Design of Experiments. Glob. Nest J. 2015, 17, 93–102. [Google Scholar]
- Salem, A.; Afshin, H.; Behsaz, H. Removal of Lead by Using Raschig Rings Manufactured with Mixture of Cement Kiln Dust, Zeolite and Bentonite. J. Hazard. Mater. 2012, 223, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Gupta, A.K. Arsenic adsorption onto iron oxide-coated cement (IOCC): Regression analysis of equilibrium data with several isotherm models and their optimization. Chem. Eng. J. 2006, 122, 93–106. [Google Scholar] [CrossRef]
- Mackie, A.; Boilard, S.; Walsh, M.E.; Lake, C.B. Physicochemical characterization of cement kiln dust for potential reuse in acidic wastewater treatment. J. Hazard. Mater. 2010, 173, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.W. Process Water Treatment in Canada’s Oil Sands Industry: II. A Review of Emerging Technologies. J. Environ. Eng. Sci. 2008, 7, 123–138. [Google Scholar] [CrossRef]
- Song, J.; Li, S.; Zhao, C.; Lu, Y.; Zhao, D.; Sun, J.; Roy, T.; Carmalt, C.J.; Deng, X.; Parkin, I.P. A Superhydrophilic Cement-Coated Mesh: An Acid, Alkali, and Organic Reagent-Free Material for Oil/Water Separation. Nanoscale 2018, 10, 1920–1929. [Google Scholar] [CrossRef] [PubMed]




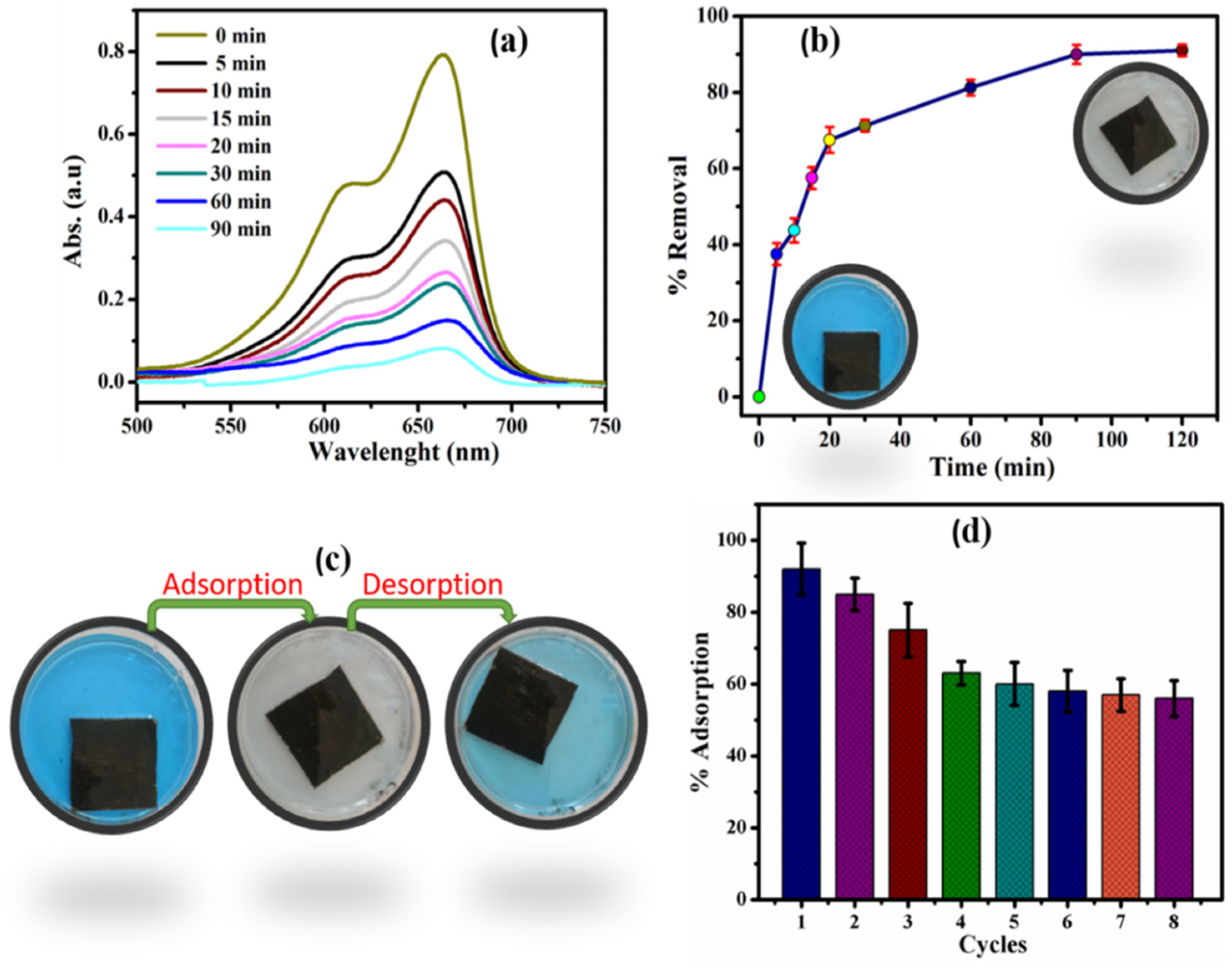
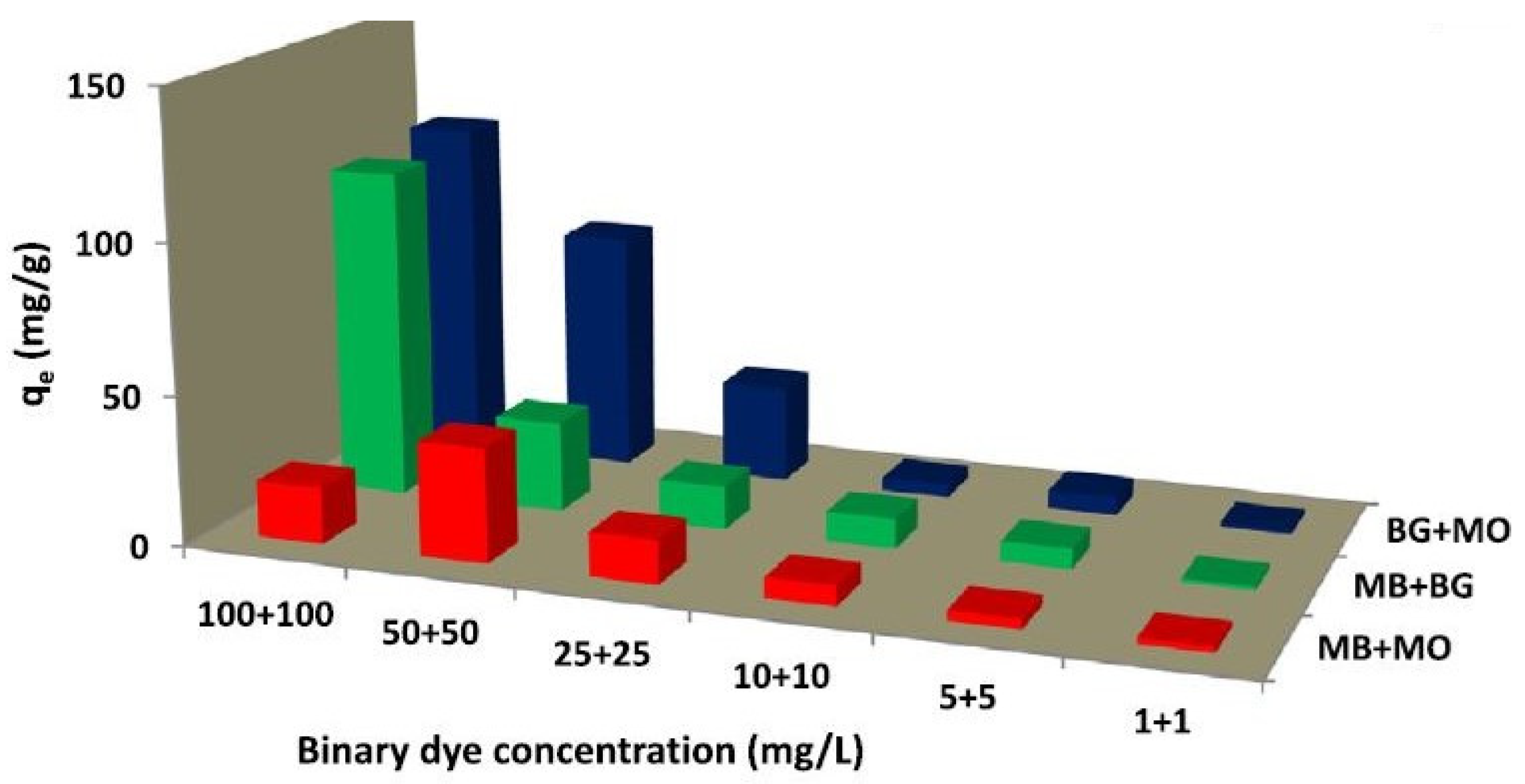
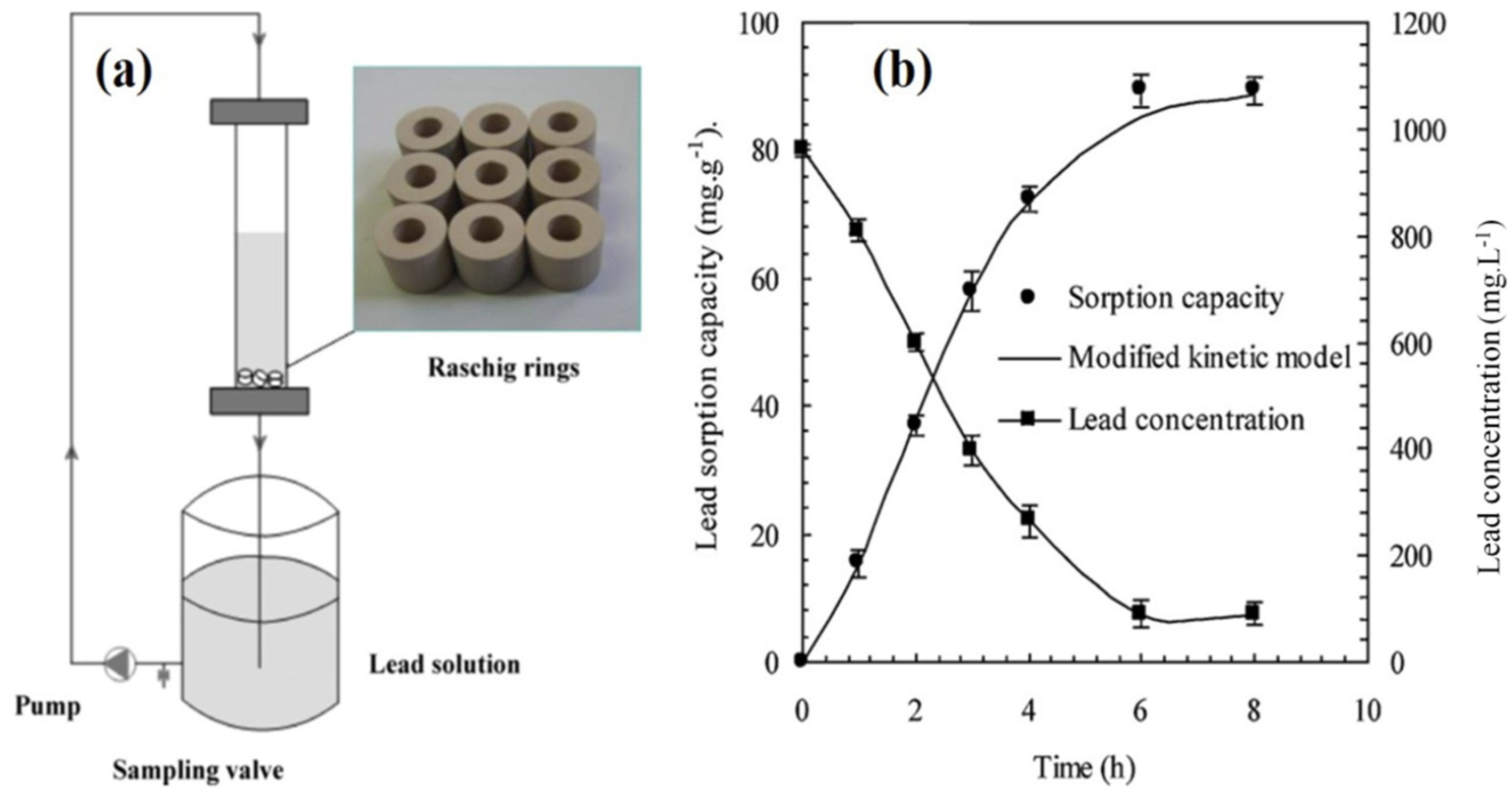
| Cement-Based Sample | External Source | Model Pollutant | Catalysis Performance | Ref. |
|---|---|---|---|---|
| Semiconductor (TiO2 and Hombikat UV 100) modified cement | UV light | Atrazine (2-chloro-4-(ethylamino)-6-(isopropyl-amino)-s-triazine) | 7.8% degradation at 7 h | [7] |
| TiO2/cement composites prepared by a smear method | UV light | Rhodamine B (RhB), methylene blue, and methyl orange | Almost 100% in 50 min | [33] |
| TiO2 with a cement binder | UV light | Yellow 17 dye | 60–95% degradation at 8 h | [38] |
| Ti-Zn-Al nanocomposites with cement-based mortars | UV light | Methylene blue (MB dye) | 28% degradation at 3.5 h | [39] |
| TiO2–cement paste | UV light | Methylene blue (MB dye) | - | [40] |
| TiO2/autoclaved cellular concrete composite | UV light | Indigo carmine dye | 100% in 350 min | [41] |
| TiO2-SiO2 nanohybrid based cement materials | UV light | Malachite green oxalate (MG), methylene blue (MB), and methyl orange (MO) | 87% degradation at 120 min | [43] |
| Sol–gel TiO2 nanoparticles for photocatalytic cement composites | UV light | Methylene blue (MB) | 76.60% | [44] |
| ZnO nanoneedle-based cement composite | UV light | Rhodamine 6G | k = 0.147 min−1 at 12 h | [47] |
| BaTiO3-rGo composition with Portland cement | Visible light | Rhodamine B | k = 0.4 min−1 at 3.5 h | [54] |
| TiO2-containing cement past and mortars | UV light | Rhodamine B | 80% degradation at 7 h | [56] |
| TiO2/SiO2 surface layers on cement panels–plates | Daylight | Red wine | - | [57] |
| TiO2 (P25) deposition on white cement | UV light | Nitrobenzenesulfonic acids | ~0.02–0.03(10−5 M h−1) | [58] |
| TiO2 (P25)-based cement composite | UV light | Benzene | Specific rate of CO2 (650 CO2/ppm) at 80 min | [59] |
| TiO2 fixed on concrete | UV light | 4-chlorophenol | Rate constant 0.277 (mg/(L h)) | [60] |
| TiO2 on pumice stone | UV light | 3-nitrobenzenesulfonic acid (3-NBSA), acid orange-7 | - | [61] |
| TiO2 and ZnO powder mixtures in cement paste | UV light | Methylene blue (MB) | Variable activity with different proportions of TiO2 and ZnO | [62] |
| Cement plates loaded with N,C-modified TiO2 | UV light | RR198 | 49.3% degradation at 100 h | [63] |
| Cement-Based Sample | Model Pollutant | Adsorbent Performance | Ref. |
|---|---|---|---|
| Zeolite–Portland cement | Heavy metal | 90% of the Cu within 30 min | [71] |
| Iron oxide-coated cement | Arsenic | 786–963.75 | [73] |
| Cement mortars hybridized with zeolite | Heavy metal | 97%. | [76] |
| Cement–carbon composite | Acidic and basic dyes | 21.50, 9.06, and 20.20 mg/g for BG, MB and MO in the single-dye system, respectively | [78] |
| Cement kiln dust | Heavy Metals | 40–99.4% | [79] |
| Cement kiln dust and coal filters | Textile industrial effluents | 97% of color, 76% of turbidity, 84% of COD, 77% of BOD and 94% of PO4−3 from raw textile wastewater | [80] |
| Cement kiln dust | Dyes | BB69 and AR114 were 2119 mg/g and 2125 mg/g, respectively. | [81] |
| Cement kiln dust | Dye | 99.4623% | [82] |
| Mixture of cement kiln dust, zeolite, and bentonite | Lead | 15.5 to 57.8 mg g−1 | [83] |
| Iron oxide-coated cement | As(V) | 505.3 mg/L | [84] |
| Cement kiln dust | Acidic wastewater | 87% | [85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, V.P.; Vaish, R.; Yousef, E.S. A Review on Cement-Based Composites for Removal of Organic/Heavy Metal Contaminants from Water. Catalysts 2022, 12, 1398. https://doi.org/10.3390/catal12111398
Singh VP, Vaish R, Yousef ES. A Review on Cement-Based Composites for Removal of Organic/Heavy Metal Contaminants from Water. Catalysts. 2022; 12(11):1398. https://doi.org/10.3390/catal12111398
Chicago/Turabian StyleSingh, Vishvendra Pratap, Rahul Vaish, and El Sayed Yousef. 2022. "A Review on Cement-Based Composites for Removal of Organic/Heavy Metal Contaminants from Water" Catalysts 12, no. 11: 1398. https://doi.org/10.3390/catal12111398




