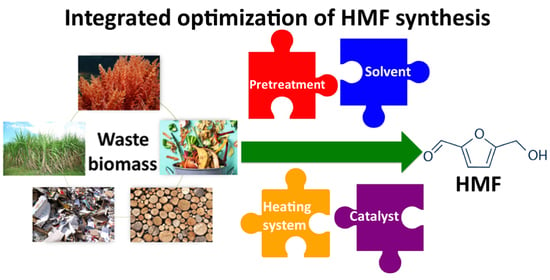
Novel Challenges on the Catalytic Synthesis of 5-Hydroxymethylfurfural (HMF) from Real Feedstocks
Abstract
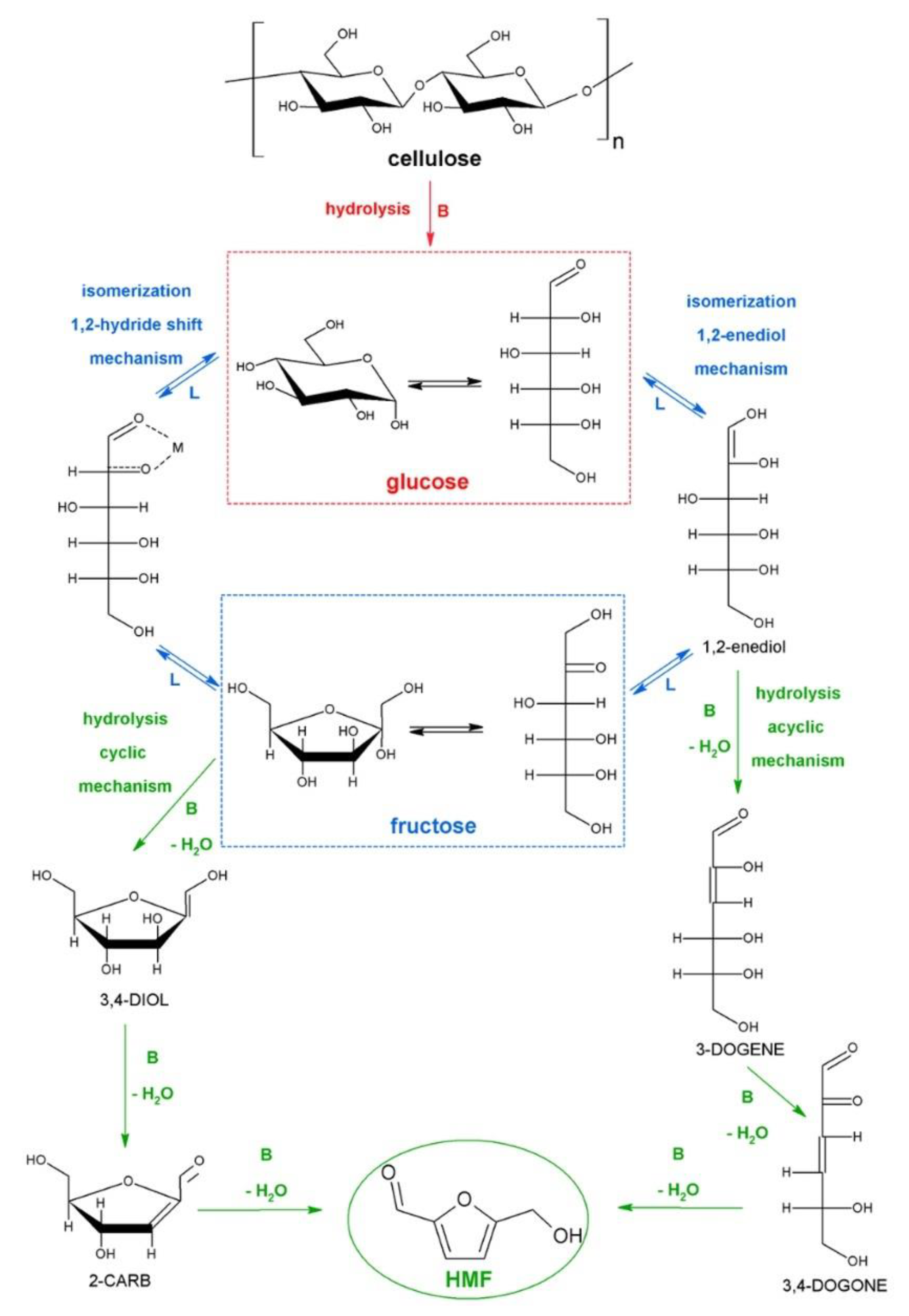
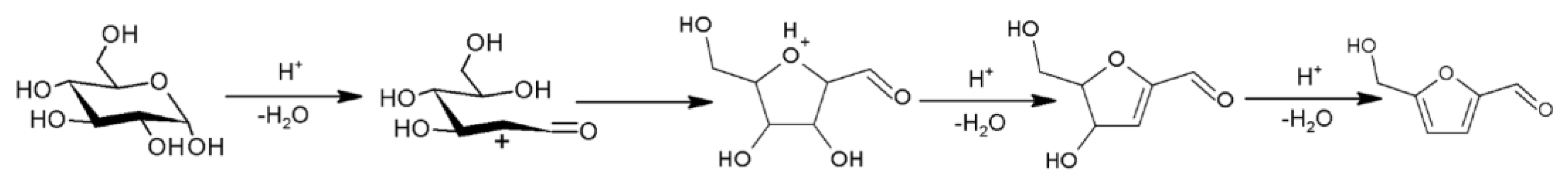
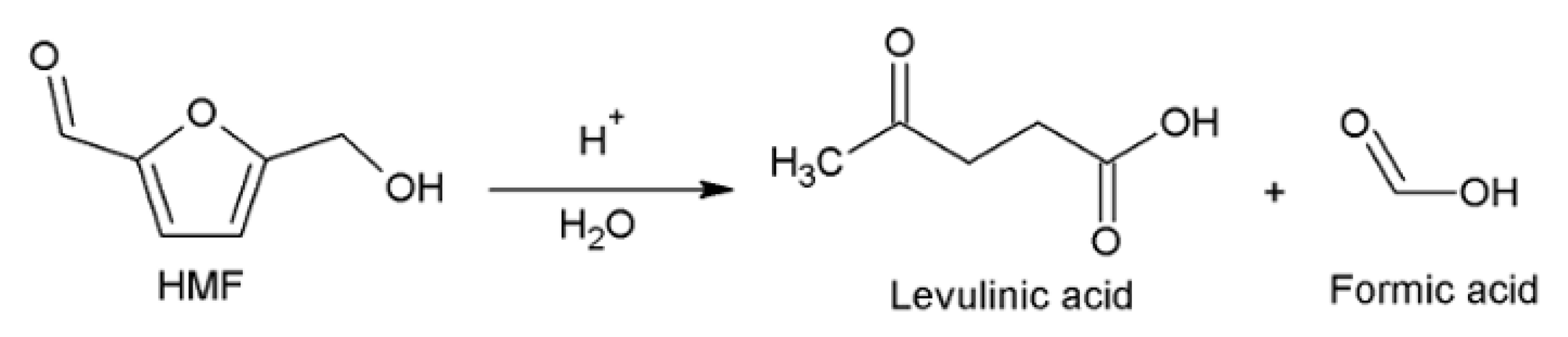
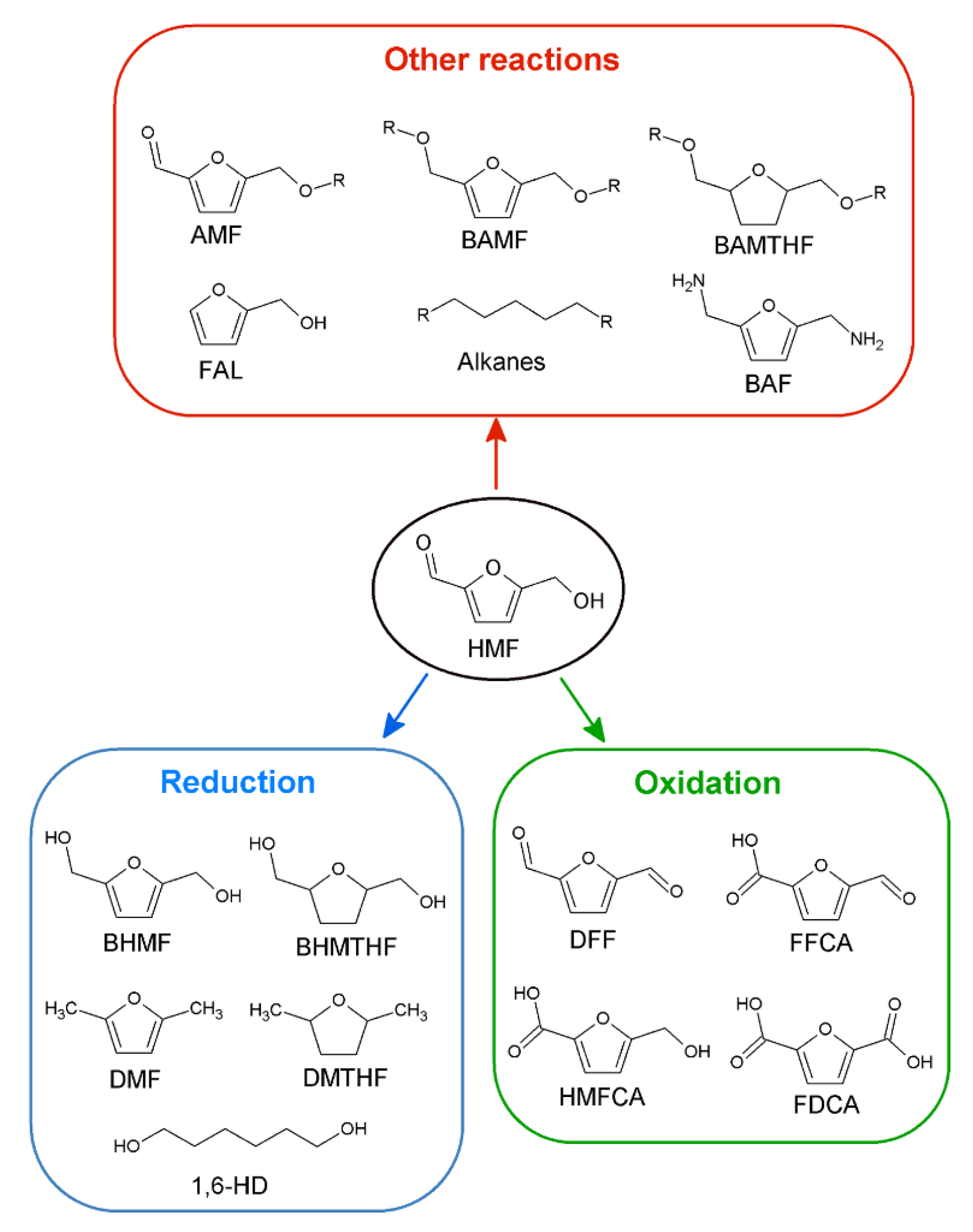
:1. Introduction
2. Synthesis of HMF from Raw Biomass
2.1. Not Pretreated Biomass
2.1.1. One-Solvent Systems
2.1.2. Ionic Liquids (ILs) and Deep Eutectic Solvents (DESs)
2.1.3. Biphasic and/or Multiple-Solvent Systems
| Entry | Feedstock (wt%) | Catalyst (wtbiomass/wtcatalyst) | Solvent | T (°C) | t (min) | Heat 1 | YHMF (mol%) | Refs. |
|---|---|---|---|---|---|---|---|---|
| 63 | Eucalyptus pulp (1.2%) | Fe2(SO4)3 (1.3) | H2O/MIBK (1/5 v/v) | 200 | 60 | Conv. | 32 | [100] |
| 64 | Mixed spruce, pine and fir pulp (1.2%) | Fe2(SO4)3 (1.3) | H2O/MIBK (1/5 v/v) | 200 | 90 | Conv. | 29 | [100] |
| 65 | Eucalyptus pulp (1.2%) | Fe2(SO4)3 (1.3) | H2O/MIBK (1/5 v/v) | 170 | 20 | MW | 27 | [100] |
| 66 | Mixed spruce, pine and fir pulp (1.2%) | Fe2(SO4)3 (1.3) | H2O/MIBK (1/5 v/v) | 170 | 20 | MW | 25 | [100] |
| 67 | Macroalgae Enteromorpha prolifera (2.1%) | FeCl3 (9.9) | H2O-NaCl/THF (1/1 v/v) | 190 | 60 | Conv. | 33 | [101] |
| 68 | Bamboo (4.8%) | NaCl (6.7) | H2O/sulfolane (1/7 v/v) | 200 | 120 | Conv. | 18 | [102] |
| 69 | Bamboo (4.8%) | MgCl2 (6.7) | H2O/sulfolane (1/7 v/v) | 200 | 120 | Conv. | 18 | [102] |
| 70 | Bamboo (4.8%) | CaCl2 (6.7) | H2O/sulfolane (1/7 v/v) | 200 | 120 | Conv. | 20 | [102] |
| 71 | Bamboo (4.8%) | AlCl3 (6.7) | H2O/sulfolane (1/7 v/v) | 200 | 120 | Conv. | 22 | [102] |
| 72 | Bamboo (4.8%) | CrCl3 (6.7) | H2O/sulfolane (1/7 v/v) | 200 | 120 | Conv. | 27 | [102] |
| 73 | Bamboo (4.8%) | ZnCl2 (6.7) | H2O/sulfolane (1/7 v/v) | 200 | 120 | Conv. | 29 | [102] |
| 74 | Bamboo (4.8%) | CuCl2 (6.7) | H2O/sulfolane (1/7 v/v) | 200 | 120 | Conv. | 30 | [102] |
| 75 | Bamboo (4.8%) | FeCl3 (6.7) | H2O/sulfolane (1/7 v/v) | 200 | 120 | Conv. | 36 | [102] |
| 76 | Bamboo (4.8%) | SnCl4 (6.7) | H2O/sulfolane (1/7 v/v) | 200 | 120 | Conv. | 41 | [102] |
| 77 | Bread waste (4.8%) | SnCl4 (3.5) | H2O/DMSO (1/1 v/v) | 140 | 20 | MW | 17 | [103] |
| 78 | Unskinned kiwi fruit (4.8%) | SnCl4 (3.5) | H2O/DMSO (1/1 v/v) | 140 | 20 | MW | 32 | [104] |
| 79 | Watermelon flesh (4.8%) | SnCl4 (3.5) | H2O/DMSO (1/1 v/v) | 140 | 20 | MW | 39 | [104] |
| 80 | Rice waste (4.8%) | SnCl4 (3.5) | H2O/DMSO (1/1 v/v) | 140 | 20 | MW | 13 | [104] |
| 81 | Rice waste (4.8%) | SnCl4 (3.5) | H2O/DMSO (1/1 v/v) | 140 | 40 | MW | 33 | [104] |
| 82 | Rice waste (4.8%) | AlCl3 (6.8) | H2O/DMSO (1/1 v/v) | 140 | 40 | MW | 29 | [104] |
| 83 | Rice waste (4.8%) | CrCl3 (5.7) | H2O/DMSO (1/1 v/v) | 140 | 40 | MW | 23 | [104] |
| 84 | Rice waste (4.8%) | AlCl3 (6.8) | H2O/DMSO (1/1 v/v) | 140 | 100 | MW | 35 | [105] |
| 85 | Rice waste (4.8%) | SnCl4 (3.5) | H2O/DMSO (1/1 v/v) | 140 | 80 | MW | 36 | [106] |
| 86 | Rice waste (4.8%) | SnCl4 (3.5) | H2O/acetone (1/1 v/v) | 140 | 20 | MW | 32 | [106] |
| 87 | Bread waste (4.8%) | SnCl4 (3.5) | H2O/DMSO (1/1 v/v) | 160 | 20 | MW | 38 | [107] |
| 88 | Bread waste (4.8%) | AlCl3 (6.8) | H2O/DMSO (1/1 v/v) | 160 | 20 | MW | 33 | [107] |
| 89 | Bread waste (4.8%) | SnCl4 (3.5) | H2O/DMSO (1/1 v/v) | 140 | 60 | MW | 35 | [108] |
| 90 | Bread waste (4.8%) | SnCl4 (3.5) | H2O/THF (1/1 v/v) | 140 | 120 | MW | 10 | [108] |
| 91 | Bread waste (4.8%) | SnCl4 (3.5) | H2O/CAN 2 (1/1 v/v) | 140 | 10 | MW | 33 | [108] |
| 92 | Bread waste (4.8%) | SnCl4 (3.5) | H2O/acetone (1/1 v/v) | 140 | 10 | MW | 33 | [108] |
| 93 | Bread waste (4.8%) | SnCl4 (3.5) | H2O/acetone (1/1 v/v) | 120 | 50 | MW | 22 | [109] |
| 94 | Bread waste (4.8%) | SnCl4 (3.5) | H2O/GVL (1/1 v/v) | 120 | 40 | MW | 31 | [109] |
| 95 | Bread waste (4.8%) | SnCl4 (3.5) | H2O/PC 3 (1/1 v/v) | 120 | 7 | MW | 26 | [109] |
| 96 | Corn waste (4.8%) | SnCl4 (3.5) | H2O/acetone (1/1 v/v) | 140 | 10 | MW | 27 | [110] |
| 97 | Taro waste (4.8%) | SnCl4 (3.5) | H2O/acetone (1/1 v/v) | 140 | 10 | MW | 30 | [110] |
| 98 | Rice waste (10.0%) | SnCl4 (3.5) | H2O-NADES 4/MIBK (1/25 v/v) | 130 | 120 | Conv. | 61 | [111] |
| 99 | Bread waste (10.0%) | SnCl4 (3.5) | H2O-NADES4/MIBK (1/25 v/v) | 130 | 120 | Conv. | 55 | [111] |
| 100 | Rice waste (4.8%) | AlCl3·6H2O (0.5) | H2O-ChCl/GVL (1/1 v/v) | 140 | 60 | Conv. | 19 | [112] |
| 101 | Molasses (12.8%) | AlCl3 (61.5) | H2O/GVL (1/4 v/v) | 160 | 180 | Conv. | 24 | [113] |
| 102 | Sunn hemp fibres (2.0%) | CuCl2 (6.3) | H2O/[BMIM]Cl 5 (1/4 v/v) | 180 | 46 | MW | 34 | [114] |
| 103 | Junegrass (3.8%) | CuCl2 (10.0) | H2O/[BMIM]Cl 5 (1/3.3 v/v) | 180 | 36 | MW | 31 | [87] |
| 104 | Fir sawdust (3.2%) | CoCl2·6H2O (1.4) | H2O/isopropanol (1/2.3 v/v) | 180 | 180 | Conv. | 19 | [115] |
| 105 | Mixed spruce, pine, and fir pulp (1.2%) | Fe2(SO4)3 (1.3) + HCl (33.3) | H2O/MIBK (1/5 v/v) | 200 | 80 | Conv. | 38 | [100] |
| 106 | Eucalyptus pulp (1.2%) | Fe2(SO4)3 (12.5) + HCl (16.7) | H2O/MIBK (1/5 v/v) | 200 | 30 | Conv. | 29 | [100] |
| 107 | Corn stover (4.8%) | AlCl3 (46.9) + HCl (41.7) | H2O/dioxane (1/4 v/v) | 200 | 5 | MW | 69 | [116] |
| 108 | Loblolly pine (4.8%) | AlCl3 (46.9) + HCl (41.7) | H2O/dioxane (1/4 v/v) | 200 | 5 | MW | 60 | [116] |
| 109 | Switchgrass (4.8%) | AlCl3 (46.9) + HCl (41.7) | H2O/dioxane (1/4 v/v) | 200 | 5 | MW | 65 | [116] |
| 110 | Hybrid poplar (4.8%) | AlCl3 (46.9) + HCl (41.7) | H2O/dioxane (1/4 v/v) | 200 | 5 | MW | 67 | [116] |
| 111 | Rice waste (4.8%) | SnCl4 (3.5) + maleic acid (31.0) | H2O/DMSO (1/1 v/v) | 140 | 40 | MW | 35 | [106] |
| 112 | Rice waste (4.8%) | SnCl4 (3.5) + maleic acid (31.0) | H2O/acetone (1/1 v/v) | 140 | 10 | MW | 30 | [106] |
| 113 | Rice waste (4.8%) | AlCl3 (6.8) + maleic acid (31.0) | H2O/DMSO (1/1 v/v) | 140 | 100 | MW | 17 | [105] |
| 114 | Sugarcane bagasse (9.0%) | AlCl3 (6.7) + oxalic acid dihydrate (1.0) + HCl (17.9) | DMSO/ 2-butanol, MIBK (1/1 v/v) | 130 | 360 | Conv. | 43 | [43] |
| 115 | Raw potato (9.0%) | AlCl3 (6.7) + oxalic acid dihydrate (1.0) + HCl (17.9) | DMSO/ 2-butanol, MIBK (1/1 v/v) | 130 | 360 | Conv. | 40 | [43] |
| Entry | Feedstock (wt%) | Catalyst (wtbiomass/wtcatalyst) | Solvent | T (°C) | t (min) | Heat 1 | YHMF (mol%) | Refs. |
|---|---|---|---|---|---|---|---|---|
| 116 | Vegetable waste (5.0%) | Amberlyst-36 (1.0) | H2O/DMSO (1/1 v/v) | 135 | 5 | MW | 17 | [66] |
| 117 | Salvia miltiorrhiza residue (0.7%) | PDVB-SO3H 2 (2.0) | H2O/GBL (1/4 v/v) | 170 | 30 | Conv. | 14 | [124] |
| 118 | Corn stalk (2.4%) | PTSA-POM 3 (2.0) | H2O/GVL (1/10 v/v) | 190 | 100 | Conv. | 20 | [125] |
| 119 | Microalgae Chlorococcum sp. (1.0%) | HZSM-5 (1.5) | H2O/MIBK (3/2 v/v) | 200 | 120 | Conv. | 44 | [74] |
| 120 | Microalgae Chlorococcum sp. (1.0%) | HZSM-5 (1.5) | H2O-NaCl/THF (3/2 v/v) | 200 | 120 | Conv. | 48 | [74] |
| 121 | Wheat straw (2.7%) | FePO4·2H2O (10.0) | H2O-NaCl/THF (1/3 v/v) | 160 | 150 | Conv. | 18 | [126] |
| 122 | Wheat straw (2.7%) | FePO4·2H2O (5.0) + NaH2PO4 (50.0) | H2O-NaCl/THF (1/3 v/v) | 160 | 60 | Conv. | 44 | [126] |
| 123 | Wheat straw (2.7%) | SnCl2-PTA/β 4 (1.7) | H2O-NaCl/THF (1/3 v/v) | 180 | 120 | Conv. | 33 | [127] |
| 124 | Corn stover (1.3%) | SO3H-NG-C 5 (2.0) | H2O/GVL (1/6.5 v/v) | 190 | 80 | Conv. | 30 | [128] |
| 125 | Bread waste (4.3%) | Sulfonated biochar (1.0) | H2O/DMSO (1/3 v/v) | 180 | 20 | MW | 38 | [129] |
| 126 | Bread waste (4.3%) | H3PO4-activated biochar (2.5) | H2O/DMSO (1/3 v/v) | 180 | 30 | MW | 38 | [130] |
| 127 | Rice waste (4.3%) | H3PO4-activated biochar (2.5) | H2O/DMSO (1/3 v/v) | 180 | 20 | MW | 24 | [130] |
| 128 | Corn stalk (20.0%) | Biochar-Mg-Sn (5.0) | Isopropanol/ AMIMCl 6 (n.a. 7) | 100 | 180 | Conv. | 82 | [91] |
| 129 | Corn stalk (20.0%) | Biochar-Mg-Sn (5.0) | DMSO/AMIMCl 6 (n.a. 7) | 100 | 180 | Conv. | 81 | [91] |
| 130 | Corn stalk (20.0%) | Biochar-Mg-Sn (5.0) | DMC 8/AMIMCl 6 (n.a. 7) | 100 | 180 | Conv. | 76 | [91] |
| 131 | Corn stalk (20.0%) | Biochar-Mg-Sn (5.0) | THF/AMIMCl 6 (n.a. 7) | 100 | 180 | Conv. | 75 | [91] |
| 132 | Corn stalk (20.0%) | Biochar-Mg-Sn (5.0) | MIBK/AMIMCl 6 (n.a. 7) | 100 | 180 | Conv. | 71 | [91] |
| 133 | Peanut shell (3.8) | PSC 9 (0.6) | EMIMCl 10/ ChCl 11-DMSO (1/1 v/v) | 150 | 60 | Conv. | 11 | [131] |
| 134 | Water hyacinth (3.8) | PSC 9 (0.6) | EMIMCl 10/ ChCl 11-DMSO (1/1 v/v) | 150 | 60 | Conv. | 6 | [131] |
| 135 | Stalk (3.8) | PSC 9 (0.6) | EMIMCl 10/ ChCl 11-DMSO (1/1 v/v) | 150 | 60 | Conv. | 15 | [131] |
| Entry | Feedstock (wt%) | Catalyst (wtbiomass/wtcatalyst) | Solvent | T (°C) | t (min) | Heat 1 | YHMF (mol%) | Refs. |
|---|---|---|---|---|---|---|---|---|
| 136 | Corn husk (1.8%) | DES 2 (0.05) | DES 2/ethyl acetate (1/2.3 v/v) | 100 | 120 | Conv. | 13 | [92] |
| 137 | Corn husk (1.8%) | DES 2 (0.05) | DES 2/MIBK (1/2.3 v/v) | 100 | 120 | Conv. | 9 | [92] |
| 138 | Corncob (1.8%) | DES 2 (0.05) | DES 2/MIBK (1/2.3 v/v) | 100 | 120 | Conv. | 11 | [92] |
| 139 | Macroalgae Ulva lactuca (1.8%) | DES 2 (0.05) | DES 2/MIBK (1/2.3 v/v) | 100 | 120 | Conv. | 7 | [92] |
| 140 | Microalgae Porphyridium cruentum (1.8%) | DES 2 (0.05) | DES 2/MIBK (1/2.3 v/v) | 100 | 120 | Conv. | 9 | [92] |
| 141 | Corn stover (2.2%) | ChH2PW12O40 (0.3) | H2O/MIBK (1/10 v/v) | 140 | 600 | Conv. | 28 3 | [132] |
| 142 | Pinewood (2.2%) | ChH2PW12O40 (0.3) | H2O/MIBK (1/10 v/v) | 140 | 600 | Conv. | 12 3 | [132] |
| 143 | Husk of xanthoceras (2.2%) | ChH2PW12O40 (0.3) | H2O/MIBK (1/10 v/v) | 140 | 600 | Conv. | 13 3 | [132] |
| 144 | Corn stover (3.2) | Ch5-AgPW 4 (1.3) | H2O/MIBK (2/3 v/v) | 170 | 180 | Conv. | 26 | [133] |
| 145 | Rice straw (3.2) | Ch5-AgPW 4 (1.3) | H2O/MIBK (2/3 v/v) | 170 | 180 | Conv. | 20 | [133] |
| 146 | Bagasse (3.2) | Ch5-AgPW 4 (1.3) | H2O/MIBK (2/3 v/v) | 170 | 180 | Conv. | 19 | [133] |
2.2. Pretreated Biomass
2.2.1. One-Solvent Systems
2.2.2. Ionic Liquids (ILs) and Deep Eutectic Solvents (DESs)
2.2.3. Biphasic and/or Multiple-Solvent Systems
3. Strategies and Future Perspectives to Wake Up the “Sleeping Giant”
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Agarwal, B.; Kailasam, K.; Sangwan, R.S.; Elumalai, S. Traversing the history of solid catalysts for heterogeneous synthesis of 5-hydroxymethylfurfural from carbohydrate sugars: A review. Renew. Sust. Energ. Rev. 2018, 82, 2408–2425. [Google Scholar] [CrossRef]
- Hou, Q.; Qi, X.; Zhen, M.; Qian, H.; Nie, Y.; Bai, C.; Zhang, S.; Bai, X.; Ju, M. Biorefinery roadmap based on catalytic production and upgrading 5-hydroxymethylfurfural. Green Chem. 2021, 23, 119–231. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, K.; Xu, H.; Zhu, L.; Wang, S. A critical review of recent advances in the production of furfural and 5-hydroxymethylfurfural from lignocellulosic biomass through homogeneous catalytic hydrothermal conversion. Renew. Sust. Energ. Rev. 2021, 139, 110706–110732. [Google Scholar] [CrossRef]
- Huang, Y.B.; Fu, Y. Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem. 2013, 15, 1095–1111. [Google Scholar] [CrossRef]
- Hu, L.; Wu, Z.; Jiang, Y.; Wang, X.; He, A.; Song, J.; Xu, J.; Zhou, S.; Zhao, Y.; Xu, J. Recent advances in catalytic and autocatalytic production of biomass-derived 5-hydroxymethylfurfural. Renew. Sust. Energ. Rev. 2020, 134, 110317–110374. [Google Scholar] [CrossRef]
- Pagán-Torres, Y.J.; Wang, T.; Gallo, J.M.R.; Shanks, B.H.; Dumesic, J.A. Production of 5-hydroxymethylfurfural from glucose using a combination of Lewis and Brønsted acid catalysts in water in a biphasic reactor with an alkylphenol solvent. ACS Catal. 2012, 2, 930–934. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, M.; Xu, H.; Wang, Y.; Yan, K. Recent advance on the catalytic system for efficient production of biomass-derived 5-hydroxymethylfurfural. Renew. Sust. Energ. Rev. 2021, 147, 111253–111271. [Google Scholar] [CrossRef]
- Antal, M.J.; Mok, W.S.; Richards, G.N. Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from D-fructose and sucrose. Carbohydr. Res. 1990, 15, 91–109. [Google Scholar] [CrossRef]
- Jadhav, H.; Pedersen, C.M.; Søllen, T.; Bols, M. 3-Deoxy-glucosone is an intermediate in the formation of furfurals from D-glucose. ChemSusChem 2011, 4, 1049–1051. [Google Scholar] [CrossRef]
- Gallo, J.M.R.; Alonso, D.M.; Mellmer, M.A.; Dumesic, J.A. Production and upgrading of 5-hydroxymethylfurfural using heterogeneous catalysts and biomass-derived solvents. Green Chem. 2013, 15, 85–90. [Google Scholar] [CrossRef]
- Delidovich, I.; Palkovits, R. Catalytic isomerization of biomass-derived aldoses: A review. ChemSusChem 2016, 9, 547–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Román-Leshkov, Y.; Davis, M.E. Activation of carbonyl-containing molecules with solid Lewis acids in aqueous media. ACS Catal. 2011, 11, 1566–1580. [Google Scholar] [CrossRef]
- Akien, G.R.; Qi, L.; Horvát, I.T. Molecular mapping of the acid catalysed dehydration of fructose. Chem. Commun. 2012, 48, 5850–5852. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, C.; Raspolli Galletti, A.M.; Fulignati, S.; Licursi, D. Amberlyst A-70: A surprisingly active catalyst for the MW-assisted dehydration of fructose and inulin to HMF in water. Catal. Commun. 2017, 97, 146–150. [Google Scholar] [CrossRef]
- van Zandvoort, I.; Wang, Y.; Rasrendra, C.B.; van Eck, E.R.H.; Bruijnincx, P.C.A.; Heeres, H.J.; Weckhuysen, B.M. Formation, molecular structure, and morphology of humins in biomass conversion: Influence of feedstock and processing conditions. ChemSusChem 2013, 6, 1745–1758. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y.; Liao, Y.; Wang, H.; Liu, Q.; Ma, L.; Wang, C. Advances in understanding the humins: Formation, prevention and application. Appl. Energ. Comb. Sci. 2022, 10, 100062–100074. [Google Scholar] [CrossRef]
- Antonetti, C.; Fulignati, S.; Licursi, D.; Raspolli Galletti, A.M. Turning point toward the sustainable production of 5-hydroxymnethyl-2-furaldehyde in water: Metal salts for its synthesis from fructose and inulin. ACS Sustainable Chem. Eng. 2019, 7, 6830–6838. [Google Scholar] [CrossRef]
- Karve, V.V.; Schertenleib, T.; Espín, J.; Trukhina, O.; Zhang, X.; Campins, M.X.; Kitao, T.; Avalos, C.E.; Uemura, T.; Queen, W.L. Hybridization of synthetic humins with a metal-organic framework for precious metal recovery and reuse. Appl. Mater. Interfaces 2021, 13, 60027–60034. [Google Scholar] [CrossRef]
- Al Ghatta, A.; Zhou, X.; Casarano, G.; Wilton-Ely, J.D.E.T.; Hallett, J.P. Characterization and valorization of humins produced by HMF degradation in ionic liquids: A valuable carbonaceous material for antimony removal. ACS Sustain. Chem. Eng. 2021, 9, 2212–2223. [Google Scholar] [CrossRef]
- Zhu, J.; Yao, C.; Maity, A.; Xu, J.; Zhan, T.; Liu, W.; Sun, M.; Wang, S.; Polshettiwar, V.; Tan, H. Nitrogen doped carbon spheres with wrinkled cages for selective oxidation of 5-hydroxymethylfurfural to 5-formyl-2-furancarboxylic acid. Chem. Commun. 2021, 57, 2005–2008. [Google Scholar] [CrossRef]
- Zhao, D.; Rodriguez-Padron, D.; Luque, R.; Len, C. Insights into the selective oxidation of 5-hydroxymnethylfurfural to 5-hydroxymethyl-2-furancarboxylic acid using silver oxide. ACS Sustain. Chem. Eng. 2020, 8, 8486–8495. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, J.; Xu, J.; Yang, K.; Zhu, Z.; Su, T.; Ge, H.; Ren, W.; Lü, H. An Anderson-type polyoxometalate triggers aerobic oxidation of 5-hydroxymnethylfyrfural to 2,5-diformylfuran. Fuel 2022, 315, 123226–123234. [Google Scholar] [CrossRef]
- Yang, W.; Tang, X.; Li, W.; Luo, X.; Zhang, C.; Shen, C. Fast and continuous synthesis of 2,5-furandicarboxylic acid in a micropacked-bed reactor. Chem. Eng. J. 2022, 442, 136110–136116. [Google Scholar] [CrossRef]
- Cong, H.; Yuan, H.; Tao, Z.; Bao, H.; Zhang, Z.; Jiang, Y.; Huang, D.; Liu, H.; Wang, T. Recent advances in catalytic conversion of biomass to 2,5-furandicarboxylic acid. Catalysts 2021, 11, 1113. [Google Scholar] [CrossRef]
- Kim, T.; Bamford, J.; Gracida-Alvarez, U.R.; Benavides, P.T. Life cycle greenhouse gas emission and water and fossil-fuel consumptions for polyethylene furanoate and its coproducts from wheat straw. ACS Sustainable Chem. Eng. 2022, 10, 2830–2843. [Google Scholar] [CrossRef]
- Tran, A.V.; Park, S.K.; Lee, H.J.; Kim, T.Y.; Kim, Y.; Suh, Y.W.; Lee, K.Y.; Kim, Y.J.; Baek, J. Efficient production of adipic acid by a two-step catalytic reaction of biomass-derived 2,5-furandicarboxylic acid. ChemSusChem 2022, 15, e202200375. [Google Scholar] [CrossRef]
- Fulignati, S.; Antonetti, C.; Wilbers, E.; Licursi, D.; Heeres, H.J.; Raspolli Galletti, A.M. Tunable HMF hydrogenation to furan diols in a flow reactor using Ru/C as catalyst. J. Ind. Eng. Chem. 2021, 100, 390.e1–390.e9. [Google Scholar] [CrossRef]
- Tang, X.; Wei, J.; Ding, N.; Sun, Y.; Zeng, X.; Hu, L.; Liu, S.; Lei, T.; Lin, L. Chemoselective hydrogenation of biomass derived 5-hydroxymethylfurfural to diols: Key intermediates for sustainable chemicals, materials and fuels. Renew. Sust. Energ. Rev. 2017, 77, 287–296. [Google Scholar] [CrossRef]
- Truong, C.C.; Mishra, D.K.; Ko, S.H.; Kim, Y.J.; Suh, Y.W. Sustainable catalytic transformation of biomass-derived 5-hydroxymethylfurfural to 2,5-bis(hydroxymethyl)tetrahydrofuran. ChemSusChem 2022, 15, e202200178. [Google Scholar] [CrossRef]
- Xiao, B.; Zheng, M.; Li, X.; Pang, J.; Sun, R.; Wang, H.; Pang, X.; Wang, A.; Wang, X.; Zhang, T. Synthesis of 1,6-hexanediol from HMF over double-layered catalysts of Pd/SiO2 + Ir-ReOx/SiO2 in a fixed-bed reactor. Green Chem. 2016, 18, 2175–2184. [Google Scholar] [CrossRef]
- Thaore, V.; Chadwick, D.; Shah, N. Sustainable production of chemical intermediates for nylon manufacture: A techno-economic analysis for renewable production of caprolactone. Chem. Eng. Res. Des. 2018, 135, 140–152. [Google Scholar] [CrossRef]
- Hoang, A.T.; Pandey, A.; Huang, Z.; Luque, R.; Ng, K.H.; Papadopoulos, A.M.; Chen, W.H.; Rajamohan, S.; Hadiyanto, H.; Nguyen, X.P.; et al. Catalyst-based synthesis of 2,5-dimethylfuran from carbohydrates as a sustainable biofuel production route. ACS Sustainable Chem. Eng. 2022, 10, 3079–3115. [Google Scholar] [CrossRef]
- Chen, S.; Ciotonea, C.; De Oliveira Vigier, K.; Jérôme, F.; Wopjcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. Hydroconversion of 5-hydroxymethylfurfural to 2,5-dimethylfuran and 2,5-dimethyltetrahydrofuran over non-promoted Ni/SBA-15. ChemCatChem 2020, 12, 2050–2059. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Wang, T.; Cao, X.; Liu, H.; Tang, X.; Sun, Y.; Zeng, X.; Lei, T.; Liu, S.; Lin, L. A flexible Cu-based catalyst system for the transformation of fructiose to furanyl ethers as potential bio-fuels. Appl. Catal. B Environ. 2019, 258, 117793–117803. [Google Scholar] [CrossRef]
- Hafizi, H.; Walker, G.; Collins, M.N. Efficent production of 5-ethoxymethylfurfural from 5-hydroxymethylfurfural and carbohydrates over Lewis/Brønsted hybrid magnetic dendritic fibrous silica core-shell catalyst. Renew. Energy 2022, 183, 459–471. [Google Scholar] [CrossRef]
- Fulignati, S.; Antonetti, C.; Tabanelli, T.; Cavani, F.; Raspolli Galletti, A.M. Integrated cascade process for the catalytic conversion of 5-hydroxymethylfurfural (HMF) to furanic and tetrahydrofuranic diethers as potential bio-fuels. ChemSusChem 2022, 15. [Google Scholar] [CrossRef]
- Hu, L.; Jiang, Y.; Wang, X.; He, A.; Xu, J.; Wu, Z. Recent advances and mechanistic insights on the production of biomass-derived 2,5-bis(alkoxymethyl)furans. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- De, S. Production of long-chain hydrocarbon biofuels from biomass-derived platform chemicals: Catalytic approaches and challenges. In Hydrocarbon Biorefinery: Sustainable Processing of Biomass for Hydrocarbon Biofuels; Maity, S.K., Gayen, K., Bhowmick, T.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 327–354. [Google Scholar] [CrossRef]
- An, N.; Ainembabazi, D.; Reid, C.; Samudrala, K.; Wilson, K.; Lee, A.F.; Voutchkova-Kostal, A. Microwave-assisted decarbonylation of biomass-derived aldehydes using Pd-doped hydrotalcites. ChemSusChem 2020, 13, 312–320. [Google Scholar] [CrossRef]
- Wei, Z.; Cheng, Y.; Zhou, K.; Zeng, Y.; Yao, E.; Li, Q.; Liu, Y.; Sun, Y. One-step reductive amination of 5.hydroxymethylfurfural into 2,5-bis(aminomethyl)furan over Raney Ni. ChemSusChem 2021, 14, 2308–2312. [Google Scholar] [CrossRef]
- Kholi, K.; Prajapati, R.; Sharma, B.K. Bio-based chemicals from renewable biomass for integrated biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.B.; Lee, Y.S.; Chung, C.H. Raw plant-based biorefinery: A new paradigm shift towards biotechnological approach to sustainable manufacturing of HMF. Biotechnol. Adv. 2019, 37, 107422–107441. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chauhan, A.S.; Das, P. Lignocellulosic biomass and carbohydrates as feed-stock for scalable production of 5-hydroxymethylfurfural. Cellulose 2021, 28, 3967–3980. [Google Scholar] [CrossRef]
- Vyskocil, J.; Kruse, A. (AVA-CO2, Karlsruhe Institute of Technology), Verfahren zur Extraction von Furfuralen aus Biomasse. DE102011053034A1, 2013. [Google Scholar]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sust. Energ. Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Vyskocil, J.; Badoux, F.; Krawielitzki, S. A Process for the Enrichment and Purification of the Aldehyde and Keto Groups Compounds. DE102015121190A1, 2015. [Google Scholar]
- Rosenfeld, C.; Konnerth, J.; Sailer-Konlachner, W.; Solt, P.; Rosenau, T.; van Herwijnen, H.W.G. Current situation of the challenging scale-up development of hydroxymethylfurfural production. ChemSusChem 2020, 13, 3544–3564. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, X.; Hu, W.; Lu, L.; Chen, J.; Zhu, Y.; Zhou, H.; Si, C. Recent advances on solid acid catalytic systems for production of 5-hydroxymethylfurfural from biomass derivatives. Fuel Process. Technol. 2022, 234, 107338–107356. [Google Scholar] [CrossRef]
- Le, H.S.; Said, Z.; Pham, M.T.; Le, T.H.; Veza, I.; Nguyen, V.N.; Deepanraj, B.; Nguyen, L.H. Production of HMF and DMF biofuel from carbohydrates through catalytic pathways as a sustainable strategy for the future energy sector. Fuel 2022, 324, 124474–124498. [Google Scholar] [CrossRef]
- Xiong, S.; Guan, Y.; Luo, C.; Zhu, L.; Wang, S. Critical review on the preparation of platform compounds from biomass or saccharides via hydrothermal conversion over carbon-based solid acid catalysts. Energy Fuels 2021, 35, 14462–14483. [Google Scholar] [CrossRef]
- Tempelman, C.H.L.; Oozeerally, R.; Degirmenci, V. Heterogeneous catalysts for the conversion of glucose into 5-hydroxymethylfurfural. Catalysts 2021, 11, 861. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, Y.; Qin, H.; Qi, Z. Advances of ionic liquids and deep eutectic solvents in green processes of biomass-derived 5-hydroxymethylfurfural. ChemSusChem 2022, 15, e202102635. [Google Scholar] [CrossRef]
- Menegazzo, F.; Ghedini, E.; Signoretto, M. 5-Hydroxymethylfurfural (HMF) production from real biomasses. Molecules 2018, 23, 2201. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xu, R.; Yang, J.; Nie, S.; Liu, D.; Liu, Y.; Si, C. Production of 5-hydroxymethylfurfural and levulinic acid from lignocellulosic biomass and catalytic upgrading. Ind. Crop. Prod. 2019, 130, 184–197. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Zhang, Y.; Naebe, M. Lignin: A review on structure, properties, and applications as a light-colored UV adsorber. ACS Sustain. Chem. Eng. 2021, 9, 1427–1442. [Google Scholar] [CrossRef]
- Meng, Y.; Zhou, Y.; Shao, Y.; Zhou, D.; Shen, D.; Long, Y. Evaluating the potential of the microwave hydrothermal method for valorizing food waste by producing 5-hydroxymethylfurfural. Fuel 2021, 306, 121769. [Google Scholar] [CrossRef]
- Abdilla-Santes, R.M.; Winkelman, J.G.M.; van Zandvoort, I.; Weckhuyden, B.M.; Bruijnincx, P.C.A.; Jurak, E.; Deuss, P.J.; Heeres, H.J. 5-Hydroxy-2-methylfurfural from sugar beet thick juice: Kinetic and modeling studies. ACS Sustain. Chem. Eng. 2021, 9, 2626–2638. [Google Scholar] [CrossRef]
- Muñoz-Valencia, R.; Portillo-Pérez, G.; Ceballos-Magaña, S.G.; Cortés-Quintero, G.C.; Nava-García, A.Y.; Dumont, M.-J.; Pineda-Urbina, K. Utilization of mango wastes as a potential feedstock for the production of HMF. Biomass Conv. Bioref. 2020. [Google Scholar] [CrossRef]
- Świątek, K.; Gaag, S.; Klier, A.; Kruse, A.; Sauer, J.; Steinbach, D. Acid hydrolysis of lignocellulosic biomass: Sugars and furfurals formation. Catalysts 2020, 10, 437. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Yu, I.K.M.; Tsang, D.C.W.; Su, Z.; Hu, C.; Wu, K.C.W.; Yip, A.C.K.; Ok, Y.S.; Poon, C.S. Influence of green solvent on levulinic acid production from lignocellulosic paper waste. Bioresour. Technol. 2020, 298, 122544–122551. [Google Scholar] [CrossRef]
- Jeong, G.T.; Ra, C.H.; Hong, Y.K.; Kim, J.K.; Kong, I.S.; Kim, S.K.; Park, D.H. Conversione of red-algae Gracilaria verrucosa to sugars, levulinic acid and 5-hydroxymethylfurfural. Bioprocess Biosyst. Eng. 2015, 38, 207–217. [Google Scholar] [CrossRef]
- Hoşgün, E.Z. One-pot hydrothermal conversion of poppy stalks over metal chloride catalysts. Biomass Conv. Bioref. 2021, 11, 2703–2710. [Google Scholar] [CrossRef]
- Jeong, G.T.; Kim, S.K. Hydrothermal conversion of microalgae Chlorella sp. into 5-hydroxymethylfurfural and levulinic aid by metal sulfate catalyst. Biomas. Bioenerg. 2021, 148, 106053–106061. [Google Scholar] [CrossRef]
- Bodachivskyi, I.; Kuzhiumparambil, U.; Williams, D.B.G. The role of the molecular formula of ZnCl2· nH2O on its catalyst activity: A sistematic study of zinc chloride hydrates in the catalytic valorisation of cellulosic biomass. Catal. Sci. Technol. 2019, 9, 4693–4701. [Google Scholar] [CrossRef]
- Chen, S.S.; Yu, I.K.M.; Tsang, D.C.W.; Yip, A.C.K.; Khan, E.; Wang, L.; Ok, Y.S.; Poon, C.S. Valorization of cellulosic food waste into levulinic acid catalyzed by heterogeneous Brønsted acids: Temperature and solvent effects. Chem. Eng. J. 2017, 327, 328–335. [Google Scholar] [CrossRef]
- Jeong, G.T.; Kim, S.K.; Park, D.H. Application of solid-acid catalyst and marine macro-algae Gracilaria verrucosa to production of fermentable sugars. Bioresour. Technol. 2015, 181, 1–6. [Google Scholar] [CrossRef]
- Li, M.; Jiang, H.; Zhang, L.; Yu, X.; Liu, H.; Yagoub, A.E.A.; Zhou, C. Synthesis of 5–HMF from an ultrasound-ionic liquid pretreated sugarcane bagasse by using a microwave-solid acid/ionic liquid system. Ind. Crop. Prod. 2020, 149, 112361–112369. [Google Scholar] [CrossRef]
- Ozsel, B.K.; Ozturk, D.; Nis, B. One-pot hydrothermal conversion of different residues to value-added chemicals using new acidic carbonaceous catalyst. Bioresour. Technol. 2019, 289, 121627–121632. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Mondal, J.; Bhaumik, A. Sulfonated porous polymeric nanofibers as an efficient solid acid catalyst for the production of 5-hydroxymethylfurfural from biomass. ChemCatChem 2015, 7, 3570–3578. [Google Scholar] [CrossRef]
- Zhang, L.; Xi, G.; Zhang, J.; Yu, H.; Wang, X. Efficient catalytic system for the direct transformation of lignocellulosic biomass to furfural and 5-hydroxymethylfurfural. Bioresour. Technol. 2017, 224, 656–661. [Google Scholar] [CrossRef]
- Mimura, N.; Sato, O.; Shirai, M.; Yamaguchi, A. 5-hydroxymethylfurfural production from glucose, fructose, cellulose, or cellulose-based waste material by using a calcium phosphate catalyst and water as a green solvent. ChemistrySelect 2017, 2, 1305–1310. [Google Scholar] [CrossRef]
- Mo, H.; Chen, X.; Liao, X.; Zhou, T. Sustainable synthesis of 5-hydroxymethylfurfural from waste cotton stalk catalyzed by solid superacid- SO42–/ZrO2. J. Cent. South Univ. 2017, 24, 1745–1753. [Google Scholar] [CrossRef]
- Wang, J.J.; Tan, Z.C.; Zhu, C.C.; Miao, G.; Kong, L.Z.; Sun, Y.H. One-pot catalytic conversion of microalgae (Chlorococcum sp.) into 5-hydroxymethylfurfural over the commercial H-ZSM-5 zeolite. Green Chem. 2016, 18, 452–460. [Google Scholar] [CrossRef]
- Alam, M.I.; De, S.; Khan, T.S.; Haider, M.A.; Saha, B. Acid functionalized ionic liquid catalyzed transformation of non-food biomass into platform chemical and fuel additive. Ind. Crop. Prod. 2018, 123, 629–637. [Google Scholar] [CrossRef]
- Al-Horani, R.A.; Desai, U.R. Chemical sulfation of small molecules-advances and challenges. Tetrahedron 2010, 66, 2907–2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Ren, J.; Li, H.; Deng, A.; Sun, R. Direct transfomration of xylan-type hemicellulose to furfural via SnCl4 catalysts in aqueous and biphasic systems. Bioresour. Technol. 2015, 183, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Martell, A.E.; Hancock, R.D. Metal Complexes in Aqueous Solutions; Plenum Press: New York, NY, USA, 1996. [Google Scholar]
- Teong, S.P.; Yi, G.; Zeng, H.; Zhang, Y. The effects of emulsion on sugar dehydration to 5-hydroxymethylfurfural in a biphasic system. Green Chem. 2015, 17, 3751–3755. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Nawaz, H.; Arêas, E.P.G. Chemistry and applications of polysaccharide solutions in strong electrolytes/dipolar aprotic solvents: An overview. Molecules 2013, 18, 1270–1313. [Google Scholar] [CrossRef] [Green Version]
- Amini, E.; Valls, C.; Roncero, M.B. Ionic liquid-assisted bioconversion of lignocellulosic biomass for the development of value-added products. J. Clean. Prod. 2021, 326, 129275–129298. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spitle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low-transition-temperature mixtures (LTTMs): A new generation of designer solvents. Angew. Chem. Int. Ed. 2013, 52, 3074–3085. [Google Scholar] [CrossRef]
- Nguyen, C.V.; Lewis, D.; Chen, W.-H.; Huang, H.-W.; Alothman, Z.A.; Yamuauchi, Y.; Wu, K.C.-W. Combined tretaments for producing 5-hydroxymethylfurfural (HMF) from lignocellulosic biomass. Catal. Today 2016, 278, 344–349. [Google Scholar] [CrossRef]
- Naz, S.; Uroos, M.; Muhammad, N. Effect of molecular structure of cation and anions of ionic liquids and co-solvents on selectivity of 5-hydroxymethylfurfural from sugars, cellulose and real biomass. J. Mol. Liq. 2021, 334, 116523–116531. [Google Scholar] [CrossRef]
- Naz, S.; Uroos, M.; Muhammad, N. One-pot production of 5-hydroxymethtylfurfural and simultaneous lignin recovery from non-food lignocellulsoic wastes using cost-effective ionic liquids. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Roy, S.; Chakraborty, S. Comparative study of the effectiveness of protic and aprotic ionic liquids in microwave-irradiated catalytic conversion of lignocellulosic June grass to biofuel precursors. Bioresour. Technol. Rep. 2019, 8, 100338–100346. [Google Scholar] [CrossRef]
- Yu, Q.; Song, Z.; Zhuang, X.; Liu, L.; Qiu, W.; Shi, J.; Wang, W.; Li, Y.; Wang, Z.; Yuan, Z. Catalytic conversion of herbal residue carbohydrates to furanic derivatives in a deep eutectic solvent accompanied by dissolution and recrystallisation of choline chloride. Cellulose 2019, 26, 8263–8277. [Google Scholar] [CrossRef]
- Tyagi, U.; Anand, N. Conversion of Babool wood residue to 5-hydroxymethylfurfural: Kinetics and process modeling. Bioresour. Technol. Rep. 2021, 14, 100674–100684. [Google Scholar] [CrossRef]
- Yan, L.; Liu, N.; Wang, Y.; Machida, H.; Qi, X. Production of 5-hydroxymethylfurfural from corn stalk catalyzed by corn stalk-derived carbonaceous solid acid catalyst. Bioresour. Technol. 2014, 173, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, X.; Hou, Q.; Zhang, S.; Ju, M. Corn stalk conversion into 5-hydroxymethylfurfural by modified biochar catalysis in a multi-functional solvent. J. Clean. Prod. 2018, 187, 380–389. [Google Scholar] [CrossRef]
- Bodachivskyi, I.; Kuzhiumparambil, U.; Williams, D.B.G. Catalytic valorization of native biomass in a deep eutectic solvent: A systematic approach toward high-yielding reactions of polysaccharides. ACS Sustainable Chem. Eng. 2020, 8, 678–685. [Google Scholar] [CrossRef]
- Abdilla-Santes, R.M.; Guo, W.; Bruijnincx, P.C.A.; Yue, J.; Deus, P.J.; Heeres, H.J. High-Yield 5-Hydroxymethylfurfural Synthesis from Crude Sugar Beet Juice in a Biphasic Microreactor. ChemSusChem 2019, 12, 4304–4312. [Google Scholar] [CrossRef]
- Amoah, J.; Hasunuma, T.; Ogino, C.; Kondo, A. 5-Hydroxymethylfurfural production from salt-induced photoautotrophically cultivated Chlorella sorokiniana. Biochem. Eng. J. 2019, 142, 117–123. [Google Scholar] [CrossRef]
- Sweygers, N.; Harrer, J.; Dewil, R.; Appels, L. A microwave-assisted process for the in-situ production of 5-hydroxymethylfurfurak and furfural from lignocellulosic polysaccharides in a biphasic reaction system. J. Clean. Prod. 2018, 187, 1014–1024. [Google Scholar] [CrossRef]
- Sweygers, N.; Depuydt, D.E.C.; Van Vuure, A.W.; Degrève, J.; Potters, G.; Dewil, R.; Appels, L. Simultaneous production of 5-hydroxymethylfurfural and furfural from bamboo (Phyllostachys nigra “Boryana”) in a biphasic reaction system. Chem. Eng. J. 2020, 386, 123957–123966. [Google Scholar] [CrossRef]
- Zhou, Y.; Lei, M.; Chen, Y.; Hu, C. Conversion of polysaccharides in Ulva prolifera to valuable chemicals in the presence of formic acid. J. Appl. Phycol. 2021, 33, 101–110. [Google Scholar] [CrossRef]
- Seemala, B.; Haritos, V.; Tanksale, A. Levulinic acid as a catlayst for the production of 5-hydroxymethylfurfural and furfural from lignocellulose biomass. ChemCatChem 2016, 8, 640–647. [Google Scholar] [CrossRef]
- Mirzaei, H.M.; Karimi, B. Sulphanilic acid as a recyclable bifunctional organocatalyst in the selective conversion of lignocellulosic biomass to 5-HMF. Green Chem. 2016, 18, 2282–2286. [Google Scholar] [CrossRef]
- Mukherjee, A.; Portillo-Perez, G.; Dumont, M.-J. Synthesis of hydroxymethylfurfural and furfural from hardwood and softwood pulp using ferric sulphate as catalyst. Front. Chem. Sci. Eng. 2019, 13, 531–542. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Zhang, R.; Hu, C. Conversion of saccharides in enteromorpha prolifera to furfurals in the presence of FeCl3. Mol. Catal. 2020, 484, 110729–110735. [Google Scholar] [CrossRef]
- Liu, C.; Wei, M.; Wang, F.; Wei, L.; Yin, X.; Jiang, J.; Wang, K. Effective and facile conversion of bamboo into platform chemicals over SnCl4 in a sulfolane/water solution. J. Energy Inst. 2020, 93, 1642–1650. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W.; Yip, A.C.K.; Chen, S.S.; Ok, Y.S.; Poon, C.S. Valorization of food waste into hydroxymethylfurfural: Dual role of metal ions in successive conversion steps. Bioresour. Technol. 2016, 219, 338–347. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W.; Yip, A.C.K.; Chen, S.S.; Ok, Y.S.; Poon, C.S. Valorization of starchy, cellulosic, and sugary food waste into hydroxymethylfurfural by one-pot catalysis. Chemosphere 2017, 184, 1099–1107. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W.; Yip, A.C.K.; Su, Z.; De Oliveira Vigier, K.; Jérôme, F.; Poon, C.S.; Ok, Y.S. Organic acid-regulated Lewis acidity for selective catalytic hydroxymethylfurfural production from rice waste: An experimental-computational study. ACS Sustainable Chem. Eng. 2019, 7, 1437–1446. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W.; Su, Z.; Yip, A.C.K.; Shang, J.; Ok, Y.S.; Kim, H.-H.; Poon, C.S. Contrasting roles of maleic acid in controlling kinetics and selectivity of Sn(IV)-catalyzed hydroxymethylfurfural synthesis. ACS Sustainable Chem. Eng. 2018, 6, 14262–14274. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W.; Yip, A.C.K.; Chen, S.S.; Wang, L.; Ok, Y.S.; Poon, C.S. Catalytic valorization of starch-rich food waste into hydroxymethylfurfural (HMF): Controlling relative kinetics for high productivity. Bioresour. Technol. 2017, 237, 222–230. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W.; Chen, S.S.; Wang, L.; Hunt, A.J.; Sherwood, J.; De Oliveira Vigier, K.; Jérôme, F.; Ok, Y.S.; Poon, C.S. Polar aprotic solvent-water mixture as the medium for the catalytic production of hydroxymethylfurfural (HMF) from bread waste. Bioresour. Technol. 2017, 245, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Yu, I.K.M.; Tsang, D.C.W.; Yip, A.C.K.; Hunt, A.J.; Sherwood, J.; Shang, J.; Song, H.; Ok, Y.S.; Poon, C.S. Propylene carbonate and γ-valerolactone as green solvents enhance Sn(IV)-catalysed hydroxymethylfurfural (HMF) production from bread waste. Green Chem. 2018, 20, 2064–2074. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Attard, T.M.; Chen, S.S.; Tsang, D.C.W.; Hunt, A.J.; Jérôme, F.; Ok, Y.S.; Poon, C.S. Supercritical carbon dioxide extraction of value-added products and thermochemical synthesis of platform chemicals from food waste. ACS Sustainable Chem. Eng. 2019, 7, 2821–2829. [Google Scholar] [CrossRef]
- Zuo, M.; Wang, X.; Wang, Q.; Zeng, X.; Lin, L. Aqueous-natural deep eutectic solvent-enhanced 5-hydroxymethylfurfural production from glucose, starch, and food wastes. ChemSusChem 2021, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Yu, X.; Chen, L.; Yagoub, A.E.A.; Zhou, C. Aqueous choline chloride/γ-valerolactone as ternary green solvent enhance Al(III)-catalyzed hydroxymethylfurfural production from rice waste. Energy Technol. 2020, 8, 2000597–2000608. [Google Scholar] [CrossRef]
- Tian, X.; Qi, B.; Zhang, S.; Luo, J.; Wan, Y. Catalytic production of 5-hydroxymethylfurfural from sucrose and molasses by aluminium chloride in green aqueous γ-valerolactone system. Biomass Convers. Biorefin. 2021, 11, 1931–1941. [Google Scholar] [CrossRef]
- Paul, S.K.; Chakraborty, S. Microwave-assisted ionic liquid-mediated rapid catalytic conversion of non-edible lignocellulosic Sunn hemp fibres to biofuels. Bioresour. Technol. 2018, 253, 85–93. [Google Scholar] [CrossRef]
- Pan, X.; Mei, S.; Huang, G.-X.; Ji, X.; Liu, W.-J.; Yu, H.-Q. Efficient conversion of the lignocellulosic biomass waste into 5-hydroxymethylfurfural-enriched bio-oil and Co nanoparticle-functionalized biochar. ACS EST Engg. 2021, 5, 895–904. [Google Scholar] [CrossRef]
- Mittal, A.; Pilath, H.M.; Johnson, D.K. Direct conversion of biomass carbohydrates to platform chemicals: 5-hydroxymethylfurfural (HMF) and furfural. Energy Fuels 2020, 34, 3284–3293. [Google Scholar] [CrossRef]
- Fan, J.; De Bruyn, M.; Budarin, V.L.; Gronnow, M.J.; Shuttleworth, P.S.; Breeden, S.; Macquarrie, D.J.; Clark, J.H. Direct microwave-assisted hydrothermal depolymerization of cellulose. J. Am. Chem. Soc. 2013, 135, 11728–11731. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.K.R.; Heltzel, J.; Lund, C.R.F. Comparison of structural features of humins formed catalytically from glucose, fructose, and 5-hydroxymethylfuraldehyde. Energy Fuels 2012, 36, 5281–5293. [Google Scholar] [CrossRef]
- Vasudevan, V.; Mushrif, S.H. Insights into the solvation of glucose in water, dimethyl sulfoxide (DMS), tetrahydrofuran (THF) and N,N-dimethylformamide (DMF) and its possible implicatiojn on the conversion of glucose to platform chemicals. RSC Adv. 2015, 5, 20756–20763. [Google Scholar] [CrossRef]
- Mushrif, S.H.; Caratzoulas, S.; Vlachos, D.G. Understanding solvent effects in the selective conversion of fructose to 5-hydroxymethyl-furfural: A molecular dynamics investigation. Phys. Chem. Chem. Phys. 2012, 14, 2637–2644. [Google Scholar] [CrossRef]
- Czompa, A.; Pásztor, B.L.; Sahar, J.A.; Mucsi, Z.; Bogdán, D.; Ludányi, K.; Varga, Z.; Mándity, I.M. Scope and limitation of propylene carbonate as a sustainable solvent in he Suzuk-Miyura reaction. RSC Adv. 2019, 9, 37818–37824. [Google Scholar] [CrossRef]
- Biancalana, L.; Fulignati, S.; Antonetti, C.; Zacchini, S.; Provinciali, G.; Pampaloni, G.; Raspolli Galletti, A.M.; Marchetti, F. Ruthernium p-cymene complexes with α-diimine ligands as catalytic precursore for the transfer hydrogenation of ethyl levulinate to γ-valerolactone. New J. Chem. 2018, 42, 17574–17586. [Google Scholar] [CrossRef]
- Kerkel, F.; Markiewicz, M.; Stolte, S.; Müller, E.; Kunz, W. The green platform molecule gamma-valerolactone – ecotoxicity, biodegradability, solvent properties, and potential applications. Green Chem. 2021, 23, 2962–2976. [Google Scholar] [CrossRef]
- Zhang, L.; He, Y.; Zhu, Y.; Liu, Y.; Wang, X. Camelia oleifera shell as an alternative feedstock for furfural production using a high surface acidity solid acid catalyst. Bioresour. Technol. 2018, 249, 536–541. [Google Scholar] [CrossRef]
- Xu, Z.; Li, W.; Du, Z.; Wu, H.; Jameel, H.; Chang, H.; Ma, L. Conversion of corn stalk furfural using a novel heterogeneous strong acid catalyst in γ-valerolactone. Bioresour. Technol. 2015, 198, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Xu, S.; Yang, L. Efficient conversion of wheat straw into furan compounds, bio-oils, and phosphate fertilizers by a combination of hydrolysis and catalytic pyrolysis. RSC Adv. 2017, 7, 1200–1205. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Pan, D.; Wu, Y.; Xu, N.; Yang, H.; Gao, L.; Li, W.; Xiao, G. Direct conversion of wheat straw components into furan compounds using a highlt efficient and reusable SnCl2-PTA/β zeolite catalyst. Ind. Eng. Chem. Res. 2019, 58, 9276–9285. [Google Scholar] [CrossRef]
- Yang, T.; Li, W.; Ogunbiyi, A.T.; An, S. Efficient catalytic conversion of corn stover to furfural and 5-hydroxymethylfurfural using glucosamine hydrochloride derived carbon solid acid in γ-valerolactone. Ind. Crop. Prod. 2021, 161, 113173. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Chen, S.S.; Tsang, D.C.W.; Wang, L.; Xiong, X.; Zhang, S.; Ok, Y.S.; Kwon, E.E.; Song, H.; et al. Production of 5-hydroxymethylfurfural from starch-rich food waste catalyzed by sulfonated biochar. Bioresour. Technol. 2018, 252, 76–82. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Tsang, D.C.W.; Zhang, S.; Ok, Y.S.; Kwon, E.E.; Song, H.; Poon, C.S. Phosphoric acid-activated wood biochar for catalytic conversion of starch-rich food waste into glucose and 5-hydroxymethylfurfural. Bioresour. Technol. 2018, 267, 242–248. [Google Scholar] [CrossRef]
- Chang, K.L.; Muega, S.C.; Ofrasio, B.I.G.; Chen, W.H.; Barte, E.G.; Abarca, R.R.M.; De Luna, M.D.G. Synthesis of 5-hydroxymethylfurfural from glucose, fructose, cellulose and agricultural wastes over sulfur-doped peanut shell catalysts in ionic liquid. Chemosphere 2022, 291, 132829–132836. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Sun, Z.; Xue, L.; Wang, X.; Jiang, Z. Highly efficient preparation of HMF from cellulose using temperature-responsive heteropolyacid catalysts in cascade reaction. Appl. Catal. B Environ. 2016, 196, 50–56. [Google Scholar] [CrossRef]
- Lai, F.; Yan, F.; Wang, P.; Li, C.; Shen, X.; Zhang, Z. Efficient conversion of carbohydrates and biomass into furan compounds by chitin/Ag co-modified H3PW12O40. J. Clean. Prod. 2021, 316, 128243–128254. [Google Scholar] [CrossRef]
- Raspolli Galletti, A.M.; Antonetti, C.; Fulignati, S.; Licursi, D. Direct alcoholysis of carbohydrate precursors and real cellulosic biomasses to alkyl levulinates: A critical review. Catalysts 2020, 10, 1221. [Google Scholar] [CrossRef]
- Sitotaw, Y.W.; Habtu, N.G.; Gebreyohannes, A.Y.; Nunes, S.P.; Gerven, T.V. Ball milling as an important pretreatment technique in lignocellulose biorefineries: A review. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Flores, E.M.M.; Cravotto, G.; Bizzi, C.A.; Santos, D.; Iop, G.D. Ultrasound-assisted biomass valorization to industrial interesting products: State-of-the-art, perspective and challenges. Ultrason. Sonochem. 2021, 72, 105455–105466. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Ong, H.C.; Mofijur, M.; Ahmed, S.F.; Ashok, B.; Bui, V.T.V.; Chau, M.Q. Insight into the recent advances of microwave pretreatment technologies for the conversion of lignocellulosic biomass into sustainable biofuel. Chemosphere 2021, 281, 130878–130899. [Google Scholar] [CrossRef]
- Ziegler-Devin, I.; Chrusciel, L.; Brosse, N. Steam explosion pretreatment of lignocellulosic biomass: A mini-review of theoretical and experimental approaches. Front. Chem. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Martín, C.; Dixit, P.; Momayez, F.; Jönsson, L.J. Hydrothermal pretreatment of lignocellulosic feedstocks to faciliotate biochemicakl conversion. Front. Bioeng. Biotechnol. 2022, 10. [Google Scholar] [CrossRef]
- Kundu, C.; Samudrala, S.P.; Kibria, M.A.; Bhattacharya, S. One-step peracetic acid pretreatment of hardwood and softwood biomass for platform chemicals production. Sci. Rep. 2021, 11, 11183. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Sharma, H.K.; Xu, C.; Qin, W. Biological pretreatment of lignocellulosic biomass for biofuels and bioproducts: An overview. Waste Biomass Valori. 2019, 10, 235–251. [Google Scholar] [CrossRef]
- Pińkowska, H.; Krzywonos, M.; Wolak, P.; Seruga, P.; Górniak, A.; Złocińska, A.; Ptak, M. Sustainable production of 5-hydroxymethylfurfuralnfrpm pectin-free sugar beet pulp in a simple aqueous phase system-optimization with Doehlert Design. Energies 2020, 13, 5649. [Google Scholar] [CrossRef]
- Kawamura, K.; Sako, K.; Ogata, T.; Tanabe, K. Environmentally friendly, hydrothermal treatment of mixed fabric wastes containing polyester, cotton, and wool fibers: Application for HMF production. Bioresour. Technol. Rep. 2020, 11, 100478–100486. [Google Scholar] [CrossRef]
- Wei, W.Q.; Wu, S.B. Conversion of eucalyptus cellulose into 5-hydroxymethylfurfural using Lewis acid catalyst in biphasic solvent system. Waste Biomass Valori. 2017, 8, 1303–1311. [Google Scholar] [CrossRef]
- Kholiya, F.; Rathod, M.R.; Gangapur, D.R.; Adimurthy, S.; Meena, R. An integrated effluent free process for the production of 5-hydroxymethylfurfural (HMF), levulinic acid (LA) and KNS-ML from aqueous seaweed extract. Carbohydr. Res. 2020, 490, 107953–107958. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Yang, M.; Shuang, E.; Liu, J.; Zhang, S.; Zhang, X.; Sheng, K.; Zhang, X. Corn stover valorization by one-step formic acid fractionation and formylation for 5-hydroxymethylfurfural and high guaiacyl lignin production. Bioresour. Technol. 2020, 299, 122586–122595. [Google Scholar] [CrossRef] [PubMed]
- Hoang, P.H.; Cuong, T.D. Simultaneous direct production of 5-hydroxymethylfurfural (HMF) and furfural from corncob biomass using porous HSO3-ZSM-5 zeolite catalyst. Energy Fuels 2021, 35, 546–551. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Du, X.; Qu, Y. Selective removal of lignin to enhance the process of preparing fermentable sugars and platform chemicals from lignocellulosic biomass. Bioresour. Technol. 2020, 303, 122846–122852. [Google Scholar] [CrossRef]
- Chen, W.C.; Lin, Y.C.; Chu, I.M.; Wang, L.F.; Tsai, S.L.; Wei, Y.H. Feasibility of enhanceing production of 5-hydroxymethylfurfural using deep eutectic solvents as reaction media in a high-pressure reactor. Biochem. Eng. J. 2020, 154, 107440–107446. [Google Scholar] [CrossRef]
- Ge, Z.; Tian, W.; Zhang, K.; Chen, M.; Qin, S.; Chen, X.; Liu, W. Preparation of cellulose and synthesis of 5-hydroxymethylfurfural from agricultural waste cottonseed hull. Appl. Eng. Agric. 2016, 32, 661–667. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.G.A.; Li, M.; Fakayode, O.A.; Yan, D.; Zhou, C.; Chen, L. Ultrasound-ionic liquid pretreatment enhanced conversion of the sugary food waste to 5-hydroxymethylfurfural in ionic liquid/solid acid catalyst system. Catal. Lett. 2020, 150, 1373–1388. [Google Scholar] [CrossRef]
- Arora, S.; Gupta, N.; Singh, V. Choline based basic ionic liquid (BIL)/acidic DES mediated cellulose rich fractionation of agricultural waste biomass and valorization to 5-HMF. Waste Biomass Valoriz. 2020, 11, 3345–3354. [Google Scholar] [CrossRef]
- Lucas-Torres, C.; Lorente, A.; Cabañas, B.; Moreno, A. Microwave heating for the catalytic conversion of melon rind waste into biofuel precursors. J. Clean. Prod. 2016, 138, 59–69. [Google Scholar] [CrossRef]
- Guo, T.; Li, X.; Liu, X.; Guo, Y.; Wang, Y. Catalytic transformation of lignocellulosic biomass into arenes, 5-hydroxymethylfurfural and furfural. ChemSusChem 2018, 11, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Sun, S.; Zhang, X.; Yang, J.; Qiu, M.; Qi, X. Mechanochemical-assisted production of 5-hydroxymethylfurfural from high concentration of cellulose. Cellulose 2020, 27, 3013–3023. [Google Scholar] [CrossRef]
- Lu, Q.; Yan, D.; Wu, P.; Chen, L.; ElGasim, A.; Yougoub, A.; Ji, Q.; Yu, X.; Zhou, C. Ultrasound-NATDES/DMSO system for corn straw biomass conversion into platform compounds. Renew. Ener. 2022, 190, 675–683. [Google Scholar] [CrossRef]
- Ganado, R.J.J.; Yu, D.E.C.; Franco, F.C. Microwave-assisted conversion of simple sugars and waste coffee grounds into 5-hydroxymethylfurfural in a highly aqueous DMSO solvent system catalyzed by a combination of Al(NO3)3 and H2SO4. Ind. Eng. Chem. Res. 2019, 58, 14621–14631. [Google Scholar] [CrossRef]
- Pereira, A.P.; Woodman, T.J.; Brahmbhatt, P.; Chuck, C.J. The optimized production of 5-(hydroxymethyl)furfural and related products from spent coffee grounds. Appl. Sci. 2019, 9, 3369. [Google Scholar] [CrossRef] [Green Version]
- Ebikade, E.; Athaley, A.; Fisher, B.; Yang, K.; Wu, C.; Ierapetritou, M.G.; Vlachos, D.G. The future is garbage: Repurposing of food waste to an integrated biorefinery. ACS Sustain. Chem. Eng. 2020, 8, 8124–8136. [Google Scholar] [CrossRef]
- Lin, C.; Wu, H.; Wang, J.; Huang, J.; Cao, F.; Zhuang, W.; Lu, Y.; Chen, J.; Jia, H.; Ouyang, P. Preparation of 5-hydroxymethylfurfural from high fructose corn syrup using organic weak acid in situ as catalyst. Ind. Eng. Chem. Res. 2020, 59, 4358–4366. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Ong, K.L.; Tsang, D.C.W.; Haque, M.A.; Kwan, T.H.; Chen, S.S.; Uisan, K.; Kulkarni, S.; Lin, C.S.K. Chemical transformation of food and beverahe waste-derived fructose to hydroxymethylfurfural as a value-added product. Catal. Today 2018, 314, 70–77. [Google Scholar] [CrossRef]
- Tempelman, C.H.L.; Jacobs, J.F.; Ramkhelawan, S.; Mok, A.; Van der Zalm, W.; Degirmenci, V. Processing of agricultural apple fruit waste into sugar rich feedstocks for the catalytic production of 5-HMF over a Sn Amberlyst-15 resin catalyst. J. Ind. Eng. Chem. 2021, 99, 443–448. [Google Scholar] [CrossRef]
- Rihko-Struckmann, L.K.; Oluyinka, O.; Sahni, A.; McBride, K.; Fachet, M.; Ludwig, K.; Sundmacher, K. Transformation of remnant algal biomass to 5-HMF and levulinic acid: Influence if a biphasic solvent system. RSC Adv. 2020, 10, 24753–24763. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, L.; Yang, J.; Wang, X.; Qin, X.; Qi, X.; Shen, F. Eco-friendly synthesis of SO3H-containing solid acid via mechanochemistry for the conversion of carbohydrates to 5-hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2020, 8, 7059–7067. [Google Scholar] [CrossRef]
- Flores-Velázquez, V.; Córdova-Pérez, G.E.; Silahua-Pavón, A.A.; Torres-Torres, J.G.; Sierra, U.; Fernández, S.; Godavarthi, S.; Ortiz-Chi, F.; Espinosa-González, C.G. Cellulose obtained from banana plant waste for catalytic production of 5-HMF: Effect of grinding on the cellulose properties. Fuel 2020, 265, 116857–116867. [Google Scholar] [CrossRef]
- Overton, J.C.; Engelberth, A.S.; Mosier, N.S. Single-vessel synthesis of 5-hydroxymethylfurfural (HMF) from milled corn. ACS Sustain. Chem. Eng. 2020, 8, 18–21. [Google Scholar] [CrossRef]
- Tyagi, U.; Anand, N. Single-pot conversion of fruit peel waste to 5-hydroxymethylfurfural catalyzed by modified activated carbon in green solvent: Kinetics and thermodynamic study. Biomass Conv. Bioref. 2022, 12, 469–489. [Google Scholar] [CrossRef]
- Tyagi, U.; Anand, N.; Kumar, D. Simultaneous pretreatment and hydrolysis of hardwood biomass species catalyzed by combination of modified activated carbon and ionic liquid in biphasic system. Bioresour. Technol. 2019, 289, 121675–121683. [Google Scholar] [CrossRef]
- Yuan, B.; Guan, J.; Peng, J.; Zhu, G.-Z.; Jiang, J.-H. Green hydrolysis of corncob cellulose into 5-hydroxymethylfurfural using hydrophobic inidazole ionic liquids with a recyclable, magnetic metalloporphyrin catalyst. Chem. Eng. J. 2017, 330, 109–119. [Google Scholar] [CrossRef]
- Motagamwala, A.H.; Huang, K.; Maravelias, C.T.; Dumesic, J.A. Solvent system for effective near-term production of hydroxymethylfurfural (HMF) with potential for long-term process improvement. Energy Environ. Sci. 2019, 12, 2212–2222. [Google Scholar] [CrossRef]
- Ji, Q.; Tan, C.P.; Yagoub, A.E.A.; Chen, L.; Yan, D.; Zhou, C. Effects of acidic deep eutectic solvent pretreatment on sugar cane bagasse for efficient 5-hydroxymethylfurfural production. Energy Technol. 2021, 9, 2100396–2100408. [Google Scholar] [CrossRef]
- Huang, L.; Peng, H.; Xiao, Z.; Wu, H.; Fu, G.; Wan, Y.; Bi, H. Production of furfural and 5-hydroxymethyl furfural from Camelia oleifera fruit shell in [Bmim]HSO4/H2O/1,4-dioxane biphasic medium. Ind. Crop. Prod. 2022, 184, 115006–115016. [Google Scholar] [CrossRef]
- Smink, D.; Juan, A.; Schuur, B.; Kersten, S.R.A. Understanding the role of choline chloride in deep eutectic solvents used for biomass delignification. Ind. Eng. Chem. Res. 2019, 58, 16348–16357. [Google Scholar] [CrossRef] [Green Version]
- Galkin, K.I.; Ananikov, V.P. When will 5-hydroxymethylfurfural, the “sleepeing giant” of sustainable chemistry, awaken? ChemSusChem 2019, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Carrión-Prieto, P.; Martín-Ramos, P.; Hernández-Navarro, S.; Sánchez-Sastre, L.F.; Marcos-Robles, J.L. Martín-Gil, Furfural, 5-HMF, acid-soluble lignin and sugar contents in C. ladanifer and E. arborea lignocellulosic biomass hydrolysates obtained from microwave-assisted treatments in different solvents. Biomass Bioenerg. 2018, 119, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Tomei, J.; Helliwell, R. Food versus fuel? Going beyond biofuels. Land Use Policy 2016, 56, 320–326. [Google Scholar] [CrossRef]
- Slak, J.; Pomeroy, B.; Kostyniuk, A.; Grilc, M.; Lokozar, B. A review of bio-refining process intensification in catalytic conversion reactions, separations and purifications of hydroxymethylfurfural (HMF) and furfural. Chem. Eng. J. 2022, 429, 132325–132341. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, T. Revisiting greenness of ionic liquids and deep eutectic solvents. Green Chem. Eng. 2021, 2, 174–186. [Google Scholar] [CrossRef]
- Hu, L.; Jiang, Y.; Wu, Z.; Wang, X.; He, A.; Xu, J.; Xu, J. State-of-the-art advances and perspective in the separation of biomass-derived 5-hydroxymethylfurfural. J. Clean. Prod. 2020, 276, 124219–124240. [Google Scholar] [CrossRef]
- Raspolli Galletti, A.M.; Licursi, D.; Ciorba, S.; Di Fidio, N.; Coccia, V.; Cotana, F.; Antonetti, C. Sustainable exploitation of residual Cynara cardunculus L. to levulinic acid and n-butyl levulinate. Catalysts 2021, 11, 1082. [Google Scholar] [CrossRef]
- Priecel, P.; Lopez-Sanchez, J.A. Advantages and limitations of microwave reactors: From chemical synthesis to the catalytic valorization of biobased chemicals. ACS Sustain. Chem. Eng. 2019, 7, 3–21. [Google Scholar] [CrossRef]

| Entry | Feedstock (wt%) | Catalyst (wtbiomass/wtcatalyst) | Solvent | T (°C) | t (min) | Heat 1 | YHMF (mol%) | Refs. |
|---|---|---|---|---|---|---|---|---|
| 1 | Food waste (4.0%) | / | H2O | 230 | 15 | MW | 7 | [57] |
| 2 | Sugar beet thick juice (14.7%) | H2SO4 (166.7) | H2O | 180 | 106 | Conv. | 27 | [58] |
| 3 | Mango pulp and skin (5.0%) | H2SO4 (1000.0) | H2O | 150 | 20 | MW | 21 | [59] |
| 4 | Spruce wood (2.4%) | H2SO4 (5.1) | H2O | 200 | 40 | Conv. | 10 | [60] |
| 5 | Beach wood (2.4%) | H2SO4 (5.1) | H2O | 200 | 40 | Conv. | 11 | [60] |
| 6 | Recycled pulp paper towel waste (5.0%) | H2SO4 (3.8) | H2O | 200 | 5 | MW | 6 | [61] |
| 7 | Macroalgae Gracilaria verrucosa sp. (6.3%) | H2SO4 (20.8) | H2O | 175 | 35 | Conv. | 18 | [62] |
| 8 | Poppy stalks (4.8%) | CuCl2 (12.5) | H2O | 200 | 60 | Conv. | 12 | [63] |
| 9 | Microalgae Chlorella sp. (5.0%) | Al2(SO4)3 (1.3) | H2O | 165 | 30 | Conv. | 23 | [64] |
| 10 | Corncob (1.5%) | ZnCl2 nH2O (0.5) + HCl (75.0) | H2O | 1°: 80 2°: 120 | 1°: 300 2°: 60 | Conv. | 30 | [65] |
| 11 | Softwood chips (1.5%) | ZnCl2 nH2O (0.5) + HCl (75.0) | H2O | 1°: 80 2°: 120 | 1°: 300 2°: 60 | Conv. | 22 | [65] |
| 12 | Algae Ulva lactuca sp. (2.5%) | ZnCl2 nH2O (0.8) + HCl (122.0) | H2O | 1°: 80 2°: 120 | 1°: 300 2°: 60 | Conv. | 25 | [65] |
| 13 | Algae Porphyridium cruentum sp. (4.3%) | ZnCl2 nH2O (1.4) + HCl (217.0) | H2O | 1°: 80 2°: 120 | 1°: 300 2°: 60 | Conv. | 35 | [65] |
| 14 | Vegetable waste (5.0%) | Amberlyst-36 (1.0) | H2O | 120 | 5 | MW | 5 | [66] |
| 15 | Macroalgae Gracilaria verrucosa sp. (11.7%) | Amberlyst-15 (6.7) | H2O | 130 | 120 | Conv. | 19 | [67] |
| 16 | Sugarcane bagasse (4.8%) | D001-cc ion-exchange resin (1.0) | H2O | 140 | 25 | MW | 9 | [68] |
| 17 | Sugarcane bagasse (4.8%) | D001-cc ion-exchange resin (1.0) | DMSO | 140 | 25 | MW | 18 | [68] |
| 18 | Waste fluff (2.0%) | BT300S 2 (2.0) | H2O | 200 | 120 | Conv. | 64 | [69] |
| 19 | Cotton linter (2.0%) | BT300S 2 (2.0) | H2O | 200 | 240 | Conv. | 29 | [69] |
| 20 | Corn straw (2.0%) | BT300S 2 (2.0) | H2O | 200 | 60 | Conv. | 52 | [69] |
| 21 | Sugarcane bagasse (1.1%) | SPPTPA 3 (5.0) | DMSO | 140 | 60 | MW | 20 4 | [70] |
| 22 | Sugarcane bagasse (1.2%) | SPPTPA 3 (5.0) | NMP 5 | 140 | 60 | MW | 19 4 | [70] |
| 23 | Corncob (1.3%) | SPPTPA 3 (4.2) | GVL | 175 | 30 | Conv. | 32 | [71] |
| 24 | Used clothing (0.7%) | Ca3(PO4)2 (0.1) | H2O | 200 | 120 | Conv. | 10 | [72] |
| 25 | Used paper (0.7%) | Ca3(PO4)2 (0.1) | H2O | 200 | 120 | Conv. | 8 | [72] |
| 26 | Japanese cedar (1.5%) | Ca3(PO4)2 (0.26) | H2O | 200 | 120 | Conv. | 14 | [72] |
| 27 | Waste cotton stalks (3.2%) | SO42−/ZrO2 (3.3) | H2O | 230 | 75 | Conv. | 27 | [73] |
| 28 | Microalgae Chlorococcum sp. (1.0%) | H-ZSM-5 (1.5) | H2O | 200 | 120 | Conv. | 39 | [74] |
| 29 | Wood ear mushroom (5.0%) | [NMP][CH3SO3] 6 (5.5) | DMA 7-LiCl | 140 | 2 | MW | 58 | [75] |
| 30 | Wood ear mushroom (5.0%) | [DMA][CH3SO3] 8 (5.5) | DMA 7-LiCl | 140 | 2 | MW | 64 | [75] |
| 31 | Wood ear mushroom (5.0%) | [BBIM-SO3H][OTf] 9 (5.5) | DMA 7-LiCl | 140 | 2 | MW | 63 | [75] |
| 32 | Wood ear mushroom (5.0%) | [BBIM-SO3H][NTf2] 10 (5.5) | DMA 7-LiCl | 140 | 2 | MW | 69 | [75] |
| Entry | Feedstock (wt%) | Catalyst (wtbiomass/wtcatalyst) | Solvent | T (°C) | t (min) | Heat 1 | YHMF (mol%) | Refs. |
|---|---|---|---|---|---|---|---|---|
| 33 | Wood chips (4.8%) | CrCl3·6H2O (1.3) | [BMIM]Cl 2 | 120 | 120 | Conv. | 41 | [84] |
| 34 | Rice straw (4.8%) | CrCl3·6H2O (1.3) | [BMIM]Cl 2 | 120 | 120 | Conv. | 35 | [84] |
| 35 | Wheat straw (10.0%) | CrCl3 (1.2) | [BMPy]Cl 3 | 130 | 120 | Conv. | 50 | [85] |
| 36 | Rice husk (10.0%) | CrCl3 (1.0) | [BMPy]Cl 4 | 130 | 60 | Conv. | 26 | [86] |
| 37 | Wheat husk (10.0%) | CrCl3 (1.0) | [BMPy]Cl 4 | 130 | 60 | Conv. | 45 | [86] |
| 38 | Corn stover (10.0%) | CrCl3 (1.0) | [BMPy]Cl 4 | 130 | 60 | Conv. | 39 | [86] |
| 39 | Sugarcane bagasse (10.0%) | CrCl3 (1.0) | [BMPy]Cl 4 | 130 | 60 | Conv. | 16 | [86] |
| 40 | Coconut shells (10.0%) | CrCl3 (1.0) | [BMPy]Cl 4 | 130 | 60 | Conv. | 26 | [86] |
| 41 | Almond shells (10.0%) | CrCl3 (1.0) | [BMPy]Cl 4 | 130 | 60 | Conv. | 30 | [86] |
| 42 | Junegrass (3.8%) | CuCl2 (8.3) | [Et3NH][HSO4] 4 | 180 | 41 | MW | 45 | [87] |
| 43 | Mixed herb residue (4.8%) | SnCl4· 5H2O (0.3) | ChCl 5/formic acid (1/8 mol/mol) | 140 | 30 | Conv. | 55 | [88] |
| 44 | Anemarrhena asphodeloides Bunge (4.8%) | SnCl4· 5H2O (0.2) | ChCl 5/formic acid (1/8 mol/mol) | 140 | 30 | Conv. | 77 | [88] |
| 45 | Caulis Polygoni Multiflori (4.8%) | SnCl4· 5H2O (0.2) | ChCl 5/formic acid (1/8 mol/mol) | 140 | 60 | Conv. | 14 | [88] |
| 46 | Sugarcane bagasse (4.8%) | D001-cc ion-exchange resin (1.0) | [BMIM]Cl 2 | 140 | 25 | MW | 21 | [68] |
| 47 | Sugarcane bagasse (4.8%) | D001-cc ion-exchange resin (1.0) | [BMIM]OAc 6 | 140 | 25 | MW | 25 | [68] |
| 48 | Babool wood (4.8%) | Sulfonated activated carbon (2.0) | [BMIM]Cl 3 | 120 | 60 | Conv. | 33 | [89] |
| 49 | Corn stalk (4.8%) | HCSS 7 (1.0) | [BMIM]Cl 2 | 150 | 30 | Conv. | 44 | [90] |
| 50 | Corn stalk (20.0%) | Biochar-Mg-Sn (5.0) | AMIMBF4 8 | 100 | 180 | Conv. | 43 | [91] |
| 51 | Corn stalk (20.0%) | Biochar-Mg-Sn (5.0) | AMIMBr 9 | 100 | 180 | Conv. | 49 | [91] |
| 52 | Corn stalk (20.0%) | Biochar-Mg-Sn (5.0) | AMIMCl 10 | 100 | 180 | Conv. | 61 | [91] |
| 53 | Corn husk (4.8%) | ChCl 5/oxalic acid (0.05) | ChCl 5/oxalic acid (1/1 mol/mol) | 80 | 60 | Conv. | 14 | [92] |
| Entry | Feedstock (wt%) | Catalyst (wtbiomass/wtcatalyst) | Solvent | T (°C) | t (min) | Heat 1 | YHMF (mol%) | Refs. |
|---|---|---|---|---|---|---|---|---|
| 54 | Sugar beet juice (3.7%) | H2SO4 (35.0) | H2O-NaCl/MeTHF 2 (1/4 v/v) | 150 | 20 | Conv. | 96 3 | [93] |
| 55 | Microalgae Chlorella sorokiniana (1.6%) | H2SO4 (3.6) | H2O-LiCl/DMSO (1/3 v/v) | 150 | 120 | Conv. | 52 | [94] |
| 56 | Bamboo (0.6%) | HCl (21.4) | H2O/MIBK (1/19 v/v) | 177 | 60 | MW | 42 | [95] |
| 57 | Bamboo culm (0.6%) | HCl (21.4) | H2O/MIBK (1/19 v/v) | 177 | 60 | MW | 37 | [96] |
| 58 | Bamboo leaves (0.6%) | HCl (21.4) | H2O/MIBK (1/19 v/v) | 177 | 60 | MW | 35 | [96] |
| 59 | Macroalgae Ulva prolifera (2.0%) | Formic acid (1.6) | H2O-NaCl/THF (1/1 v/v) | 200 | 60 | Conv. | 31 | [97] |
| 60 | Pinewood (2.0%) | Levulinic acid (4.0) | H2O/MeTHF 2 (1/1 v/v) | 200 | 60 | Conv. | 21 | [98] |
| 61 | Straw (1.0%) | Sulfanilic acid (6.0) | H2O, DMSO/ 2-butanol, MIBK (1/2 v/v) | 150 | 60 | Conv. | 41 | [99] |
| 62 | Barley husk (1.0%) | Sulfanilic acid (7.1) | H2O, DMSO/ 2-butanol, MIBK (1/2 v/v) | 150 | 60 | Conv. | 41 | [99] |
| Entry | Feedstock (wt%) | Catalyst (wtbiomass/wtcatalyst) | Solvent | T (°C) | t (min) | Heat 1 | YHMF (mol%) | Refs. |
|---|---|---|---|---|---|---|---|---|
| 147 | Sugar beet pulp 2 (5.0%) | / | H2O | 193 | 51 | Conv. | 10 | [143] |
| 148 | Polyester/cotton mixed fabric waste 3 (1.0%) | Citric acid (1.0) | H2O | 225 | 60 | Conv. | 12 4 | [144] |
| 149 | Eucalyptus 5 (1.0%) | InCl3 (7.3) | H2O | 200 | 120 | Conv. | 14 | [145] |
| 150 | Seaweed Gracilaria dura 6 (4.8%) | KHSO4 (250.0) | H2O | 110 | 540 | Conv. | 61 | [146] |
| 151 | Corn stover 7 (1.0%) | AlCl3 (3.8) + Maleic acid (4.3) | H2O | 180 | 20 | MW | 16 | [147] |
| 152 | Used clothing 3 (0.7%) | Ca3(PO4)2 (0.1) | H2O | 200 | 120 | Conv. | 31 | [72] |
| 153 | Used paper 3 (0.7%) | Ca3(PO4)2 (0.1) | H2O | 200 | 120 | Conv. | 22 | [72] |
| 154 | Japanese cedar 3 (1.5%) | Ca3(PO4)2 (0.26) | H2O | 200 | 120 | Conv. | 36 | [72] |
| 155 | Microalgae Chlorococcum 3 (1.0%) | H-ZSM-5 (1.5) | H2O | 200 | 120 | Conv. | 47 | [74] |
| 156 | Corncob 8 (10.0%) | HSO3−ZSM-5 (3.3) | H2O | 150 | 300 | Conv. | 24 | [148] |
| 157 | Corn stalk 9 (1.6%) | SO42−/ZrO2 (5.0) | H2O | 230 | 120 | Conv. | 60 | [149] |
| Entry | Feedstock (wt%) | Catalyst (wtbiomass/wtcatalyst) | Solvent | T (°C) | t (min) | Heat 1 | YHMF (mol%) | Refs. |
|---|---|---|---|---|---|---|---|---|
| 158 | Wood dust 2 (n.a. 3) | H2SO4 (20.0 4) | ChCl 5/citric acid (2/1 mol/mol) | 130 | 5 | Conv. | 24 6 | [150] |
| 159 | Wood chips 7 (4.8%) | CrCl3·6H2O (1.3) | [BMIM]Cl 8 | 120 | 120 | Conv. | 79 | [84] |
| 160 | Rice straw 7 (4.8%) | CrCl3·6H2O (1.3) | [BMIM]Cl 8 | 120 | 120 | Conv. | 76 | [84] |
| 161 | Waste cottonseed hull 9 (4.0%) | CuCl2·2H2O (5.0) | [EMIM]Ac 10 + [BMIM][TOS] 11 (1/6 v/v) | 120 | 150 | Conv. | 42 | [151] |
| 162 | Waste cottonseed hull 9 (4.0%) | H4[Si(W3O10)4]·xH2O (5.0) | [EMIM]Ac 10 + [BMIM][TOS] 11 (1/6 v/v) | 120 | 150 | Conv. | 40 | [151] |
| 163 | Apple waste 12 (6.3%) | D001-cc ion-exchange resin (1.0) | [BMIM]Cl 8 | 140 | 60 | Conv. | 45 | [152] |
| 164 | Orange waste 12 (6.3%) | D001-cc ion-exchange resin (1.0) | [BMIM]Cl 8 | 140 | 60 | Conv. | 42 | [152] |
| 165 | Sugarcane bagasse 13 (4.8%) | D001-cc ion-exchange resin (1.0) | [BMIM]OAc 14 | 140 | 25 | MW | 66 | [68] |
| 166 | Wheat straw 9 (2.0%) | ChCl 5/p-TSA (0.02) | ChCl 5/p-TSA (n.a. 3) | 80 | 30 | Conv. | 72 | [153] |
| 167 | Rice husk 9 (2.0%) | ChCl 5/p-TSA (0.02) | ChCl 5/p-TSA (n.a. 3) | 80 | 30 | Conv. | 68 | [153] |
| 168 | Bagasse 9 (2.0%) | ChCl 5/p-TSA (0.02) | ChCl 5/p-TSA (n.a. 3) | 80 | 30 | Conv. | 70 | [153] |
| Entry | Feedstock (wt%) | Catalyst (wtbiomass/wtcatalyst) | Solvent | T (°C) | t (min) | Heat 1 | YHMF (mol%) | Refs. |
|---|---|---|---|---|---|---|---|---|
| 169 | Melon rind 2 (2.7%) | H2SO4 (10.2) | H2O-NaCl/THF (1/3 v/v) | 180 | 30 | Conv. | 14 | [154] |
| 170 | Melon rind 2 (2.7%) | H2SO4 (10.2) | H2O-NaCl/THF (1/3 v/v) | 180 | 30 | MW | 13 | [154] |
| 171 | Eucalyptus 3 (1.0%) | InCl3 (7.3) | H2O-NaCl/THF (1/3 v/v) | 200 | 120 | Conv. | 43 | [145] |
| 172 | Eucalyptus 3 (1.0%) | AlCl3 (12.1) | H2O-NaCl/THF (1/3 v/v) | 200 | 120 | Conv. | 40 | [145] |
| 173 | Eucalyptus 3 (1.0%) | FeCl3 (9.8) | H2O-NaCl/THF (1/3 v/v) | 200 | 120 | Conv. | 38 | [145] |
| 174 | Bagasse 3 (1.0%) | InCl3 (7.3) | H2O-NaCl/THF (1/3 v/v) | 200 | 120 | Conv. | 46 | [145] |
| 175 | Birch wood 4 (2.2%) | Salts (0.3) | Seawater/THF (1/6 v/v) | 200 | 300 | Conv. | 43 | [155] |
| 176 | Beech wood 4 (2.2%) | Salts (0.3) | Seawater/THF (1/6 v/v) | 200 | 300 | Conv. | 44 | [155] |
| 177 | Pine wood 4 (2.2%) | Salts (0.3) | Seawater/THF (1/6 v/v) | 200 | 300 | Conv. | 46 | [155] |
| 178 | Corn stalks 4 (2.2%) | Salts (0.3) | Seawater/THF (1/6 v/v) | 200 | 300 | Conv. | 50 | [155] |
| 179 | Molasses 5 (12.8%) | AlCl3 (61.5) | H2O/GVL (1/4 v/v) | 160 | 180 | Conv. | 28 | [113] |
| 180 | Corn straw 6 (1.0%) | Al2(SO4)3 (2.8) | H2O-NaCl/GVL (1/4 v/v) | 165 | 50 | Conv. | 30 | [156] |
| 181 | Rice straw 6 (1.0%) | Al2(SO4)3 (2.8) | H2O-NaCl/GVL (1/4 v/v) | 165 | 50 | Conv. | 35 | [156] |
| 182 | Cow dung 6 (1.0%) | Al2(SO4)3 (2.8) | H2O-NaCl/GVL (1/4 v/v) | 165 | 50 | Conv. | 35 | [156] |
| 183 | Poplar sawdust 6 (1.0%) | Al2(SO4)3 (2.8) | H2O-NaCl/GVL (1/4 v/v) | 165 | 50 | Conv. | 66 | [156] |
| 184 | Corn waste 7 (4.8%) | SnCl4 (3.5) | H2O/acetone (1/1 v/v) | 140 | 10 | MW | 27 | [110] |
| 185 | Taro waste 7 (4.8%) | SnCl4 (3.5) | H2O/acetone (1/1 v/v) | 140 | 10 | MW | 32 | [110] |
| 186 | Corn straw 8 (2.5%) | SnCl4 (0.7) | DES 9/DMSO (1/1 v/v) | 140 | 60 | Conv. | 28 | [157] |
| 187 | Waste coffee grounds 2 (2.4%) | Al(NO3)3·9H2O (2.7) + H2SO4 (8.5) | H2O/DMSO (3/2 v/v) | n.a. 10 | 20 | MW | 14 11 | [158] |
| 188 | Spent coffee grounds 12 (5.0% 13) | AlCl3 (79.0) + HCl (465.0) | H2O-NaCl/GVL (1/2 v/v) | 170 | 20 | Conv. | 8 11 | [159] |
| 189 | Pistachio hull 12 (5.0% 13) | AlCl3 (79.0) + HCl (465.0) | H2O-NaCl/GVL (1/2 v/v) | 170 | 20 | Conv. | 8 11 | [159] |
| 190 | Potato peels 14 (4.0%) | 1°: LiBr (0.2) + H2SO4 (8.2) 2°: AlCl3 (2.0 15) | 1°: H2O 2°: H2O/2-butanol (1/3 v/v) | 1°: 140 2°: 160 | 1°: 60 2°: 180 | Conv. | 54 | [160] |
| 191 | Corn stover 16 (1.0%) | AlCl3 (3.8) + Maleic acid (4.3) | H2O/acetonitrile (2/1 v/v) | 180 | 20 | MW | 31 | [147] |
| 192 | High-fructose corn syrup 17 (2.2%) | CaCl2 (1.1) + Gluconic acid (1.2) | H2O/MeTHF (1/4 v/v) | 150 | 120 | Conv. | 82 | [161] |
| 193 | High-fructose corn syrup 17 (2.2%) | CaCl2 (0.2) + Gluconic acid (1.2) | H2O/MeTHF (1/4 v/v) | 150 | 10 | MW | 86 | [161] |
| Entry | Feedstock (wt%) | Catalyst (wtbiomass/wtcatalyst) | Solvent | T (°C) | t (min) | Heat 1 | YHMF (mol%) | Refs. |
|---|---|---|---|---|---|---|---|---|
| 194 | Food and beverage waste-derived high-fructose syrup 2 (3.8%) | Amberlyst-36 (1.0) | H2O/DMSO (1/1 v/v) | 140 | 40 | MW | 71 3 | [162] |
| 195 | Apple waste 4 (4.0) | Sn-amberlyst-15 (0.3) | H2O/MIBK (1/1 v/v) | 120 | 120 | Conv. | 29 | [163] |
| 196 | Melon rind 5 (2.7%) | Montmorillonite KSV (1.0) | H2O-NaCl/THF (1/3 v/v) | 180 | 30 | Conv. | 22 | [154] |
| 197 | Melon rind 5 (2.7%) | Montmorillonite KSV (1.0) | H2O-NaCl/THF (1/3 v/v) | 180 | 30 | MW | 28 | [154] |
| 198 | Microalgae Dunaliella salina 5 (4.6%) | H-ZSM-5 (6.0) | H2O-NaCl/THF (1/1.7 v/v) | 180 | 60 | Conv. | 39 | [164] |
| 199 | Corncob 5 (10.0%) | HSO3-ZSM-5 (3.3) | H2O/DMSO (1/3 v/v) | 150 | 300 | Conv. | 63 | [148] |
| 200 | Corncob 5 (10.0%) | HSO3-ZSM-5 (2.5) | H2O/THF (1/3 v/v) | 160 | 300 | Conv. | 63 | [148] |
| 201 | Rice straw 6 (1.0%) | APG-SO3H 7 (2.0) | H2O-NaCl/GVL (1/4 v/v) | 180 | 480 | Conv. | 31 | [165] |
| 202 | Banana plant waste 8 (2.0%) | Al2O3-TiO2-W (2.5) | H2O-NaCl/THF (1/3 v/v) | 175 | 180 | Conv. | 76 | [166] |
| 203 | Banana plant waste 6,8 (2.0%) | Al2O3-TiO2-W (2.5) | H2O-NaCl/THF (1/3 v/v) | 175 | 60 | Conv. | 80 | [166] |
| 204 | Yellow dent corn 6 (30.0%) | Activated carbon (3.9) + Maleic acid (73.8) + AlCl3 (32.1) | H2O/DMSO/ acetonitrile (2/2/1 v/v) | 180 | 3 | Conv. | 85 | [167] |
| 205 | Pineapple peels 9 (2.0%) | Sulfonated activated carbon (2.0) | H2O/[BMIM]Cl 10 (4/1 v/v) | 120 | 60 | Conv. | 50 | [168] |
| 206 | Banana peels 9 (2.0%) | Sulfonated activated carbon (2.0) | H2O/[BMIM]Cl 10 (4/1 v/v) | 120 | 60 | Conv. | 45 | [168] |
| 207 | Catalpa 6,9 (2.4%) | Sulfonated activated carbon (1.0) + AlCl3 (1.2) | H2O/[BMIM]Cl 10 (n.a. 11) | 120 | 60 | Conv. | 86 | [169] |
| 208 | Indian rosewood 6,9 (2.4%) | Sulfonated activated carbon (1.0) + AlCl3 (1.2) | H2O/[BMIM]Cl 10 (n.a. 11) | 120 | 60 | Conv. | 70 | [169] |
| 209 | Chinaberry 6,9 (2.4%) | Sulfonated activated carbon (1.0) + AlCl3 (1.2) | H2O/[BMIM]Cl 10 (n.a. 11) | 120 | 60 | Conv. | 62 | [169] |
| 210 | Babool 6,9 (2.4%) | Sulfonated activated carbon (1.0) + AlCl3 (1.2) | H2O/[BMIM]Cl 10 (n.a. 11) | 120 | 60 | Conv. | 75 | [169] |
| 211 | Corncob 9 (3.2%) | MCMP 12-Al (30.3) | H2O/ [moMIM][PF6] 13 (1/3 v/v) | 200 | 180 | Conv. | 52 | [170] |
| 212 | Corncob 9 (3.2%) | MCMP 12-Al (30.3) + MCMP 12-Cr (30.3) + MCMP 12-Mg (30.3) | H2O/ [moMIM][PF6] 13 (1/3 v/v) | 200 | 180 | Conv. | 68 | [170] |
| Entry | Feedstock (wt%) | Catalyst (wtbiomass/wtcatalyst) | Solvent | T (°C) | t (min) | Heat 1 | YHMF (mol%) | Refs. |
|---|---|---|---|---|---|---|---|---|
| 213 | Sugarcane bagasse 2 (8.5%) | DES 3 (0.1) | H2O/DES 3 (n.a. 4) | 110 | 240 | Conv. | 57 | [172] |
| 214 | Camelia oleifera fruit shell 5 (1.6%) | [BMIM]HSO4 6 (0.2) | H2O/1,4-dioxane (1/10 v/v) | 180 | 20 | MW | 21 | [173] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fulignati, S.; Licursi, D.; Di Fidio, N.; Antonetti, C.; Raspolli Galletti, A.M. Novel Challenges on the Catalytic Synthesis of 5-Hydroxymethylfurfural (HMF) from Real Feedstocks. Catalysts 2022, 12, 1664. https://doi.org/10.3390/catal12121664
Fulignati S, Licursi D, Di Fidio N, Antonetti C, Raspolli Galletti AM. Novel Challenges on the Catalytic Synthesis of 5-Hydroxymethylfurfural (HMF) from Real Feedstocks. Catalysts. 2022; 12(12):1664. https://doi.org/10.3390/catal12121664
Chicago/Turabian StyleFulignati, Sara, Domenico Licursi, Nicola Di Fidio, Claudia Antonetti, and Anna Maria Raspolli Galletti. 2022. "Novel Challenges on the Catalytic Synthesis of 5-Hydroxymethylfurfural (HMF) from Real Feedstocks" Catalysts 12, no. 12: 1664. https://doi.org/10.3390/catal12121664