Kinetics of Strontium Carbonate Formation on a Ce-Doped SrFeO3 Perovskite
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Perovskite Oxide Synthesis
3.2. Formation of Carbonates
3.3. Powder Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilley, R.J.D. Perovskites: Structure-Property Relationships, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Li, C.; Soh, K.C.K.; Wu, P. Formability of ABO3 Perovskites. J. Alloys Compd. 2004, 372, 40–48. [Google Scholar] [CrossRef]
- Twu, J.; Gallagher, P.K. Preparation of Bulk and Supported Perovskites. In Properties and Applications of Perovskite-Type Oxides; Tejuca, L.G., Fierro, J.L.G., Eds.; CRC Press: New York, NY, USA, 1993; pp. 1–23. [Google Scholar]
- Grabowska, E. Selected Perovskite Oxides: Characterization, Preparation and Photocatalytic Properties—A Review. Appl. Catal. B Environ. 2016, 186, 97–126. [Google Scholar] [CrossRef]
- Chen, H.; Ku, J.; Wang, L. Thermal Catalysis under Dark Ambient Conditions in Environmental Remediation: Fundamental Principles, Development, and Challenges. Chin. J. Catal. 2019, 40, 1117–1134. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Hwang, J.; Rao, R.R.; Giordano, L.; Katayama, Y.; Yu, Y.; Shao-Horn, Y. Perovskites in Catalysis and Electrocatalysis. Science 2017, 358, 751–756. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Sharma, M.; Powar, S.; Kabachkov, E.N.; Vaish, R. Impact of Remnant Surface Polarization on Photocatalytic and Antibacterial Performance of BaTiO3. J. Eur. Ceram. Soc. 2019, 39, 2915–2922. [Google Scholar] [CrossRef]
- Srilakshmi, C.; Rao, G.M.; Saraf, R. Effect of the Nature of a Transition Metal Dopant in BaTiO3 Perovskite on the Catalytic Reduction of Nitrobenzene. RSC Adv. 2015, 5, 45965–45973. [Google Scholar] [CrossRef]
- Janowska, K.; Boffa, V.; Jørgensen, M.K.; Quist-Jensen, C.; Hubac, F.; Deganello, F.; Coelho, F.E.B.; Magnacca, G. Thermocatalytic Membrane Distillation for Clean Water Production. NPJ Clean Water 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Markov, A.A.; Nikitin, S.S.; Leonidov, I.A.; Patrakeev, M.V. Oxygen and Electron Transport in Ce0.1Sr0.9FeO3−δ. Solid State Ion. 2020, 344, 115131. [Google Scholar] [CrossRef]
- Lu, Q.; Huberman, S.; Zhang, H.; Song, Q.; Wang, J.; Vardar, G.; Hunt, A.; Waluyo, I.; Chen, G.; Yildiz, B. Bi-Directional Tuning of Thermal Transport in SrCoOx with Electrochemically Induced Phase Transitions. Nat. Mater. 2020, 19, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Matras, D.; Vamvakeros, A.; Jacques, S.D.M.; Middelkoop, V.; Vaughan, G.; Agote Aran, M.; Cernik, R.J.; Beale, A.M. In Situ X-ray Diffraction Computed Tomography Studies Examining the Thermal and Chemical Stabilities of Working Ba0.5Sr0.5Co0.8Fe0.2O3−δ Membranes during Oxidative Coupling of Methane. Phys. Chem. Chem. Phys. 2020, 22, 18964–18975. [Google Scholar] [CrossRef]
- Colomer, M.T.; Ortiz, A.L.; López-Domínguez, V.; Alonso, J.M.; García, M.A. Preparation, Thermal and Phase Evolution and Functional Properties of Non-Stoichiometric Strontium-Doped Lanthanum Manganite Perovskite Ceramics. J. Eur. Ceram. Soc. 2017, 37, 3527–3533. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Liang, J.; Luo, Y.; Chen, G.; Shi, X.; Lu, S.; Gao, S.; Hu, J.; Liu, Q.; et al. A-Site Perovskite Oxides: An Emerging Functional Material for Electrocatalysis and Photocatalysis. J. Mater. Chem. A 2021, 9, 6650–6670. [Google Scholar] [CrossRef]
- Tummino, M.L.; Laurenti, E.; Deganello, F.; Bianco Prevot, A.; Magnacca, G. Revisiting the Catalytic Activity of a Doped SrFeO3 for Water Pollutants Removal: Effect of Light and Temperature. Appl. Catal. B Environ. 2017, 207, 174–181. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Deganello, F.; Liotta, L.F.; Longo, A.; Casaletto, M.P.; Scopelliti, M. Cerium Effect on the Phase Structure, Phase Stability and Redox Properties of Ce-Doped Strontium Ferrates. J. Solid State Chem. 2006, 179, 3406–3419. [Google Scholar] [CrossRef]
- Srilakshmi, C.; Saraf, R.; Shivakumara, C. Effective Degradation of Aqueous Nitrobenzene Using the SrFeO3−δ Photocatalyst under UV Illumination and Its Kinetics and Mechanistic Studies. Ind. Eng. Chem. Res. 2015, 54, 7800–7810. [Google Scholar] [CrossRef]
- Yan, A.; Liu, B.; Dong, Y.; Tian, Z.; Wang, D.; Cheng, M. A Temperature Programmed Desorption Investigation on the Interaction of Ba0.5Sr0.5Co0.8Fe0.2O3−δ Perovskite Oxides with CO2 in the Absence and Presence of H2O and O2. Appl. Catal. B Environ. 2008, 80, 24–31. [Google Scholar] [CrossRef]
- Yang, G.; Su, C.; Chen, Y.; Dong, F.; Tade, M.O.; Shao, Z. Cobalt-Free SrFe0.9Ti0.1O3−δ as a High-Performance Electrode Material for Oxygen Reduction Reaction on Doped Ceria Electrolyte with Favorable CO2 Tolerance. J. Eur. Ceram. Soc. 2015, 35, 2531–2539. [Google Scholar] [CrossRef]
- Rethwisch, D.G.; Dumesic, J.A. Effect of Metal-Oxygen Bond Strength on Properties of Oxides. 1. Infrared Spectroscopy of Adsorbed CO and CO2. Langmuir 1986, 2, 73–79. [Google Scholar] [CrossRef]
- Singh, K.P.; Singh, G.; Vaish, R. Utilizing the Localized Surface Piezoelectricity of Centrosymmetric Sr1-XFexTiO3 (X≤0.2) Ceramics for Piezocatalytic Dye Degradation. J. Eur. Ceram. Soc. 2021, 41, 326–334. [Google Scholar] [CrossRef]
- Wu, S.; Yin, S.; Cao, H.; Lu, Y.; Yin, J.; Li, B. Glucosan Controlled Biomineralization of SrCO3 Complex Nanostructures with Superhydrophobicity and Adsorption Properties. J. Mater. Chem. 2011, 21, 8734–8741. [Google Scholar] [CrossRef]
- Lutz, H.D.; Eckers, W.; Schneider, G.; Haeuseler, H. Raman and Infrared Spectra of Barium and Strontium Hydroxides and Hydroxide Hydrates. Spectrochim. Acta. Part A Mol. Spectrosc. 1981, 37, 561–567. [Google Scholar] [CrossRef]
- Grinter, D.C.; Allan, M.; Yang, H.J.; Salcedo, A.; Murgida, G.E.; Shaw, B.; Pang, C.L.; Idriss, H.; Ganduglia-Pirovano, M.V.; Thornton, G. Ce=O Terminated CeO2. Angew. Chem. 2021, 133, 13954–13958. [Google Scholar] [CrossRef]
- Mihaylov, M.Y.; Ivanova, E.Z.; Vayssilov, G.N.; Hadjiivanov, K.I. Revisiting Ceria-NOx Interaction: FTIR Studies. Catal. Today 2020, 357, 613–620. [Google Scholar] [CrossRef]
- Durães, L.; Costa, B.F.O.; Vasques, J.; Campos, J.; Portugal, A. Phase Investigation of As-Prepared Iron Oxide/Hydroxide Produced by Sol–Gel Synthesis. Mater. Lett. 2005, 59, 859–863. [Google Scholar] [CrossRef]
- Markov, L.; Blaskov, V.; Klissurski, D.; Nikolov, S. The Thermal Decomposition Mechanism of Iron(III) Hydroxide Carbonate to α-Fe2O3. J. Mater. Sci. 1990, 25, 3096–3100. [Google Scholar] [CrossRef]
- Savoye, S.; Legrand, L.; Sagon, G.; Lecomte, S.; Chausse, A.; Messina, R.; Toulhoat, P. Experimental Investigations on Iron Corrosion Products Formed in Bicarbonate/Carbonate-Containing Solutions at 90 °C. Corros. Sci. 2001, 43, 2049–2064. [Google Scholar] [CrossRef]
- Ullah, R.; Li, H.; Zhu, Y. Terahertz and FTIR Spectroscopy of ‘Bisphenol A’. J. Mol. Struct. 2014, 1059, 255–259. [Google Scholar] [CrossRef]
- Dinescu, R.; Preda, M. Thermal Decomposition of Strontium Hydroxide. J. Therm. Anal. 1973, 5, 465–473. [Google Scholar] [CrossRef]
- Balek, V.; Šubrt, J. Thermal Behaviour of Iron(III) Oxide Hydroxides. Pure Appl. Chem. 1995, 67, 1839–1842. [Google Scholar] [CrossRef]
- Ptáček, P.; Bartoníčková, E.; Švec, J.; Opravil, T.; Šoukal, F.; Frajkorová, F. The Kinetics and Mechanism of Thermal Decomposition of SrCO3 Polymorphs. Ceram. Int. 2015, 41, 115–126. [Google Scholar] [CrossRef]
- Robbins, S.A.; Rupard, R.G.; Weddle, B.J.; Maull, T.R.; Gallagher, P.K. Some Observations on the Use of Strontium Carbonate as a Temperature Standard for DTA. Thermochim. Acta 1995, 269, 43–49. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Zhu, M.; Cheng, F.; Zhang, D. Decomposition of Key Minerals in Coal Gangues during Combustion in O2/N2 and O2/CO2 Atmospheres. Appl. Therm. Eng. 2019, 148, 977–983. [Google Scholar] [CrossRef]
- Belete, T.T.; van de Sanden, M.C.M.; Gleeson, M.A. Effects of Transition Metal Dopants on the Calcination of CaCO3 under Ar, H2O and H2. J. CO2 Util. 2019, 31, 152–166. [Google Scholar] [CrossRef] [Green Version]
- Reller, A.; Padeste, C.; Hug, P. Formation of Organic Carbon Compounds from Metal Carbonates. Nature 1987, 329, 527–529. [Google Scholar] [CrossRef]
- Pintar, A.; Berčič, G.; Besson, M.; Gallezot, P. Catalytic Wet-Air Oxidation of Industrial Effluents: Total Mineralization of Organics and Lumped Kinetic Modelling. Appl. Catal. B Environ. 2004, 47, 143–152. [Google Scholar] [CrossRef]
- Østergaard, M.B.; Strunck, A.B.; Jørgensen, M.K.; Boffa, V. Abatement of Oil Residues from Produced Water Using a Thermocatalytic Packed Bed Reactor. J. Environ. Chem. Eng. 2021, 9, 106749. [Google Scholar] [CrossRef]
- Deganello, F.; Marcí, G.; Deganello, G. Citrate-Nitrate Auto-Combustion Synthesis of Perovskite-Type Nanopowders: A Systematic Approach. J. Eur. Ceram. Soc. 2009, 29, 439–450. [Google Scholar] [CrossRef]
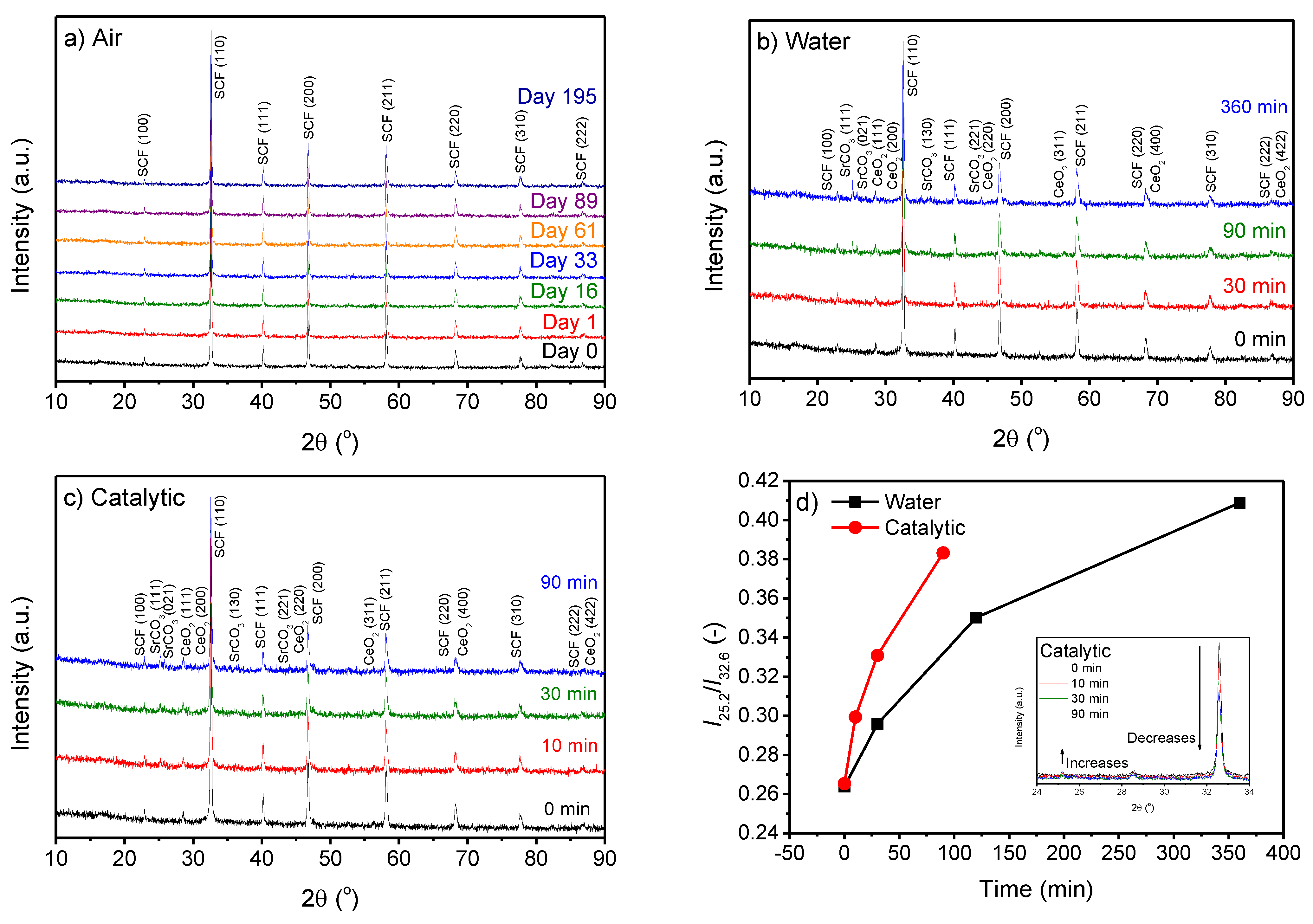


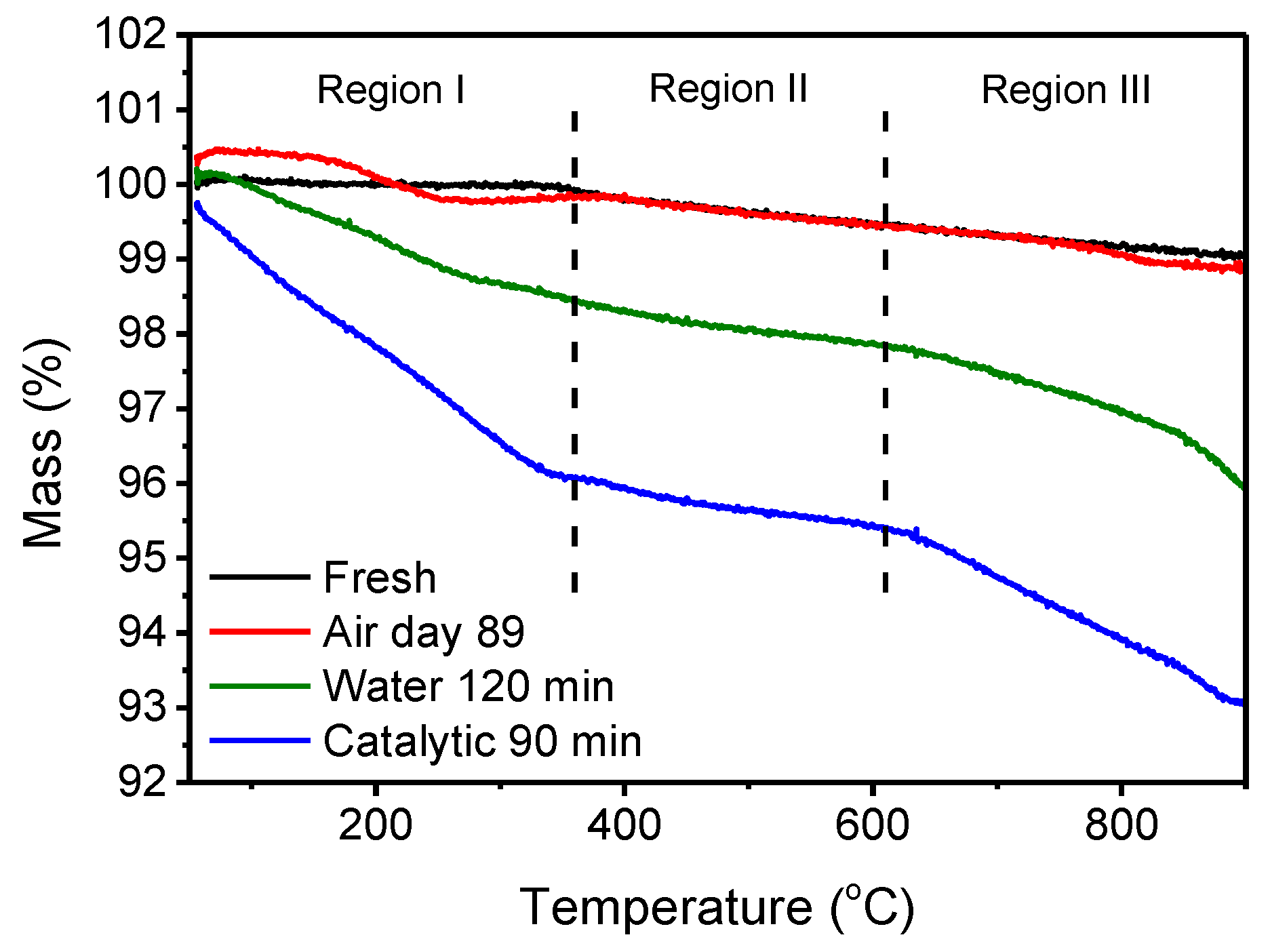
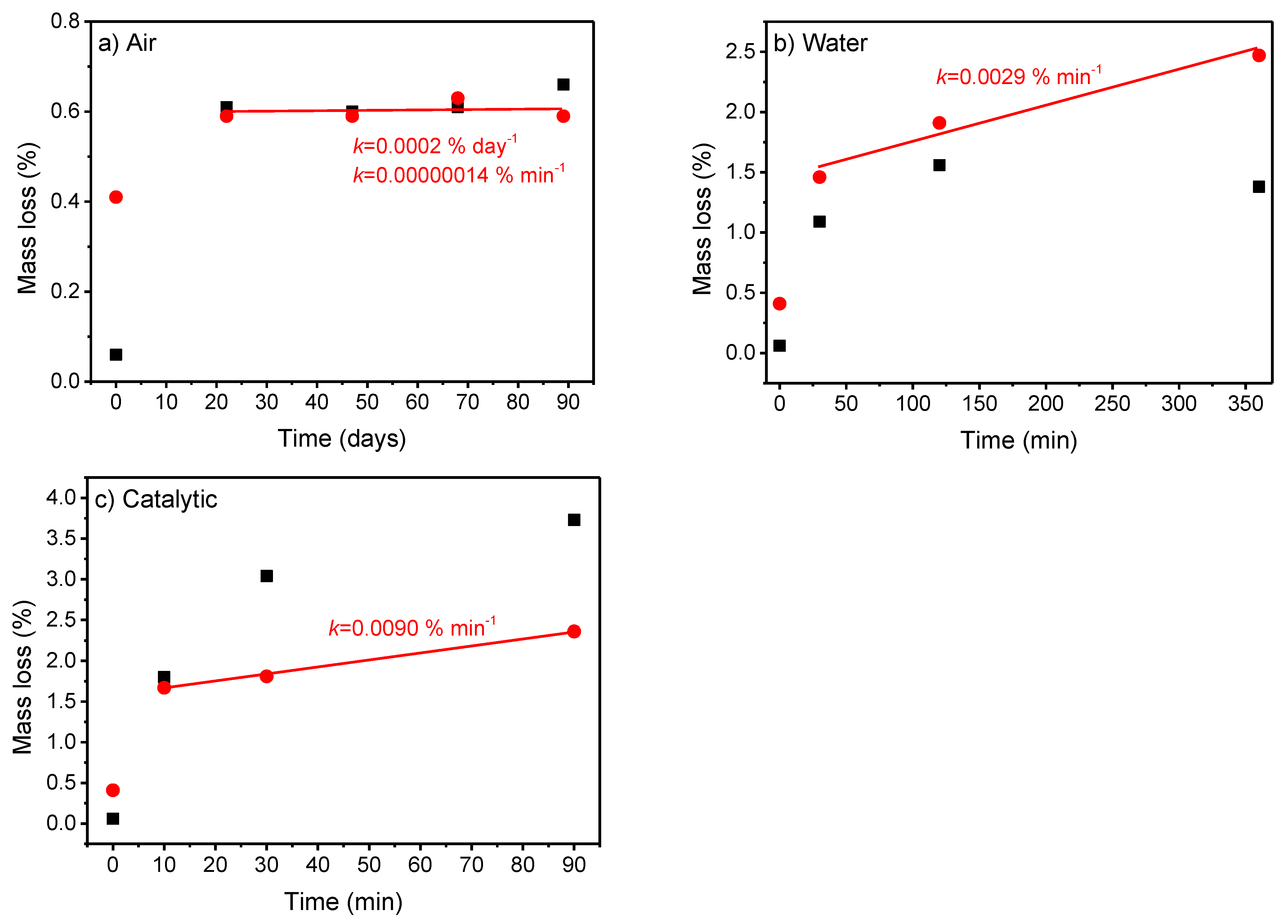
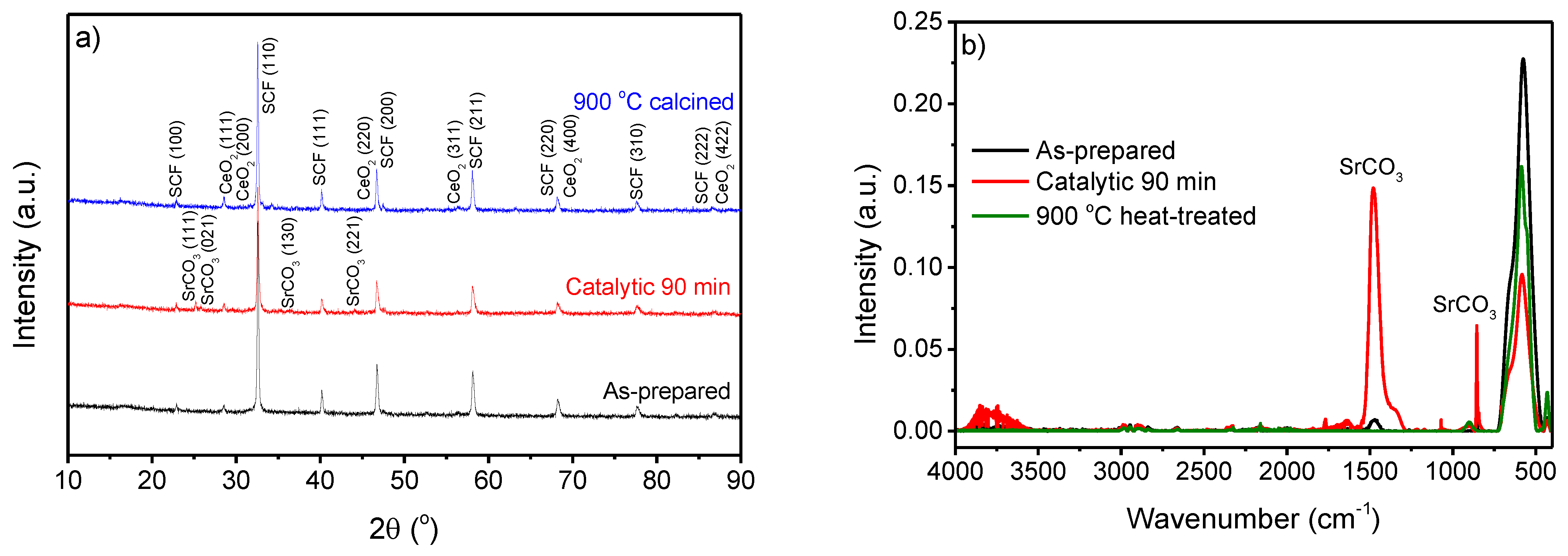
| Storage | Time | Peak Position | FWHM | Crystallite Size (Å) |
|---|---|---|---|---|
| Fresh perovskite | - | 32.599 | 0.166 | 514 |
| Air | 1 days | 32.603 | 0.170 | 502 |
| Air | 16 days | 32.606 | 0.167 | 511 |
| Air | 33 days | 32.607 | 0.166 | 514 |
| Air | 61 days | 32.607 | 0.167 | 511 |
| Air | 89 days | 32.602 | 0.163 | 524 |
| Water | 30 min | 32.583 | 0.207 | 410 |
| Water | 120 min | 32.576 | 0.230 | 368 |
| Water | 360 min | 32.584 | 0.229 | 369 |
| Catalytic | 10 min | 32.586 | 0.201 | 422 |
| Catalytic | 30 min | 32.560 | 0.205 | 414 |
| Catalytic | 90 min | 32.582 | 0.208 | 408 |
| Storage | Time | Mass Loss (%) | nSrCO3 (%) | |||
|---|---|---|---|---|---|---|
| 50–360 °C | 360–610 °C | 610–900 °C | Total | |||
| Fresh perovskite | - | 0.06 | 0.48 | 0.41 | 0.95 | 2.19 |
| Air | 26 days | 0.61 | 0.40 | 0.59 | 1.6 | 3.16 |
| Air | 47 days | 0.60 | 0.41 | 0.59 | 1.6 | 3.16 |
| Air | 68 days | 0.61 | 0.44 | 0.63 | 1.68 | 3.38 |
| Air | 89 days | 0.66 | 0.37 | 0.59 | 1.62 | 3.16 |
| Water | 30 min | 1.09 | 0.60 | 1.46 | 3.15 | 7.89 |
| Water | 120 min | 1.56 | 0.74 | 1.91 | 4.21 | 10.38 |
| Water | 360 min | 1.38 | 1.24 | 2.47 | 5.09 | 13.49 |
| Catalytic | 10 min | 1.80 | 0.60 | 1.67 | 4.07 | 9.05 |
| Catalytic | 30 min | 3.04 | 0.63 | 1.81 | 5.48 | 9.82 |
| Catalytic | 90 min | 3.73 | 0.68 | 2.38 | 6.79 | 12.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Østergaard, M.B.; Strunck, A.B.; Boffa, V.; Jørgensen, M.K. Kinetics of Strontium Carbonate Formation on a Ce-Doped SrFeO3 Perovskite. Catalysts 2022, 12, 265. https://doi.org/10.3390/catal12030265
Østergaard MB, Strunck AB, Boffa V, Jørgensen MK. Kinetics of Strontium Carbonate Formation on a Ce-Doped SrFeO3 Perovskite. Catalysts. 2022; 12(3):265. https://doi.org/10.3390/catal12030265
Chicago/Turabian StyleØstergaard, Martin B., Azeem B. Strunck, Vittorio Boffa, and Mads K. Jørgensen. 2022. "Kinetics of Strontium Carbonate Formation on a Ce-Doped SrFeO3 Perovskite" Catalysts 12, no. 3: 265. https://doi.org/10.3390/catal12030265






