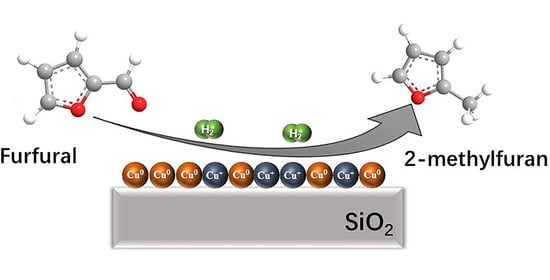
Preparation of Highly Active Cu/SiO2 Catalysts for Furfural to 2-Methylfuran by Ammonia Evaporation Method
Abstract
:1. Introduction
2. Results and Discussion
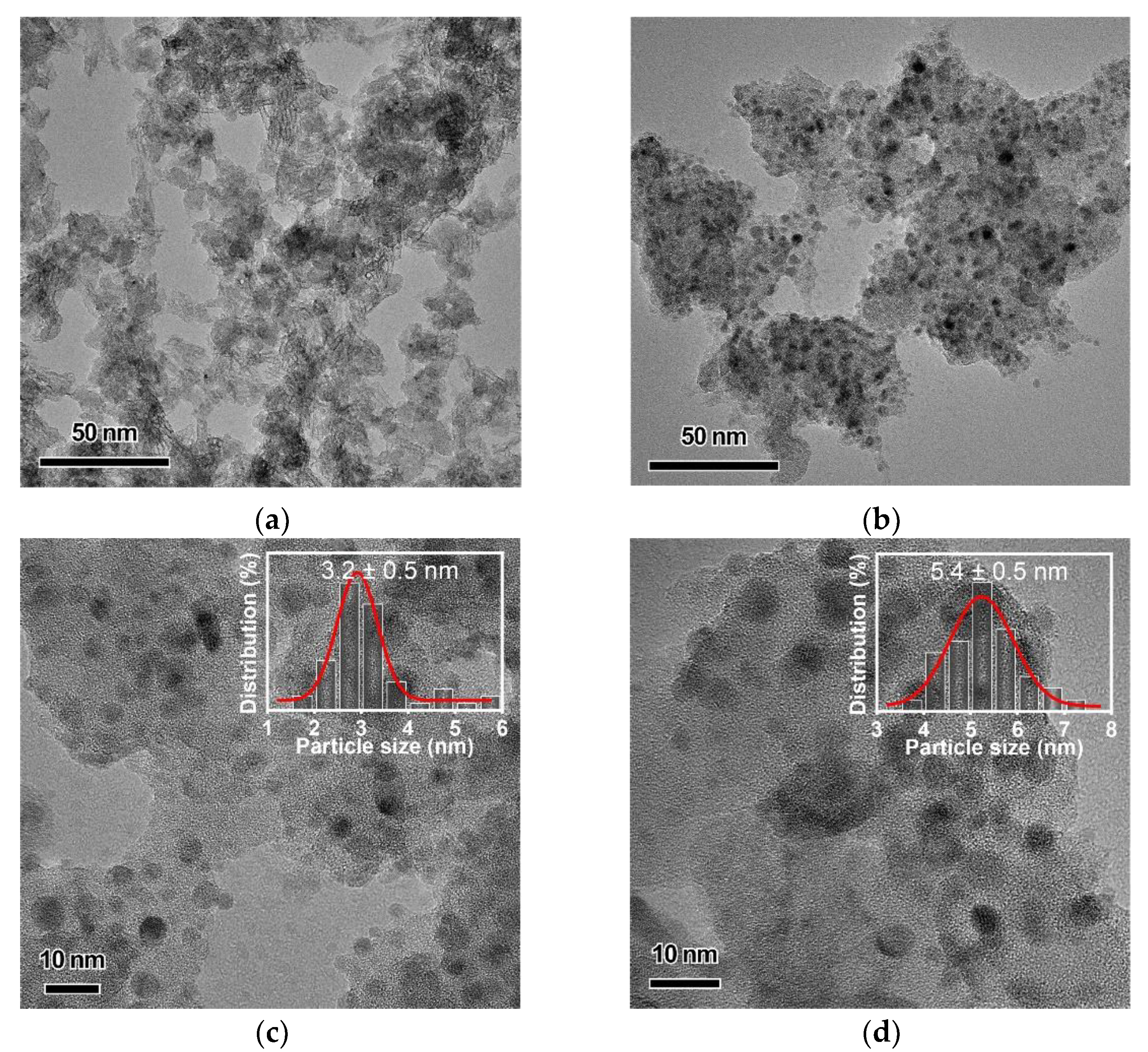
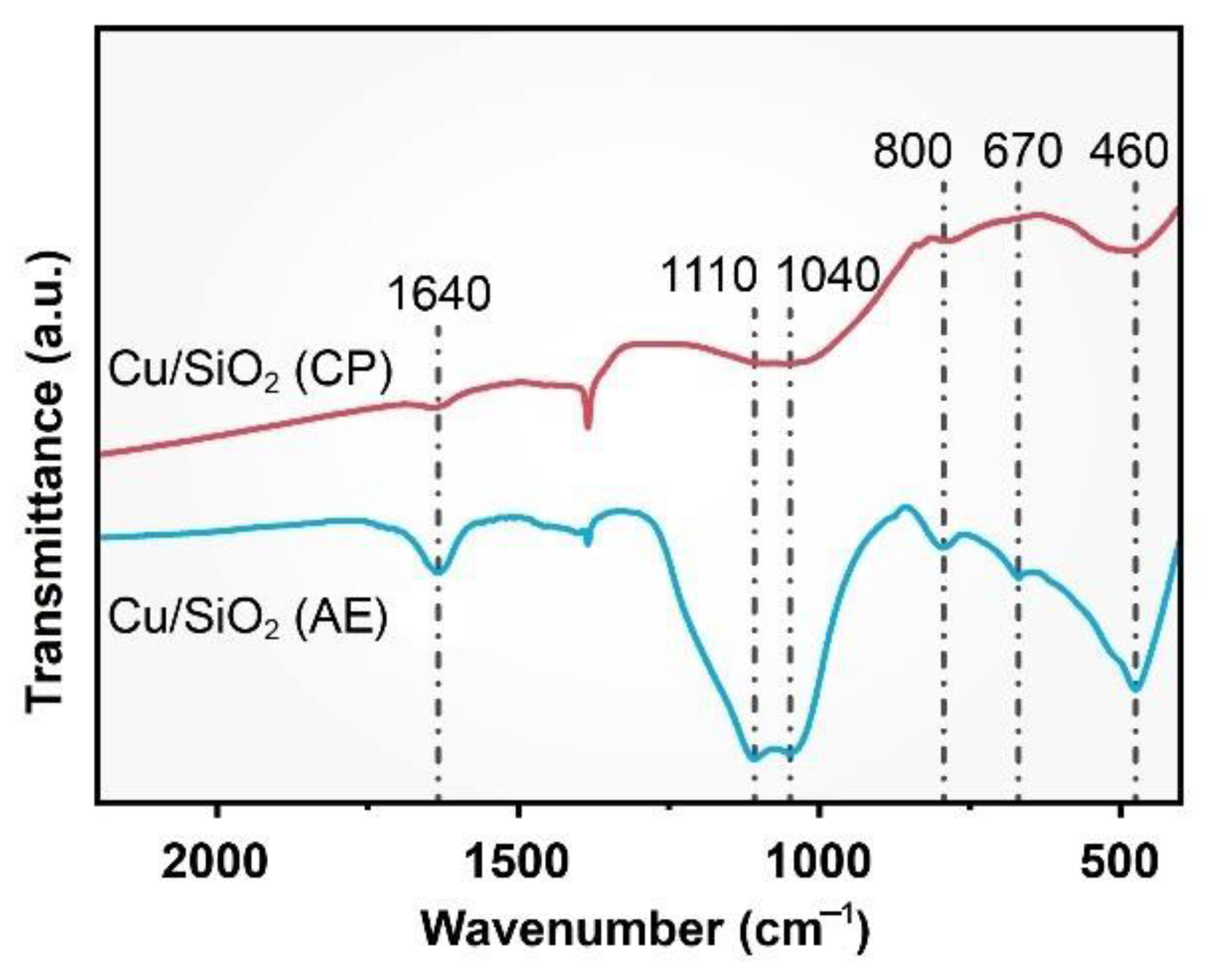
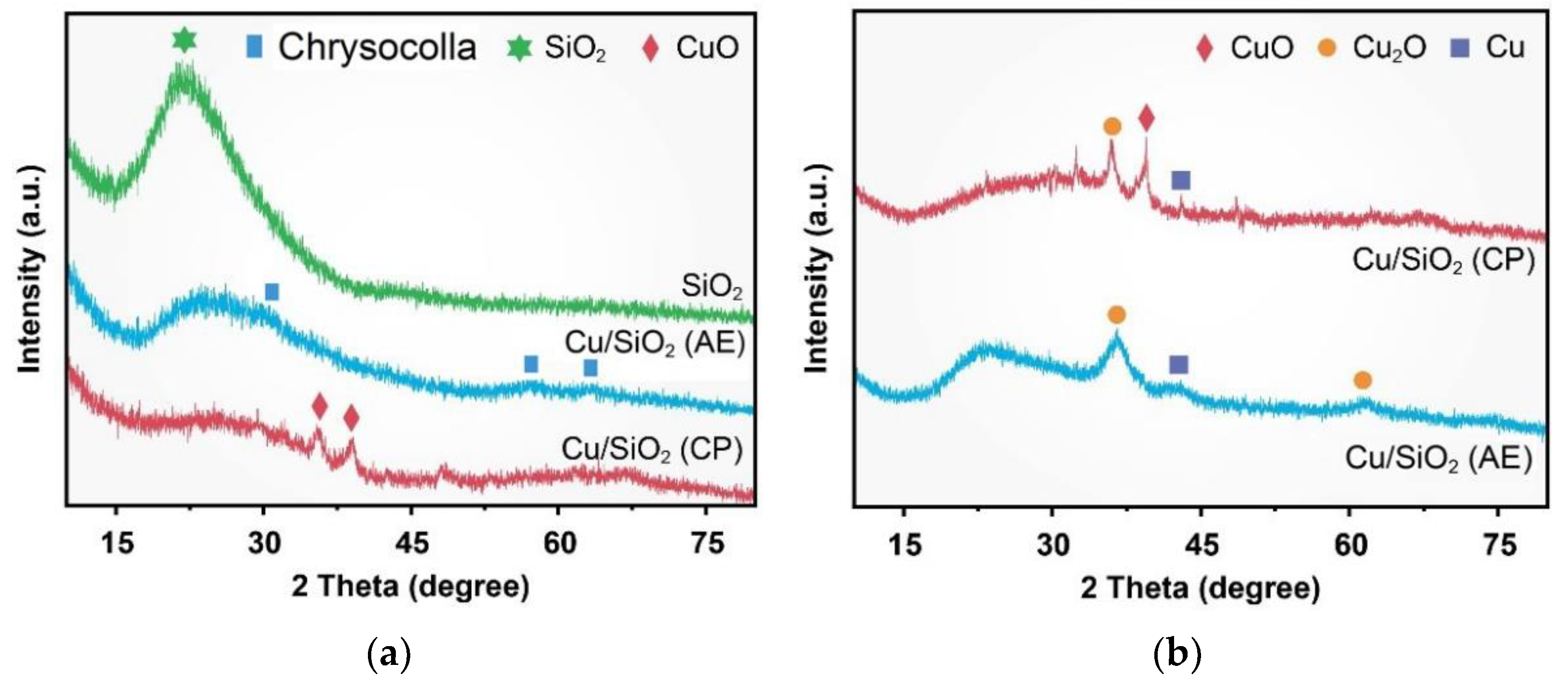
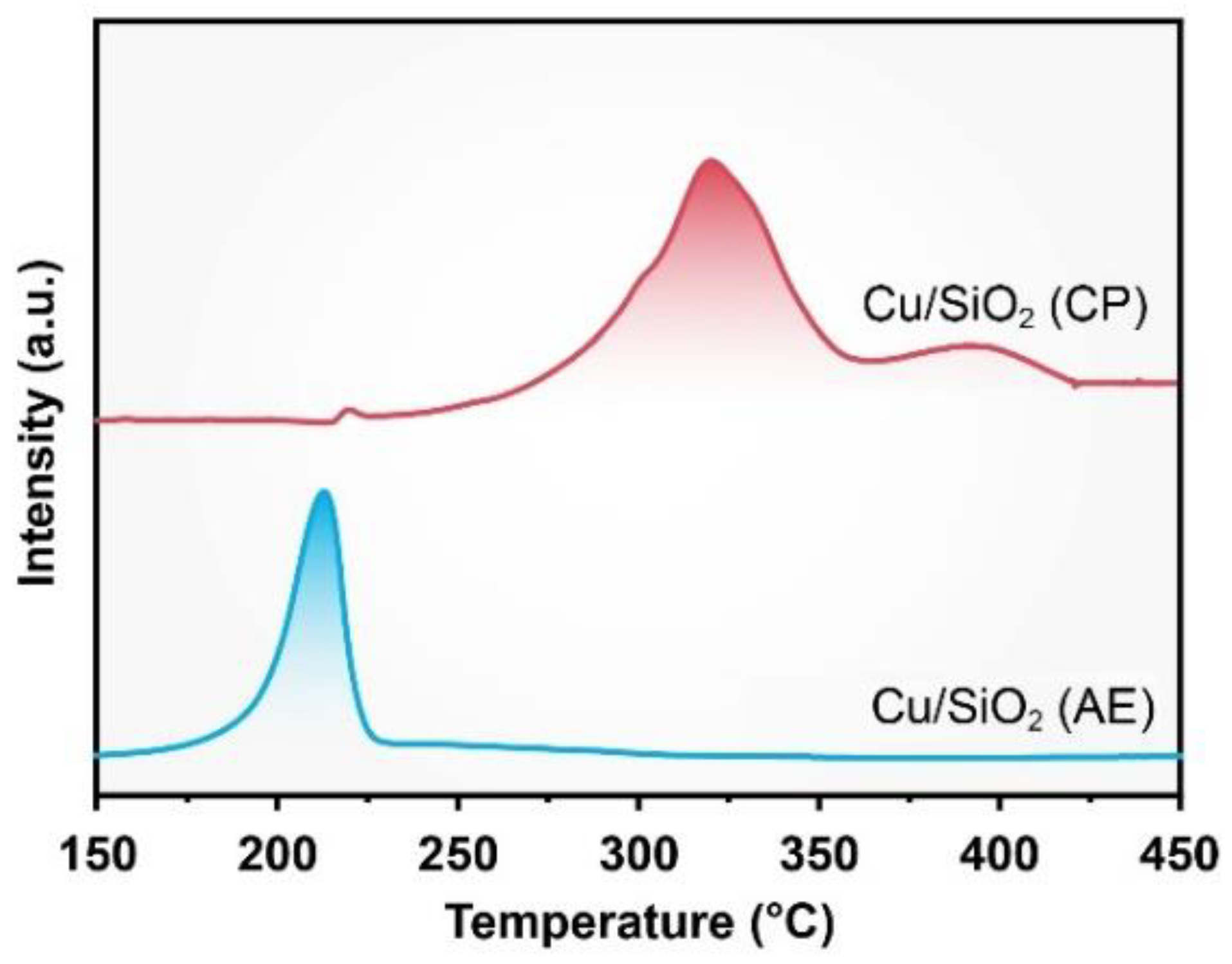
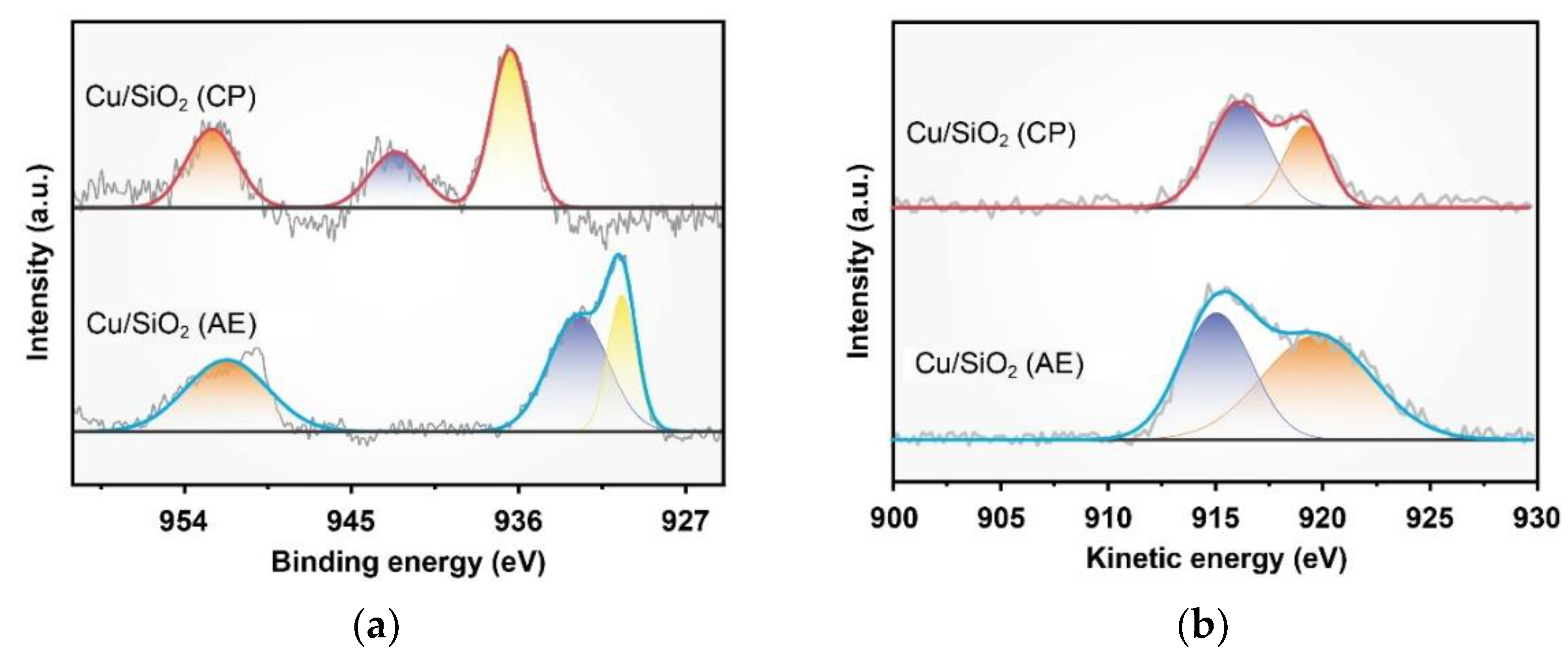
2.1. Characterization of Catalysts
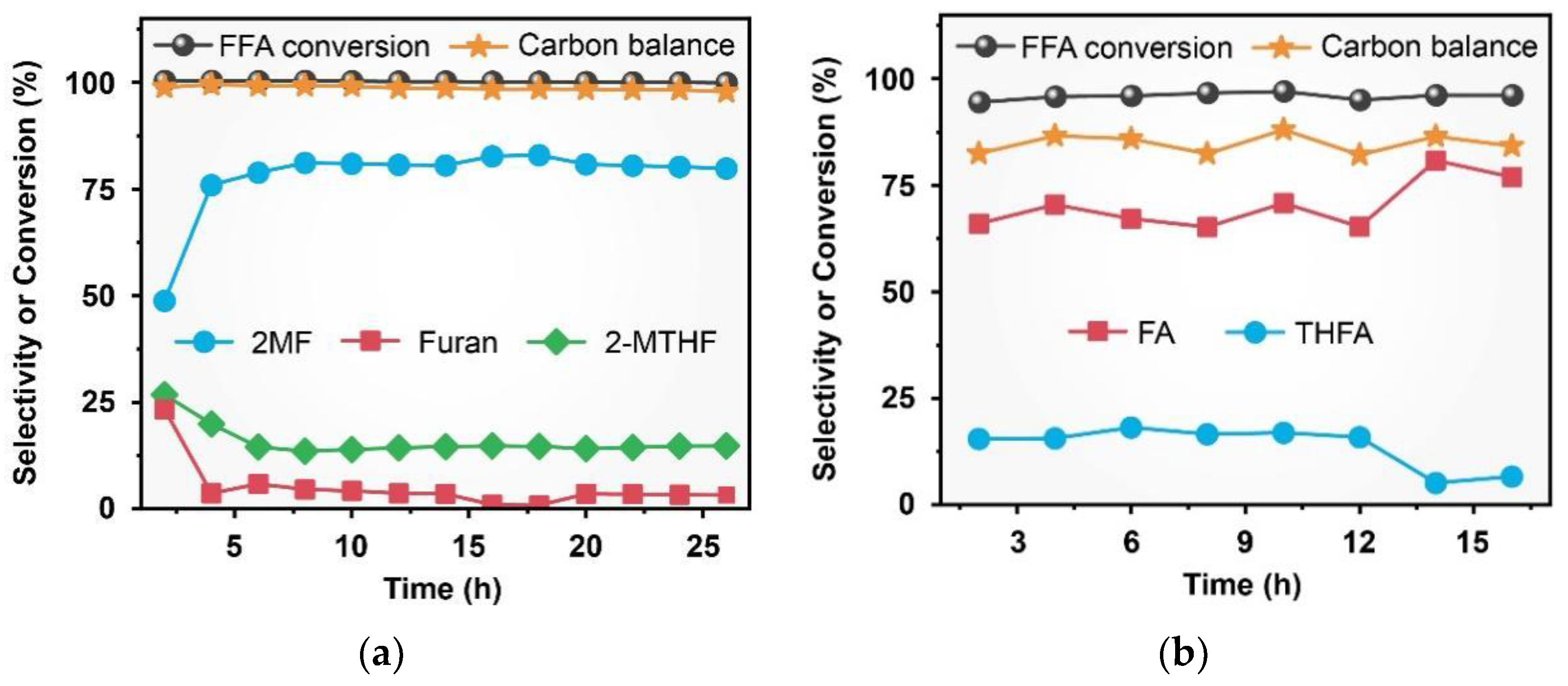
2.2. Catalytic Activity and Stability
3. Materials and Methods
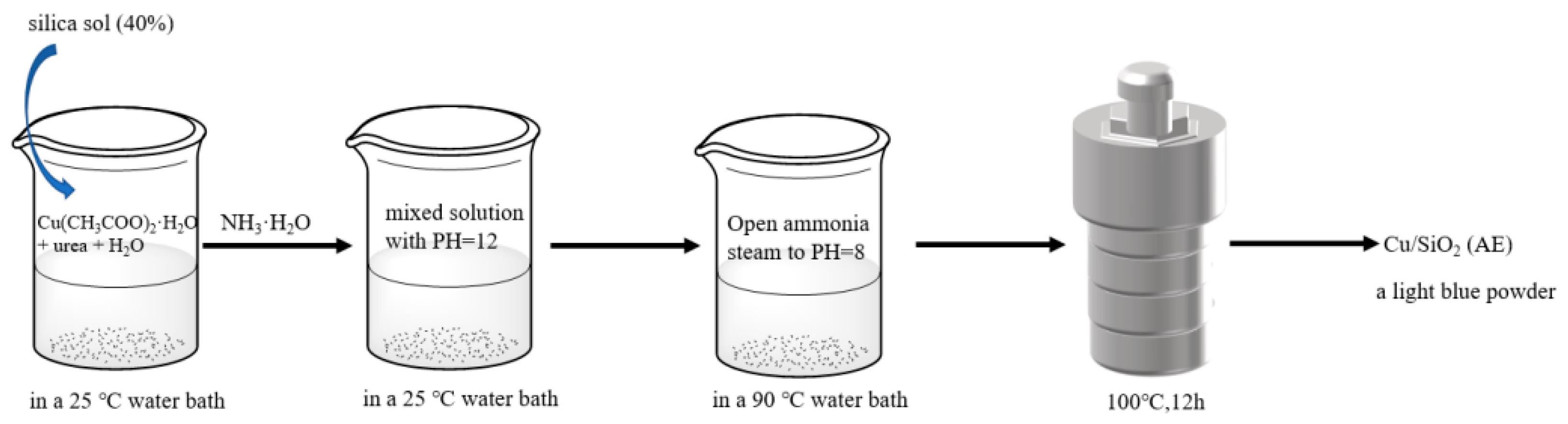
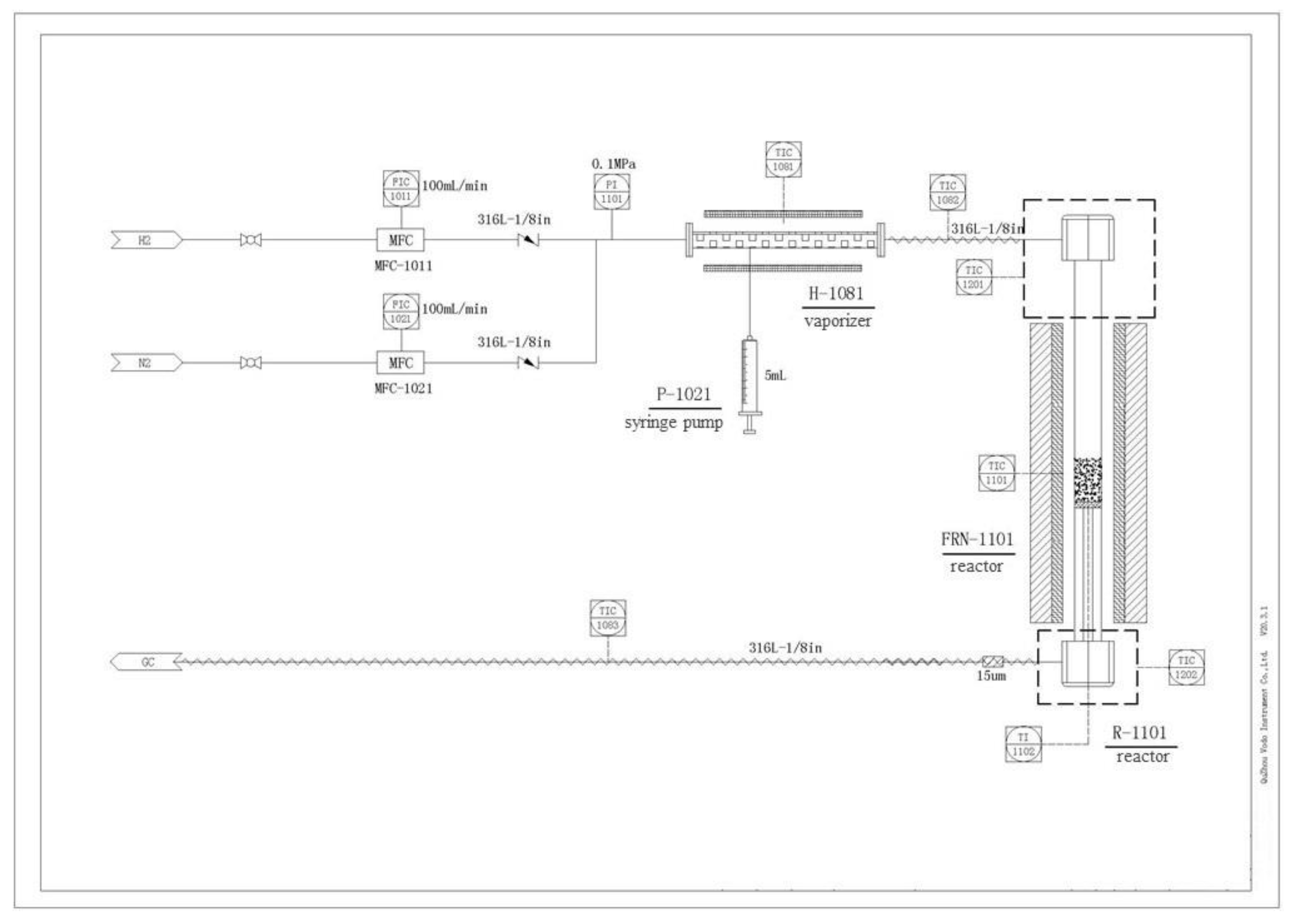
3.1. Catalyst Preparation
3.2. Characterization
3.3. Catalytic Performance Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hronec, M.; Fulajtarová, K. Selective transformation of furfural to cyclopentanone. Catal. Commun. 2012, 24, 100–104. [Google Scholar] [CrossRef]
- Jia, P.; Lan, X.; Li, X.; Wang, T. Highly Selective Hydrogenation of Furfural to Cyclopentanone over a NiFe Bimetallic Catalyst in a Methanol/Water Solution with a Solvent Effect. ACS Sustain. Chem. Eng. 2019, 7, 15221–15229. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Schnibpp, L.E.; Geller, H.H.; Korff, R.W. The Preparation of Acetopropyl Alcohol and 1,4-Pentanediol from Methylfuran. J. Am. Chem. Soc. 1947, 69, 3. [Google Scholar]
- Pace, V.; Hoyos, P.; Castoldi, L.; Maria, D.D.P.; Alcantara, A.R. 2-Methyltetrahydrofuran (2-MeTHF): A Biomass-Derived Solvent with Broad Application in Organic Chemistry. ChemSusChem 2012, 5, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, H.; Daniel, R.; Ghafourian, A.; Herreros, J.M.; Shuai, S.; Ma, X. Combustion characteristics and emissions of 2-methylfuran compared to 2,5-dimethylfuran, gasoline and ethanol in a DISI engine. Fuel 2013, 103, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Soledad, Z.M.; Martin, G.; Gustavo, M.; Carlos, Q. Furfural hydrodeoxygenation on iron and platinum catalysts. Appl. Catal. A-Gen. 2019, 587, 117217. [Google Scholar]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Wang, B.; Li, C.; He, B.; Qi, J.; Liang, C. Highly stable and selective Ru/NiFe2O4 catalysts for transfer hydrogenation of biomass-derived furfural to 2-methylfuran. J. Energy Chem. 2017, 26, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Tang, Y.; Mo, L.; Chen, P.; Lou, H.; Zheng, X. One-step hydrogenation-esterification of furfural and acetic acid over bifunctional Pd catalysts for bio-oil upgrading. Bioresour Technol. 2011, 102, 8241–8246. [Google Scholar] [CrossRef]
- Yan, K.; Chen, A. Selective hydrogenation of furfural and levulinic acid to biofuels on the ecofriendly Cu-Fe catalyst. Fuel 2014, 115, 101–108. [Google Scholar] [CrossRef]
- Bhogeswararao, S.; Srinivas, D. Catalytic conversion of furfural to industrial chemicals over supported Pt and Pd catalysts. J. Catal. 2015, 327, 65–77. [Google Scholar] [CrossRef]
- Brands, D.S.; Poels, E.K.; Bliek, A. Ester hydrogenolysis over promoted Cu/SiO2 catalysts. Appl. Catal. A-Gen. 1999, 184, 279–289. [Google Scholar] [CrossRef]
- Ding, T.; Tian, H.; Liu, J.; Wu, W.; Yu, J. Highly active Cu/SiO2 catalysts for hydrogenation of diethyl malonate to 1,3-propanediol. Chin. J. Catal. 2016, 37, 484–493. [Google Scholar] [CrossRef]
- Burnett, L.W.; Johns, I.B.; Holdern, R.F.; Hixon, R.M. Production of 2-Methylfuran by Vapor-Phase Hydrogenation of Furfural. Ind. Eng. Chem. 1948, 40, 502–505. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.; Zhu, Y.; Zheng, H.; Li, Y. Promoting effect of boron oxide on Cu/SiO2 catalyst for glycerol hydrogenolysis to 1,2-propanediol. J. Catal. 2013, 303, 70–79. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, D.; Zhu, M.; Yu, F.; Dai, B. Effect of Different Nano-Sized Silica Sols as Supports on the Structure and Properties of Cu/SiO2 for Hydrogenation of Dimethyl Oxalate. Catalysts 2017, 7, 75. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wang, D.; Dai, B. Promotive Effect of Sn2+ on Cu0/Cu+ Ratio and Stability Evolution of Cu/SiO2 Catalyst in the Hydrogenation of Dimethyl Oxalate. Catalysts 2017, 7, 122. [Google Scholar] [CrossRef] [Green Version]
- Rao, R.; Dandekar, A.; Baker, R.T.K.; Vannice, M.A. Properties of copper chromite catalysts in hydrogenation reactions. J. Catal. 1997, 171, 406–419. [Google Scholar] [CrossRef]
- Adkins, H.; Conner, R. The catalytic hydrogenation of organic compounds over copper chromite. J. Am. Chem. Soc. 1931, 53, 1091–1095. [Google Scholar] [CrossRef]
- Yang, X.; Xiang, X.; Chen, H.; Zheng, H.; Li, Y.W.; Zhu, Y. Efficient Synthesis of Furfuryl Alcohol and 2-Methylfuran from Furfural over Mineral-Derived Cu/ZnO Catalysts. ChemCatChem 2017, 9, 3023–3030. [Google Scholar] [CrossRef]
- Chang, X.; Liu, A.F.; Cai, B.; Luo, J.Y.; Pan, H.; Huang, Y.B. Catalytic Transfer Hydrogenation of Furfural to 2-Methylfuran and 2-Methyltetrahydrofuran over Bimetallic Copper–Palladium Catalysts. ChemSusChem 2016, 9, 3330–3337. [Google Scholar] [CrossRef]
- Sheng, H.B.; Lobo, R.F. Iron-Promotion of Silica-Supported Copper Catalysts for Furfural Hydrodeoxygenation. ChemCatChem 2016, 8, 3402–3408. [Google Scholar] [CrossRef]
- Park, S.; Kannapu, H.P.R.; Jeong, C.; Kim, J.; Suh, Y.W. Highly Active Mesoporous Cu-Al2O3 Catalyst for the Hydrodeoxygenation of Furfural to 2-methylfuran. ChemCatChem 2020, 12, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Li, L.; Sun, H.; Zhao, C. Selective Deoxygenation of Aqueous Furfural to 2-Methylfuran over Cu0/Cu2O·SiO2 Sites via a Copper Phyllosilicate Precursor without Extraneous Gas. ACS Sustain. Chem. Eng. 2018, 6, 12096–12103. [Google Scholar] [CrossRef]
- Dong, F.; Zhu, Y.; Zheng, H.; Zhu, Y.; Li, X.; Li, Y. Cr-free Cu-catalysts for the selective hydrogenation of biomass-derived furfural to 2-methylfuran: The synergistic effect of metal and acid sites. J. Mol. Catal. A Chem. 2015, 398, 140–148. [Google Scholar] [CrossRef]
- Chen, L.; Guo, P.; Qiao, M.; Yan, S.; Li, H.; Shen, W.; Xu, H.; Fan, K. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2008, 257, 172–180. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, F.; Kang, H.; Chen, J.; Zhang, X.; Xia, C. Highly dispersed silica-supported copper nanoparticles prepared by precipitation-gel method: A simple but efficient and stable catalyst for glycerol hydrogenolysis. Chem. Mater. 2008, 20, 5090–5099. [Google Scholar] [CrossRef]
- Natesakhawat, S.; Lekse, J.W.; Baltrus, J.P.; Ohodnicki, P.R.; Howard, B.H.; Deng, X.; Matranga, C. Active Sites and Structure–Activity Relationships of Copper-Based Catalysts for Carbon Dioxide Hydrogenation to Methanol. ACS Catal. 2012, 2, 1667–1676. [Google Scholar] [CrossRef]
- Chaminand, J.; Djakovitch, L.A.; Gallezot, P.; Marion, P.; Pinel, C.; Rosier, C.C. Glycerol hydrogenolysis on heterogeneous catalysts. Green Chem. 2004, 6, 359–361. [Google Scholar] [CrossRef]
- Toupance, T.; Kermarec, M.; Lambert, J.F.; Louis, C. Conditions of formation of copper phyllosilicates in silica-supported copper catalysts prepared by selective adsorption. J. Phys. Chem. B 2002, 106, 2277–2286. [Google Scholar] [CrossRef]
- Toupance, T.; Kermarec, M.; Louis, C. Metal particle size in silica-supported copper catalysts. Influence of the conditions of preparation and of thermal pretreatments. J. Phys. Chem. B 2000, 104, 965–972. [Google Scholar] [CrossRef]
- Wen, C.; Yin, A.; Cui, Y.; Yang, X.; Dai, W.L.; Fan, K. Enhanced catalytic performance for SiO2–TiO2 binary oxide supported Cu-based catalyst in the hydrogenation of dimethyloxalate. Appl. Catal. A-Gen. 2013, 458, 82–89. [Google Scholar] [CrossRef]
- Zhao, S.; Yue, H.; Zhao, Y.; Wang, B.; Geng, Y.; Lv, J.; Wang, S.; Gong, J.; Ma, X. Chemoselective synthesis of ethanol via hydrogenation of dimethyl oxalate on Cu/SiO2: Enhanced stability with boron dopant. J. Catal. 2013, 297, 142–150. [Google Scholar] [CrossRef]
- Yin, A.; Guo, X.; Dai, W.L.; Fan, K. The Nature of Active Copper Species in Cu-HMS Catalyst for Hydrogenation of Dimethyl Oxalate to Ethylene Glycol: New Insights on the Synergetic Effect between Cu0 and Cu+. J. Phys. Chem. C 2009, 113, 11003–11013. [Google Scholar] [CrossRef]
- Zhang, B.; Hui, S.; Zhang, S.; Ji, Y.; Li, W.; Fang, D. Effect of copper loading on texture, structure and catalytic performance of Cu/SiO2 catalyst for hydrogenation of dimethyl oxalate to ethylene glycol. J. Nat. Gas Chem. 2012, 21, 563–570. [Google Scholar] [CrossRef]
- He, Z.; Lin, H.; He, P.; Yuan, Y. Effect of boric oxide doping on the stability and activity of a Cu–SiO2 catalyst for vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2011, 277, 54–63. [Google Scholar] [CrossRef]
- Wang, D.; Yang, G.; Ma, Q.; Wu, M.; Tan, Y.; Yoneyama, Y.; Tsubaki, N. Confinement Effect of Carbon Nanotubes: Copper Nanoparticles Filled Carbon Nanotubes for Hydrogenation of Methyl Acetate. ACS Catal. 2012, 2, 1958–1966. [Google Scholar] [CrossRef]
- Gong, J.L.; Yue, H.R.; Zhao, Y.J.; Zhao, S.; Zhao, L.; Lv, J.; Wang, S.P.; Ma, X.B. Synthesis of Ethanol via Syngas on Cu/SiO2 Catalysts with Balanced Cu0–Cu+ Sites. J. Am. Chem. Soc. 2012, 134, 13922–13925. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Zhu, Y.L.; Teng, B.T.; Bai, Z.Q.; Zhang, C.H.; Xiang, H.W.; Li, Y.W. Towards understanding the reaction pathway in vapour phase hydrogenation of furfural to 2-methylfuran. J. Mol. Catal. A Chem. 2006, 246, 18–23. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A.; Franco-Duro, F.I.; Pozo, M.; Moreno-Tost, R.; Maireles-Torres, P. Promotion effect of Ce or Zn oxides for improving furfuryl alcohol yield in the furfural hydrogenation using inexpensive Cu-based catalysts. Mol. Catal. 2018, 455, 121–131. [Google Scholar] [CrossRef]
- Sitthisa, S.; Sooknoi, T.; Ma, Y.; Balbuena, P.B.; Resasco, D.E. Kinetics and mechanism of hydrogenation of furfural on Cu/SiO2 catalysts. J. Catal. 2011, 277, 1–13. [Google Scholar] [CrossRef]



| Catalyst | Cu Loading a (wt%) | SBET b (m2∙g−1) | Pore Volume c (nm) | I670/I800 d | XCu+ e (%) |
|---|---|---|---|---|---|
| Cu/SiO2 (CP) | 24 | 316.6 | 5.2 | 0 | 22 |
| Cu/SiO2 (AE) | 22 | 462.3 | 6.7 | 0.128 | 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Liu, Y.; Liu, Q.; Liu, Z.; Peng, Z. Preparation of Highly Active Cu/SiO2 Catalysts for Furfural to 2-Methylfuran by Ammonia Evaporation Method. Catalysts 2022, 12, 276. https://doi.org/10.3390/catal12030276
Fu X, Liu Y, Liu Q, Liu Z, Peng Z. Preparation of Highly Active Cu/SiO2 Catalysts for Furfural to 2-Methylfuran by Ammonia Evaporation Method. Catalysts. 2022; 12(3):276. https://doi.org/10.3390/catal12030276
Chicago/Turabian StyleFu, Xinxin, Yan Liu, Qiaoyun Liu, Zhongyi Liu, and Zhikun Peng. 2022. "Preparation of Highly Active Cu/SiO2 Catalysts for Furfural to 2-Methylfuran by Ammonia Evaporation Method" Catalysts 12, no. 3: 276. https://doi.org/10.3390/catal12030276