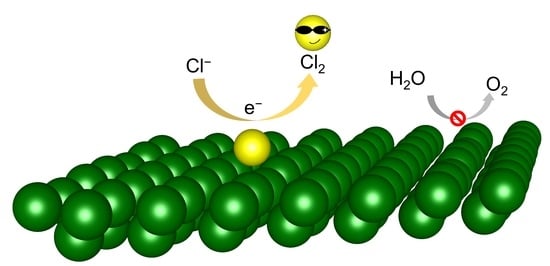
β-Arsenene Monolayer: A Promising Electrocatalyst for Anodic Chlorine Evolution Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cl Adsorption on Group-VA Monolayers
2.2. CER Activity of Group-VA Monolayers
2.3. CER Selectivity
3. Computational Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sohrabnejad-Eskan, I.; Goryachev, A.; Exner, K.S.; Kibler, L.A.; Hensen, E.J.M.; Hofmann, J.P.; Over, H. Temperature-Dependent Kinetic Studies of the Chlorine Evolution Reaction over RuO2(110) Model Electrodes. ACS Catal. 2017, 7, 2403–2411. [Google Scholar] [CrossRef]
- Karlsson, R.K.; Cornell, A. Selectivity between Oxygen and Chlorine Evolution in the Chlor-Alkali and Chlorate Processes. Chem. Rev. 2016, 116, 2982–3028. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Wiley, D.; Zhao, S.; Tang, Z. Recent advances in electrocatalytic chloride oxidation for chlorine gas production. J. Mater. Chem. A 2021, 9, 18974–18993. [Google Scholar] [CrossRef]
- Bhalothia, D.; Lin, C.-Y.; Yan, C.; Yang, Y.-T.; Chen, T.-Y. Effects of Pt metal loading on the atomic restructure and oxygen reduction reaction performance of Pt-cluster decorated Cu@Pd electrocatalysts. Sustain. Energy Fuels 2019, 3, 1668–1681. [Google Scholar] [CrossRef]
- Bhalothia, D.; Krishnia, L.; Yang, S.-S.; Yan, C.; Hsiung, W.-H.; Wang, K.-W.; Chen, T.-Y. Recent Advancements and Future Prospects of Noble Metal-Based Heterogeneous Nanocatalysts for Oxygen Reduction and Hydrogen Evolution Reactions. Appl. Sci. 2020, 10, 7708. [Google Scholar] [CrossRef]
- Bhalothia, D.; Huang, T.-H.; Chang, C.-W.; Lin, T.-H.; Wu, S.-C.; Wang, K.-W.; Chen, T.-Y. High-Performance and Stable Hydrogen Evolution Reaction Achieved by Pt Trimer Decoration on Ultralow-Metal Loading Bimetallic PtPd Nanocatalysts. ACS Appl. Energy Mater. 2020, 3, 11142–11152. [Google Scholar] [CrossRef]
- Novak, D.; Tilak, B.; Conway, B. Fundamental and applied aspects of anodic chlorine production. Mod. Asp. Electrochem. 1982, 195–318. [Google Scholar]
- Trasatti, S. Electrocatalysis: Understanding the success of DSA®. Electrochim. Acta 2000, 45, 2377–2385. [Google Scholar] [CrossRef]
- Vos, J.G.; Wezendonk, T.A.; Jeremiasse, A.W.; Koper, M.T.M. MnOx/IrOx as Selective Oxygen Evolution Electrocatalyst in Acidic Chloride Solution. J. Am. Chem. Soc. 2018, 140, 10270–10281. [Google Scholar] [CrossRef] [Green Version]
- Vos, J.G.; Koper, M.T.M. Measurement of competition between oxygen evolution and chlorine evolution using rotating ring-disk electrode voltammetry. J. Electroanal. Chem. 2018, 819, 260–268. [Google Scholar] [CrossRef]
- Exner, K.S.; Anton, J.; Jacob, T.; Over, H. Microscopic Insights into the Chlorine Evolution Reaction on RuO2(110): A Mechanistic Ab Initio Atomistic Thermodynamics Study. Electrocatalysis 2015, 6, 163–172. [Google Scholar] [CrossRef]
- Exner, K.S.; Anton, J.; Jacob, T.; Over, H. Controlling selectivity in the chlorine evolution reaction over RuO2-based catalysts. Angew. Chem. Int. Ed. 2014, 53, 11032–11035. [Google Scholar] [CrossRef] [PubMed]
- Vos, J.G.; Liu, Z.; Speck, F.D.; Perini, N.; Fu, W.; Cherevko, S.; Koper, M.T.M. Selectivity Trends Between Oxygen Evolution and Chlorine Evolution on Iridium-Based Double Perovskites in Acidic Media. ACS Catal. 2019, 9, 8561–8574. [Google Scholar] [CrossRef] [Green Version]
- Exner, K.S. Controlling Stability and Selectivity in the Competing Chlorine and Oxygen Evolution Reaction over Transition Metal Oxide Electrodes. ChemElectroChem 2019, 6, 3401–3409. [Google Scholar] [CrossRef]
- Exner, K.S.; Anton, J.; Jacob, T.; Over, H. Full Kinetics from First Principles of the Chlorine Evolution Reaction over a RuO2 (110) Model Electrode. Angew. Chem. Int. Ed. 2016, 55, 7501–7504. [Google Scholar] [CrossRef]
- Lim, T.; Kim, J.H.; Kim, J.; Baek, D.S.; Shin, T.J.; Jeong, H.Y.; Lee, K.-S.; Exner, K.S.; Joo, S.H. General Efficacy of Atomically Dispersed Pt Catalysts for the Chlorine Evolution Reaction: Potential-Dependent Switching of the Kinetics and Mechanism. ACS Catal. 2021, 11, 12232–12246. [Google Scholar] [CrossRef]
- Exner, K.S. Beyond Dimensionally Stable Anodes: Single-Atom Catalysts with Superior Chlorine Selectivity. ChemElectroChem 2020, 7, 1528–1530. [Google Scholar] [CrossRef]
- Exner, K.S. Design criteria for the competing chlorine and oxygen evolution reactions: Avoid the OCl adsorbate to enhance chlorine selectivity. Phys. Chem. Chem. Phys. 2020, 22, 22451–22458. [Google Scholar] [CrossRef]
- Exner, K.S. Beyond the Traditional Volcano Concept: Overpotential-Dependent Volcano Plots Exemplified by the Chlorine Evolution Reaction over Transition-Metal Oxides. J. Phys. Chem. C 2019, 123, 16921–16928. [Google Scholar] [CrossRef]
- Gupta, U.; Rao, C.N.R. Hydrogen generation by water splitting using MoS2 and other transition metal dichalcogenides. Nano Energy 2017, 41, 49–65. [Google Scholar] [CrossRef]
- Faber, M.S.; Jin, S. Earth-abundant inorganic electrocatalysts and their nanostructures for energy conversion applications. Energy Environ. Sci. 2014, 7, 3519–3542. [Google Scholar] [CrossRef]
- Qiu, W.; Xie, X.Y.; Qiu, J.; Fang, W.H.; Liang, R.; Ren, X.; Ji, X.; Cui, G.; Asiri, A.M.; Cui, G.; et al. High-performance artificial nitrogen fixation at ambient conditions using a metal-free electrocatalyst. Nat. Commun. 2018, 9, 3485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Ji, X.; Ren, X.; Ma, Y.; Shi, X.; Tian, Z.; Asiri, A.M.; Chen, L.; Tang, B.; Sun, X. Electrochemical Ammonia Synthesis via Nitrogen Reduction Reaction on a MoS2 Catalyst: Theoretical and Experimental Studies. Adv. Mater. 2018, 30, e1800191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhu, Y.; Tang, C.; Chen, Y.; Qian, B.; Hu, Z.; Chang, Y.C.; Pao, C.W.; Lin, Q.; Kazemi, S.A.; et al. High-Efficiency Electrosynthesis of Hydrogen Peroxide from Oxygen Reduction Enabled by a Tungsten Single Atom Catalyst with Unique Terdentate N1O2 Coordination. Adv. Funct. Mater. 2021, 2110224. [Google Scholar] [CrossRef]
- Khan, K.; Yan, X.; Yu, Q.; Bae, S.-H.; White, J.J.; Liu, J.; Liu, T.; Sun, C.; Kim, J.; Cheng, H.-M.; et al. Stone-Wales defect-rich carbon-supported dual-metal single atom sites for Zn-air batteries. Nano Energy 2021, 90, 106488. [Google Scholar] [CrossRef]
- Ramalingam, V.; Varadhan, P.; Fu, H.C.; Kim, H.; Zhang, D.; Chen, S.; Song, L.; Ma, D.; Wang, Y.; Alshareef, H.N.; et al. Heteroatom-Mediated Interactions between Ruthenium Single Atoms and an MXene Support for Efficient Hydrogen Evolution. Adv. Mater. 2019, 31, e1903841. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Q.; Wang, Y.; Liu, P.F.; Zheng, L.R.; Wang, G.; Yang, H.G.; Wong, P.K.; Zhang, H.; Zhao, H. Cobalt Covalent Doping in MoS2 to Induce Bifunctionality of Overall Water Splitting. Adv. Mater. 2018, 30, e1801450. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Ye, G.J.; Ge, Q.; Ou, X.; Wu, H.; Feng, D.; Chen, X.H.; Zhang, Y. Black phosphorus field-effect transistors. Nat Nanotechnol 2014, 9, 372–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bat-Erdene, M.; Xu, G.; Batmunkh, M.; Bati, A.S.R.; White, J.J.; Nine, M.J.; Losic, D.; Chen, Y.; Wang, Y.; Ma, T.; et al. Surface oxidized two-dimensional antimonene nanosheets for electrochemical ammonia synthesis under ambient conditions. J. Mater. Chem. A 2020, 8, 4735–4739. [Google Scholar] [CrossRef]
- Ren, X.; Li, Z.; Qiao, H.; Liang, W.; Liu, H.; Zhang, F.; Qi, X.; Liu, Y.; Huang, Z.; Zhang, D.; et al. Few-Layer Antimonene Nanosheet: A Metal-Free Bifunctional Electrocatalyst for Effective Water Splitting. ACS Appl. Energy Mater. 2019, 2, 4774–4781. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, M.; Li, F.; Yan, Z.; Li, Y.; Kan, E.; Liu, W.; Chen, Z.; Zeng, H. Semiconducting Group 15 Monolayers: A Broad Range of Band Gaps and High Carrier Mobilities. Angew. Chem. Int. Ed. 2016, 55, 1666–1669. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M.; Sofer, Z. 2D Monoelemental Arsenene, Antimonene, and Bismuthene: Beyond Black Phosphorus. Adv. Mater. 2017, 29, 1605299. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Wang, E.G.; Ting, C.S.; Su, W.P. Tight-binding theory of the electronic structures for rhombohedral semimetals. Phys. Rev. B 1993, 48, 17271–17279. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.; Jung, G.Y.; Kim, J.H.; Park, S.O.; Park, J.; Kim, Y.T.; Kang, S.J.; Jeong, H.Y.; Kwak, S.K.; Joo, S.H. Atomically dispersed Pt-N4 sites as efficient and selective electrocatalysts for the chlorine evolution reaction. Nat. Commun. 2020, 11, 412. [Google Scholar] [CrossRef]
- Exner, K.S.; Sohrabnejad-Eskan, I.; Over, H. A Universal Approach To Determine the Free Energy Diagram of an Electrocatalytic Reaction. ACS Catal. 2018, 8, 1864–1879. [Google Scholar] [CrossRef]
- Exner, K.S.; Anton, J.; Jacob, T.; Over, H. Chlorine Evolution Reaction on RuO2(110): Ab initio Atomistic Thermodynamics Study-Pourbaix Diagrams. Electrochim. Acta 2014, 120, 460–466. [Google Scholar] [CrossRef]
- Menzel, N.; Ortel, E.; Mette, K.; Kraehnert, R.; Strasser, P. Dimensionally Stable Ru/Ir/TiO2-Anodes with Tailored Mesoporosity for Efficient Electrochemical Chlorine Evolution. ACS Catal. 2013, 3, 1324–1333. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, H.; Li, Y.; Zhang, H.; Zhang, G.; Lu, Z.; Sun, X.; Jiang, L. Superaerophobic RuO2 -Based Nanostructured Electrode for High-Performance Chlorine Evolution Reaction. Small 2017, 13, 1602240. [Google Scholar] [CrossRef]
- Chen, R.; Trieu, V.; Natter, H.; Stöwe, K.; Maier, W.F.; Hempelmann, R.; Bulan, A.; Kintrup, J.; Weber, R. In situ Supported Nanoscale RuxTi1−xO2 on Anatase TiO2 with Improved Electroactivity. Chem. Mater. 2010, 22, 6215–6217. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, P.; Wang, Z.; Liu, Y.; Zheng, Z.; Zhang, Q.; Zhang, X.; Dai, Y.; Whangbo, M.-H.; Huang, B. Co3O4 nanobelt arrays assembled with ultrathin nanosheets as highly efficient and stable electrocatalysts for the chlorine evolution reaction. J. Mater. Chem. A 2018, 6, 12718–12723. [Google Scholar] [CrossRef]
- Moreno-Hernandez, I.A.; Brunschwig, B.S.; Lewis, N.S. Crystalline nickel, cobalt, and manganese antimonates as electrocatalysts for the chlorine evolution reaction. Energy Environ. Sci. 2019, 12, 1241–1248. [Google Scholar] [CrossRef]
- Liu, J.; Hinsch, J.J.; Yin, H.; Liu, P.; Zhao, H.; Wang, Y. TMN4 complex embedded graphene as efficient and selective electrocatalysts for chlorine evolution reactions. J. Electroanal. Chem. 2022, 907, 116071. [Google Scholar] [CrossRef]
- Dickens, C.F.; Nørskov, J.K. A Theoretical Investigation into the Role of Surface Defects for Oxygen Evolution on RuO2. J. Phys. Chem. C 2017, 121, 18516–18524. [Google Scholar] [CrossRef]
- Rao, R.R.; Kolb, M.J.; Halck, N.B.; Pedersen, A.F.; Mehta, A.; You, H.; Stoerzinger, K.A.; Feng, Z.; Hansen, H.A.; Zhou, H.; et al. Towards identifying the active sites on RuO2(110) in catalyzing oxygen evolution. Energy Environ. Sci. 2017, 10, 2626–2637. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Dobson, J.F.; Gould, T. Calculation of dispersion energies. J. Phys. Condens. Matter. 2012, 24, 073201. [Google Scholar] [CrossRef]
- Bjorkman, T.; Gulans, A.; Krasheninnikov, A.V.; Nieminen, R.M. Are we van der Waals ready? J. Phys. Condens. Matter 2012, 24, 424218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobson, J.F.; White, A.; Rubio, A. Asymptotics of the Dispersion Interaction: Analytic Benchmarks for van der Waals Energy Functionals. Phys. Rev. Lett. 2006, 96, 073201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.; Wang, Y.; Zhang, H.; Liu, P.; Zhao, H. Adsorption and oxidation of oxalic acid on anatase TiO2 (001) surface: A density functional theory study. J. Colloid Interface Sci. 2015, 454, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Dronskowski, R.; Bloechl, P.E. Crystal orbital Hamilton populations (COHP): Energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 1993, 97, 8617–8624. [Google Scholar] [CrossRef]
- Deringer, V.L.; Tchougreeff, A.L.; Dronskowski, R. Crystal orbital Hamilton population (COHP) analysis as projected from plane-wave basis sets. J. Phys. Chem. A 2011, 115, 5461–5466. [Google Scholar] [CrossRef]
- Maintz, S.; Deringer, V.L.; Tchougreeff, A.L.; Dronskowski, R. Analytic projection from plane-wave and PAW wavefunctions and application to chemical-bonding analysis in solids. J. Comput. Chem. 2013, 34, 2557–2567. [Google Scholar] [CrossRef]
- Maintz, S.; Deringer, V.L.; Tchougreeff, A.L.; Dronskowski, R. LOBSTER: A tool to extract chemical bonding from plane-wave based DFT. J. Comput. Chem. 2016, 37, 1030–1035. [Google Scholar] [CrossRef] [Green Version]
- Nelson, R.; Ertural, C.; George, J.; Deringer, V.L.; Hautier, G.; Dronskowski, R. LOBSTER: Local orbital projections, atomic charges, and chemical-bonding analysis from projector-augmented-wave-based density-functional theory. J. Comput. Chem. 2020, 41, 1931–1940. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the Exchange Current for Hydrogen Evolution. J. Electrochem. Soc. 2005, 152, J23. [Google Scholar] [CrossRef] [Green Version]





| Phases | a (Å) | b (Å) | d (Å) | Bonds | L (Å) | |
|---|---|---|---|---|---|---|
| P | α | 4.59 | 3.30 | 2.11 | in-plane | 2.22 |
| out-plane | 2.26 | |||||
| β | 3.27 | 3.27 | 1.24 | P−P | 2.26 | |
| As | α | 4.70 | 3.69 | 2.41 | in-plane | 2.51 |
| out-plane | 2.50 | |||||
| β | 3.60 | 3.60 | 1.40 | As−As | 2.51 | |
| Sb | α | 4.76 | 4.38 | 2.83 | in-plane | 2.94 |
| out-plane | 2.86 | |||||
| β | 4.11 | 4.11 | 1.65 | Sb−Sb | 2.89 | |
| Bi | α | 4.90 | 4.58 | 2.99 | in-plane | 3.09 |
| out-plane | 3.02 | |||||
| β | 4.33 | 4.33 | 1.73 | Bi−Bi | 3.04 | |
| Structures | rCl−X (Å) | ΔECl* (eV) | ΔGCl* (eV) | ||
|---|---|---|---|---|---|
| α | P | 2.24 | −0.315 | 0.055 | |
| As | 2.39 | −0.602 | −0.232 | ||
| Sb | 2.55 | −1.226 | −0.856 | ||
| Bi | 2.70 | −1.336 | −0.966 | ||
| β | P | Site 1 | 2.21 | −0.134 | 0.236 |
| Site 2 | 3.04 | 0.582 | 0.952 | ||
| As | Site 1 | 2.37 | −0.302 | 0.068 | |
| Site 2 | 3.07 | 0.271 | 0.641 | ||
| Sb | Site 1 | 2.51 | −0.585 | −0.215 | |
| Site 3 | 2.98 | −0.602 | −0.232 | ||
| Bi | Site 1 | 2.59 | −0.797 | −0.427 | |
| Site 2 | 3.20 | −0.799 | −0.429 | ||
| Site 3 | 3.08 | −1.091 | −0.721 | ||
| Electrocatalyst | ηexp (mV) @ 10 mA cm−2 | ηtd (V) | Ref. |
|---|---|---|---|
| commercial Ru/Ir-based | 105 | N/A | [34] |
| RuO2 (110) | 82 | 0.13 | [1,12,14,36] |
| Ru/Ir/TiO2 | >240 | N/A | [37] |
| RuO2@TiO2 | ~80 | N/A | [38] |
| Ru0.3Ti0.7O2 | ~80 | N/A | [39] |
| Co3O4 | 200 | N/A | [40] |
| CoSb2Ox | ~300 | N/A | [41] |
| Pt1/CNT | 50 | 0.09 | [16,34] |
| PtO2 (110) | N/A | 0.20 | [34] |
| PtNT/CNT | 120 | N/A | [34] |
| β-arsenene | N/A | 0.068 | This work |
| Structures | rCl−X (Å) | qCl (e) | qX (e) | Fes (eV/Å) | |
|---|---|---|---|---|---|
| A | P | 2.24 | −0.35 | 0.19 | 0.19 |
| As | 2.39 | −0.43 | 0.20 | 0.22 | |
| Sb | 2.55 | −0.51 | 0.26 | 0.29 | |
| Bi | 2.70 | −0.56 | 0.29 | 0.32 | |
| Β | P | 2.21 | −0.29 | 0.25 | 0.21 |
| As | 2.37 | −0.37 | 0.25 | 0.24 | |
| Sb | 2.51 | −0.44 | 0.35 | 0.35 | |
| Bi | 2.59 | −0.48 | 0.38 | 0.39 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Hinsch, J.J.; Yin, H.; Liu, P.; Zhao, H.; Wang, Y. β-Arsenene Monolayer: A Promising Electrocatalyst for Anodic Chlorine Evolution Reaction. Catalysts 2022, 12, 296. https://doi.org/10.3390/catal12030296
Liu J, Hinsch JJ, Yin H, Liu P, Zhao H, Wang Y. β-Arsenene Monolayer: A Promising Electrocatalyst for Anodic Chlorine Evolution Reaction. Catalysts. 2022; 12(3):296. https://doi.org/10.3390/catal12030296
Chicago/Turabian StyleLiu, Junxian, Jack Jon Hinsch, Huajie Yin, Porun Liu, Huijun Zhao, and Yun Wang. 2022. "β-Arsenene Monolayer: A Promising Electrocatalyst for Anodic Chlorine Evolution Reaction" Catalysts 12, no. 3: 296. https://doi.org/10.3390/catal12030296