Investigation on the Role of Pd, Pt, Rh in Methane Abatement for Heavy Duty Applications
Abstract
:1. Introduction
2. Methods
2.1. Measurements on Engine Test Bench
2.1.1. Catalysts
2.1.2. Engine Test Bench Setup
2.1.3. λ Ramp Measurements
2.1.4. Cold Start Measurements
2.1.5. Slow/Fast λ Oscillations
2.2. Temperature Ramp Measurements in Model Gas Reactor
3. Results and Discussions
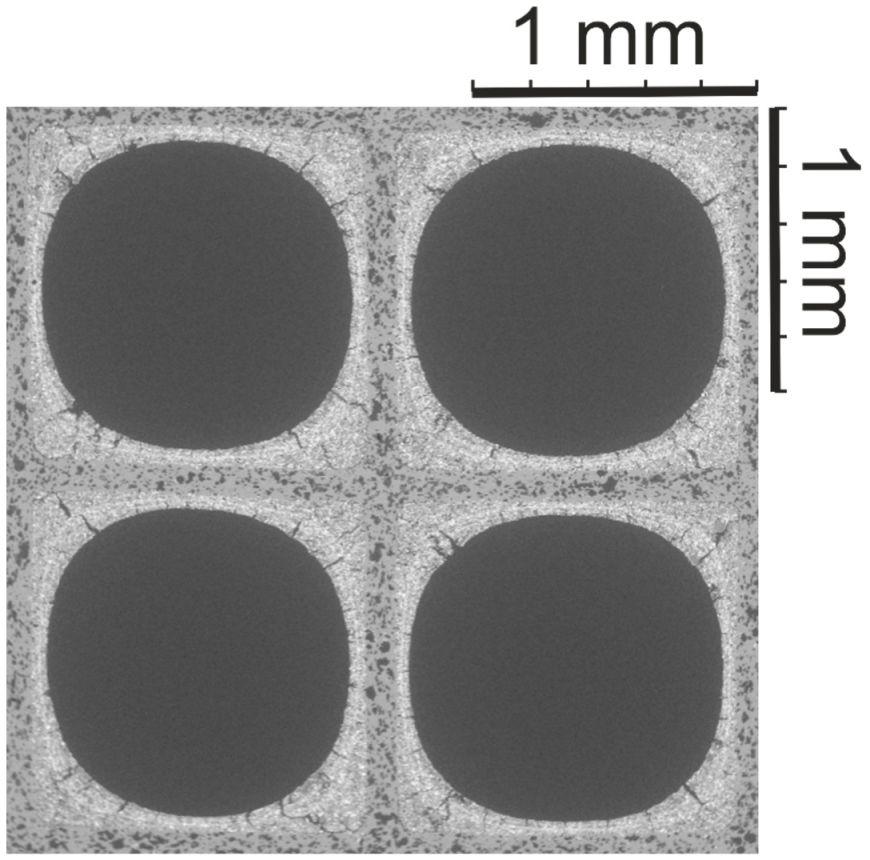
3.1. SEM Washcoat Characterization
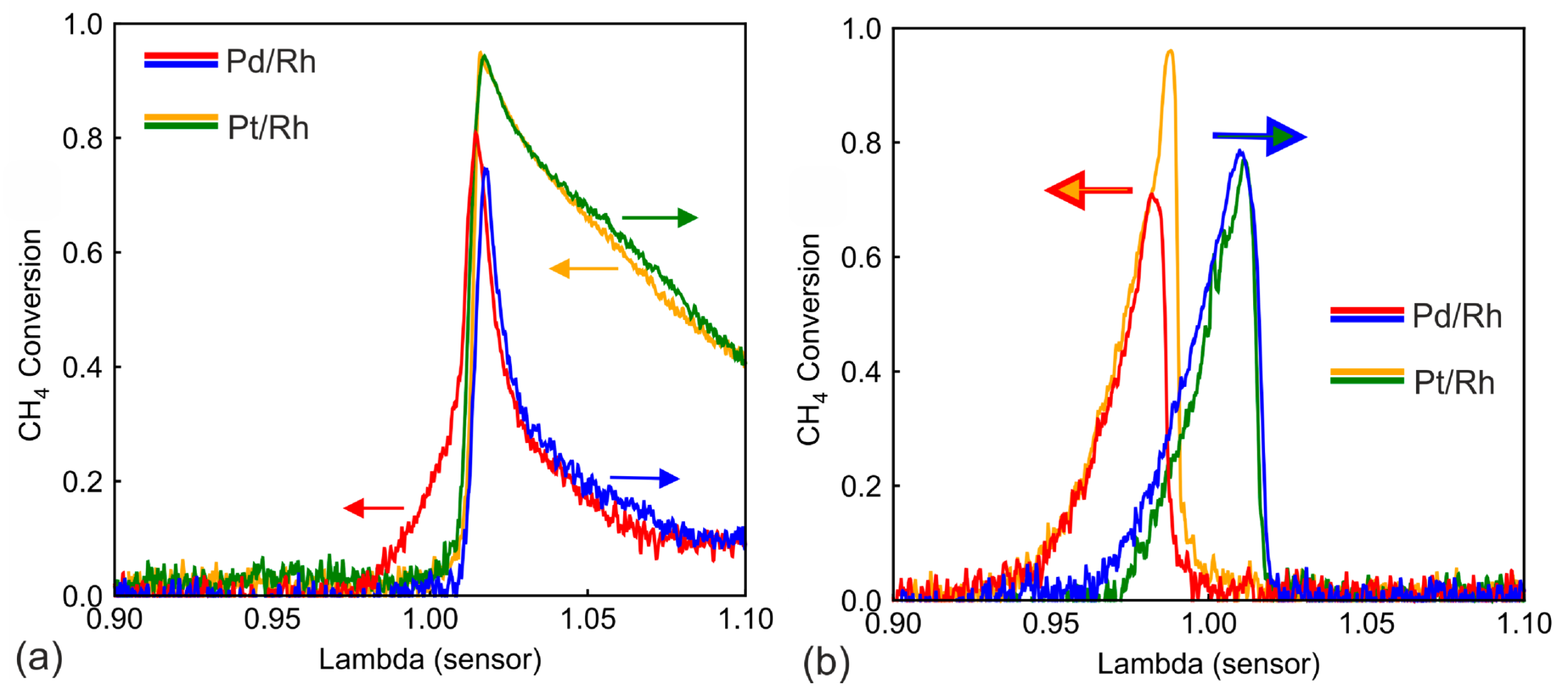
3.2. λ Ramp in the Mid Temperature Range
3.3. λ Ramps at Low and High Temperature
3.4. Analysis of Low Temperature Behavior Based on Cold Start and Temperature Ramps
3.5. Slow λ Oscillation
3.6. Conversion Characteristics of Pt/Pd/Rh Catalyst
3.7. λ Oscillations in Pt/Pd/Rh Catalyst
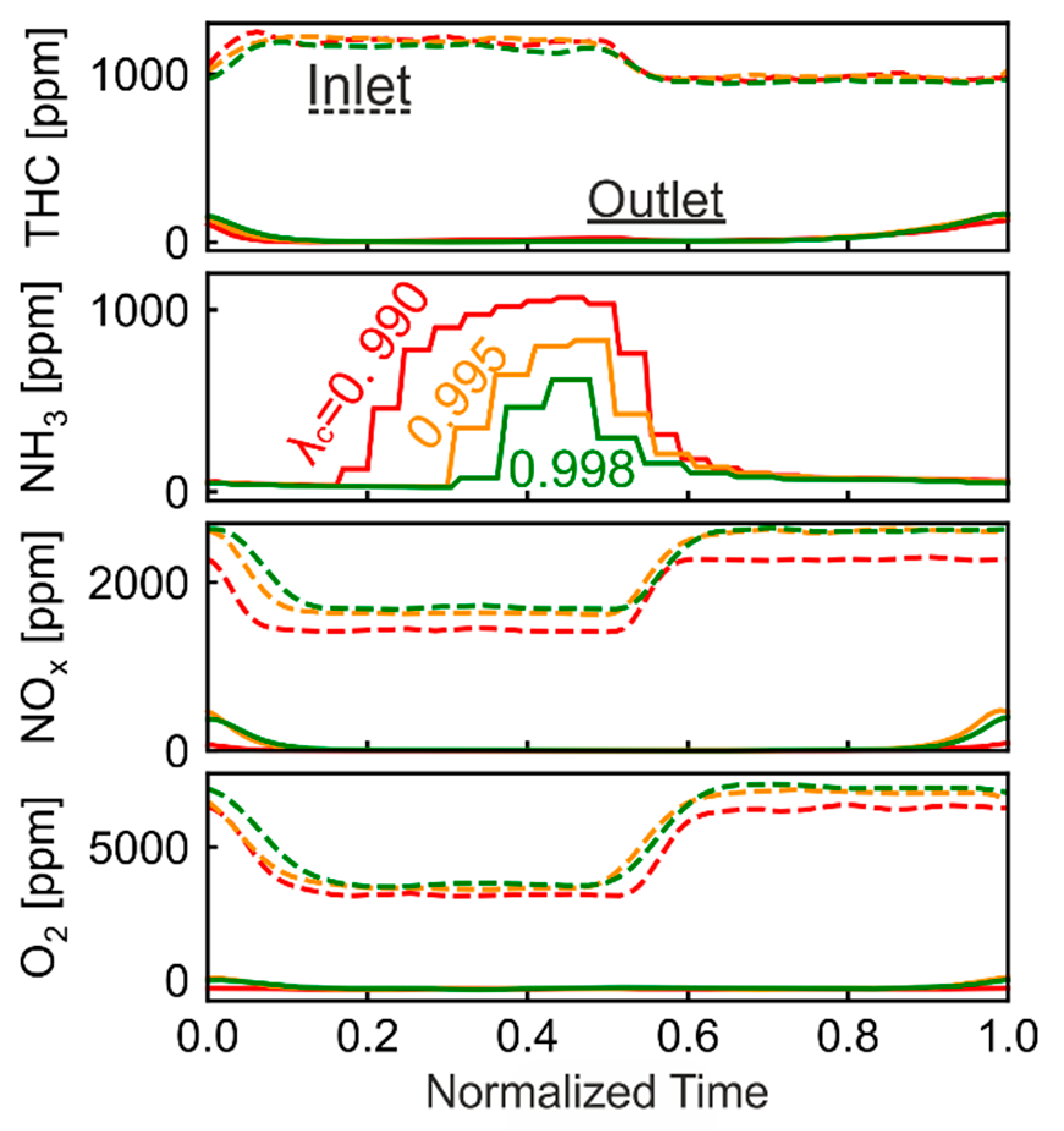
3.7.1. Optimization of λ Oscillation
3.7.2. Optimized Oscillation for the WHSC Cycle
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| λ | O2 [ppm] | NO [ppm] | H2 [ppm] | CO [ppm] | THC [ppm] | CO2 [ppm] |
|---|---|---|---|---|---|---|
| 0.90 | 2518 | 696 | 18,543 | 23,741 | 1617 | 77,330 |
| 0.91 | 2506 | 785 | 16,997 | 21,958 | 1538 | 78,733 |
| 0.91 | 2581 | 897 | 15,552 | 20,305 | 1517 | 80,033 |
| 0.93 | 2575 | 994 | 14,074 | 18,636 | 1456 | 81,367 |
| 0.94 | 2572 | 1132 | 12,587 | 16,805 | 1387 | 82,827 |
| 0.95 | 2737 | 1228 | 11,341 | 15,444 | 1347 | 83,834 |
| 0.96 | 2815 | 1369 | 9939 | 13,687 | 1291 | 85,183 |
| 0.97 | 2964 | 1519 | 8610 | 12,032 | 1237 | 86,393 |
| 0.98 | 3295 | 1666 | 7409 | 10,625 | 1194 | 87,353 |
| 0.9 | 3669 | 1856 | 6163 | 9077 | 1159 | 88,405 |
| 1.00 | 4198 | 2067 | 4891 | 7428 | 1105 | 89,414 |
| 1.01 | 4989 | 2294 | 3713 | 5937 | 1058 | 90,181 |
| 1.02 | 6033 | 2510 | 2852 | 4816 | 995 | 90,510 |
| 1.03 | 7049 | 2737 | 2130 | 3764 | 970 | 90,833 |
| 1.04 | 8004 | 2853 | 1751 | 3178 | 935 | 90,888 |
| 1.05 | 9308 | 3019 | 1397 | 2689 | 921 | 90,671 |
| 1.06 | 10,308 | 3127 | 993 | 2108 | 889 | 90,678 |
| 1.07 | 11,481 | 3253 | 720 | 1613 | 883 | 90,465 |
| 1.08 | 13,141 | 3419 | 684 | 1548 | 885 | 89,782 |
| 1.09 | 14,751 | 3455 | 664 | 1568 | 878 | 89,004 |
| 1.10 | 16,248 | 3582 | 558 | 1320 | 881 | 88,409 |
References
- Kiani, A.; Lejeune, M.; Li, C.; Patel, J.; Feron, P. Liquefied synthetic methane from ambient CO2 and renewable H2–A technoeconomic study. J. Nat. Gas Sci. Eng. 2021, 94, 104079. [Google Scholar] [CrossRef]
- Bassano, C.; Deiana, P.; Lietti, L.; Visconti, C.G. P2G movable modular plant operation on synthetic methane production from CO2 and hydrogen from renewables sources. Fuel 2019, 253, 1071–1079. [Google Scholar] [CrossRef]
- Prussi, M.; Padella, M.; Conton, M.; Postma, E.D.; Lonza, L. Review of technologies for biomethane production and assessment of Eu transport share in 2030. J. Clean. Prod. 2019, 222, 565–572. [Google Scholar] [CrossRef]
- Çabukoglu, E.; Georges, G.; Küng, L.; Pareschi, G.; Boulouchos, K. Battery electric propulsion: An option for heavy-duty vehicles? Results from a Swiss case-study. Transp. Res. Part C Emerg. Technol. 2018, 88, 107–123. [Google Scholar] [CrossRef]
- Keller, V.; Lyseng, B.; Wade, C.; Scholtysik, S.; Fowler, M.; Donald, J.; Palmer-Wilson, K.; Robertson, B.; Wild, P.; Rowe, A. Electricity system and emission impact of direct and indirect electrification of heavy-duty transportation. Energy 2019, 172, 740–751. [Google Scholar] [CrossRef]
- López, J.M.; Gómez, Á.; Aparicio, F.; Javier Sánchez, F. Comparison of GHG emissions from diesel, biodiesel and natural gas refuse trucks of the City of Madrid. Appl. Energy 2009, 86, 610–615. [Google Scholar] [CrossRef]
- Gremminger, A.; Pihl, J.; Casapu, M.; Grunwaldt, J.-D.; Toops, T.J.; Deutschmann, O. PGM based catalysts for exhaust-gas after-treatment under typical diesel, gasoline and gas engine conditions with focus on methane and formaldehyde oxidation. Appl. Catal. B Environ. 2020, 265, 118571. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, M.; Shao, S.; Li, G. Ammonia emissions of a natural gas engine at the stoichiometric operation with TWC. Appl. Therm. Eng. 2018, 130, 1363–1372. [Google Scholar] [CrossRef]
- Li, M.; Tian, H.; Wei, Z.; Zhang, Q.; Shen, B. Ammonia and nitrous oxide emissions of a stoichiometric natural gas engine operating with high caloric value and low caloric value fuels. Fuel 2021, 285, 119166. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 133/2014. Available online: http://data.europa.eu/eli/reg/2014/133/oj (accessed on 24 March 2022).
- Nevalainen, P.; Kinnunen, N.M.; Kirveslahti, A.; Kallinen, K.; Maunula, T.; Keenan, M.; Suvanto, M. Formation of NH3 and N2O in a modern natural gas three-way catalyst designed for heavy-duty vehicles: The effects of simulated exhaust gas composition and ageing. Appl. Catal. A Gen. 2018, 552, 30–37. [Google Scholar] [CrossRef]
- Shelef, M.; Graham, G.W. Why Rhodium in Automotive Three-Way Catalysts? Catal. Rev. 1994, 36, 433–457. [Google Scholar] [CrossRef]
- Zheng, T.; Lu, B.; Harle, G.; Yang, D.; Wang, C.; Zhao, Y. A comparative study of Rh-only, Pd-only and Pd/Rh catalysts. Appl. Catal. A Gen. 2020, 602, 117649. [Google Scholar] [CrossRef]
- Anderson, R.B.; Stein, K.C.; Feenan, J.J.; Hofer, L.J.E. Catalytic Oxidation of Methane. Ind. Eng. Chem. 1961, 53, 809–812. [Google Scholar] [CrossRef]
- Gélin, P.; Primet, M. Complete oxidation of methane at low temperature over noble metal based catalysts: A review. Appl. Catal. B Environ. 2002, 39, 1–37. [Google Scholar] [CrossRef]
- Franken, T.; Roger, M.; Petrov, A.W.; Clark, A.H.; Agote-Arán, M.; Krumeich, F.; Kröcher, O.; Ferri, D. Effect of Short Reducing Pulses on the Dynamic Structure, Activity, and Stability of Pd/Al2O3 for Wet Lean Methane Oxidation. ACS Catal. 2021, 11, 4870–4879. [Google Scholar] [CrossRef]
- Bounechada, D.; Groppi, G.; Forzatti, P.; Kallinen, K.; Kinnunen, T. Effect of periodic lean/rich switch on methane conversion over a Ce-Zr promoted Pd-Rh/Al2O3 catalyst in the exhausts of natural gas vehicles. Appl. Catal. B Environ. 2012, 119, 91–99. [Google Scholar] [CrossRef]
- Chin, Y.H.C.; García-Diéguez, M.; Iglesia, E. Dynamics and thermodynamics of Pd-PdO phase transitions: Effects of pd cluster size and kinetic implications for catalytic methane combustion. J. Phys. Chem. C 2016, 120, 1446–1460. [Google Scholar] [CrossRef]
- Matam, S.K.; Aguirre, M.H.; Weidenkaff, A.; Ferri, D. Revisiting the Problem of Active Sites for Methane Combustion on Pd/Al2O3 by Operando XANES in a Lab-Scale Fixed-Bed Reactor. J. Phys. Chem. C 2010, 114, 9439–9443. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Hobson, M.C.; Kennelly, T.; Waterman, E.M. Catalytic chemistry of supported palladium for combustion of methane. Appl. Catal. A Gen. 1992, 81, 227–237. [Google Scholar] [CrossRef]
- Chen, X.; Schwank, J.W.; Fisher, G.B.; Cheng, Y.; Jagner, M.; Mccabe, R.W.; Katz, M.B.; Graham, G.W.; Pan, X. Nature of the two-step temperature-programmed decomposition of PdO supported on alumina. Appl. Catal. A Gen. 2014, 475, 420–426. [Google Scholar] [CrossRef]
- Craciun, R.; Shereck, B.; Gorte, R.J. Kinetic studies of methane steam reforming on ceria-supported Pd. Catal. Lett. 1998, 51, 149–153. [Google Scholar] [CrossRef]
- Ligthart, D.A.J.M.; Van Santen, R.A.; Hensen, E.J.M. Influence of particle size on the activity and stability in steam methane reforming of supported Rh nanoparticles. J. Catal. 2011, 280, 206–220. [Google Scholar] [CrossRef]
- Feio, L.S.F.; Hori, C.E.; Damyanova, S.; Noronha, F.B.; Cassinelli, W.H.; Marques, C.M.P.; Bueno, J.M.C. The effect of ceria content on the properties of Pd/CeO2/Al2O3 catalysts for steam reforming of methane. Appl. Catal. A Gen. 2007, 316, 107–116. [Google Scholar] [CrossRef]
- Wang, X.; Gorte, R.J. A study of steam reforming of hydrocarbon fuels on Pd/ceria. Appl. Catal. A Gen. 2002, 224, 209–218. [Google Scholar] [CrossRef]
- Duarte, R.B.; Safonova, O.V.; Krumeich, F.; Makosch, M.; Van Bokhoven, J.A. Oxidation state of Ce in CeO2-promoted Rh/Al2O3 catalysts during methane steam reforming: H2O activation and alumina stabilization. ACS Catal. 2013, 3, 1956–1964. [Google Scholar] [CrossRef]
- Ferri, D.; Elsener, M.; Kröcher, O. Methane oxidation over a honeycomb Pd-only three-way catalyst under static and periodic operation. Appl. Catal. B Environ. 2018, 220, 67–77. [Google Scholar] [CrossRef]
- Wang, M.; Dimopoulos Eggenschwiler, P.; Franken, T.; Ferri, D.; Kröcher, O. Reaction pathways of methane abatement in Pd-Rh three-way catalyst in heavy duty applications: A combined approach based on exhaust analysis, model gas reactor and DRIFTS measurements. Chem. Eng. J. 2021, 422, 129932. [Google Scholar] [CrossRef]
- Wang, M.; Dimopoulos Eggenschwiler, P.; Ferri, D.; Kröcher, O. Experimental and modeling-based analysis of reaction pathways on catalysts for natural gas engines under periodic lean/rich oscillations. Chem. Eng. J. 2022, 430, 132848. [Google Scholar] [CrossRef]
- Bounechada, D.; Groppi, G.; Forzatti, P.; Kallinen, K.; Kinnunen, T. Enhanced methane conversion under periodic operation over a Pd/Rh based TWC in the exhausts from NGVs. Top. Catal. 2013, 56, 372–377. [Google Scholar] [CrossRef]
- Auckenthaler, T.; Onder, C.; Geering, H. Modelling of a Solid-Electrolyte Oxygen Sensor. SAE Tech. Pap. 2002, 2162–2171. [Google Scholar] [CrossRef]
- Weng, X.; Ren, H.; Chen, M.; Wan, H. Effect of surface oxygen on the activation of methane on palladium and platinum surfaces. ACS Catal. 2014, 4, 2598–2604. [Google Scholar] [CrossRef]
- Chin, Y.H.; Iglesia, E. Elementary steps, the role of chemisorbed oxygen, and the effects of cluster size in catalytic CH4-O2 reactions on palladium. J. Phys. Chem. C 2011, 115, 17845–17855. [Google Scholar] [CrossRef]
- Xiong, H.; Lester, K.; Ressler, T.; Schlögl, R.; Allard, L.F.; Datye, A.K. Metastable Pd↔ PdO Structures During High Temperature Methane Oxidation. Catal. Lett. 2017, 147, 1095–1103. [Google Scholar] [CrossRef]
- Matam, S.K.; Chiarello, G.L.; Lu, Y.; Weidenkaff, A.; Ferri, D. PdO x/Pd at Work in a Model Three-Way Catalyst for Methane Abatement Monitored by Operando XANES. Top. Catal. 2013, 56, 239–242. [Google Scholar] [CrossRef]
- Qi, W.; Ran, J.; Wang, R.; Du, X.; Shi, J.; Niu, J.; Zhang, P.; Ran, M. Kinetic consequences of methane combustion on Pd, Pt and Pd-Pt catalysts. RSC Adv. 2016, 6, 109834–109845. [Google Scholar] [CrossRef]
- Burch, R.; Ramli, A. A comparative investigation of the reduction of No by CH4 on Pt, Pd, and Rh catalysts. Appl. Catal. B Environ. 1998, 15, 49–62. [Google Scholar] [CrossRef]
- Adams, E.C.; Skoglundh, M.; Folic, M.; Bendixen, E.C.; Gabrielsson, P.; Carlsson, P.A. Ammonia formation over supported platinum and palladium catalysts. Appl. Catal. B Environ. 2015, 165, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Adams, E.C.; Skoglundh, M.; Gabrielsson, P.; Laurell, M.; Carlsson, P.A. Ammonia formation over Pd/Al2O3 modified with cerium and barium. Catal. Today 2016, 267, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Renème, Y.; Dhainaut, F.; Granger, P. Kinetics of the NO/H2/O2 reactions on natural gas vehicle catalysts-Influence of Rh addition to Pd. Appl. Catal. B Environ. 2012, 111, 424–432. [Google Scholar] [CrossRef]
- Liu, X.; Ruettinger, W.; Xu, X.; Farrauto, R. Deactivation of Pt/CeO2 water-gas shift catalysts due to shutdown/startup modes for fuel cell applications. Appl. Catal. B Environ. 2005, 56, 69–75. [Google Scholar] [CrossRef]
- Fu, Q.; Deng, W.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Activity and stability of low-content gold–cerium oxide catalysts for the water–gas shift reaction. Appl. Catal. B Environ. 2005, 56, 57–68. [Google Scholar] [CrossRef]
- Hilaire, S.; Wang, X.; Luo, T.; Gorte, R.J.; Wagner, J. A comparative study of water-gas-shift reaction over ceria-supported metallic catalysts. Appl. Catal. A Gen. 2004, 258, 271–276. [Google Scholar] [CrossRef]
- Jakobsen, J.G.; Jakobsen, M.; Chorkendorff, I.; Sehested, J. Methane Steam Reforming Kinetics for a Rhodium-Based Catalyst. Catal. Lett. 2010, 140, 90–97. [Google Scholar] [CrossRef]
- Tavazzi, I.; Beretta, A.; Groppi, G.; Forzatti, P. Development of a molecular kinetic scheme for methane partial oxidation over a Rh/α-Al2O3 catalyst. J. Catal. 2006, 241, 1–13. [Google Scholar] [CrossRef]
- Papetti, V.; Dimopoulos Eggenschwiler, P.; Della Torre, A.; Montenegro, G.; Onorati, A.; Ortona, A.; Koltsakis, G. Instationary heat and mass transfer phenomena in additive manufactured open cell polyhedral structures for automotive catalysis. Chem. Eng. Sci. 2021, 234, 116448. [Google Scholar] [CrossRef]
- Roy, S.; Hegde, M.S.; Sharma, S.; Lalla, N.P.; Marimuthu, A.; Madras, G. Low temperature NOx and N2O reduction by H2: Mechanism and development of new nano-catalysts. Appl. Catal. B Environ. 2008, 84, 341–350. [Google Scholar] [CrossRef]
- Ding, W.; Li, W. First-principles study of NO reduction by CO on transition metal atoms-doped CeO2(111). Chin. J. Catal. 2014, 35, 1937–1943. [Google Scholar] [CrossRef]
- Zsiga, N.; Skopil, M.A.; Wang, M.; Klein, D.; Soltic, P. Comparison of Turbocharging and Pressure Wave Supercharging of a Natural Gas Engine for Light Commercial Trucks and Vans. Energies 2021, 14, 5306. [Google Scholar] [CrossRef]
- Xu, X.; Shuai, K.; Xu, B. Review on Copper and Palladium Based Catalysts for Methanol Steam Reforming to Produce Hydrogen. Catalysts 2017, 7, 183. [Google Scholar] [CrossRef]
- Agreement Concerning the Establishing of Global Technical Regulations for Wheeled Vehicles, Equipment and Parts Which Can Be Fitted and/or Be Used on Wheeled Vehicles. Available online: https://unece.org/fileadmin/DAM/trans/main/wp29/wp29wgs/wp29gen/wp29registry/ECE-TRANS-180a4e.pdf (accessed on 24 March 2022).











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Dimopoulos Eggenschwiler, P.; Franken, T.; Agote-Arán, M.; Ferri, D.; Kröcher, O. Investigation on the Role of Pd, Pt, Rh in Methane Abatement for Heavy Duty Applications. Catalysts 2022, 12, 373. https://doi.org/10.3390/catal12040373
Wang M, Dimopoulos Eggenschwiler P, Franken T, Agote-Arán M, Ferri D, Kröcher O. Investigation on the Role of Pd, Pt, Rh in Methane Abatement for Heavy Duty Applications. Catalysts. 2022; 12(4):373. https://doi.org/10.3390/catal12040373
Chicago/Turabian StyleWang, Moyu, Panayotis Dimopoulos Eggenschwiler, Tanja Franken, Miren Agote-Arán, Davide Ferri, and Oliver Kröcher. 2022. "Investigation on the Role of Pd, Pt, Rh in Methane Abatement for Heavy Duty Applications" Catalysts 12, no. 4: 373. https://doi.org/10.3390/catal12040373






