BF3–Catalyzed Diels–Alder Reaction between Butadiene and Methyl Acrylate in Aqueous Solution—An URVA and Local Vibrational Mode Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
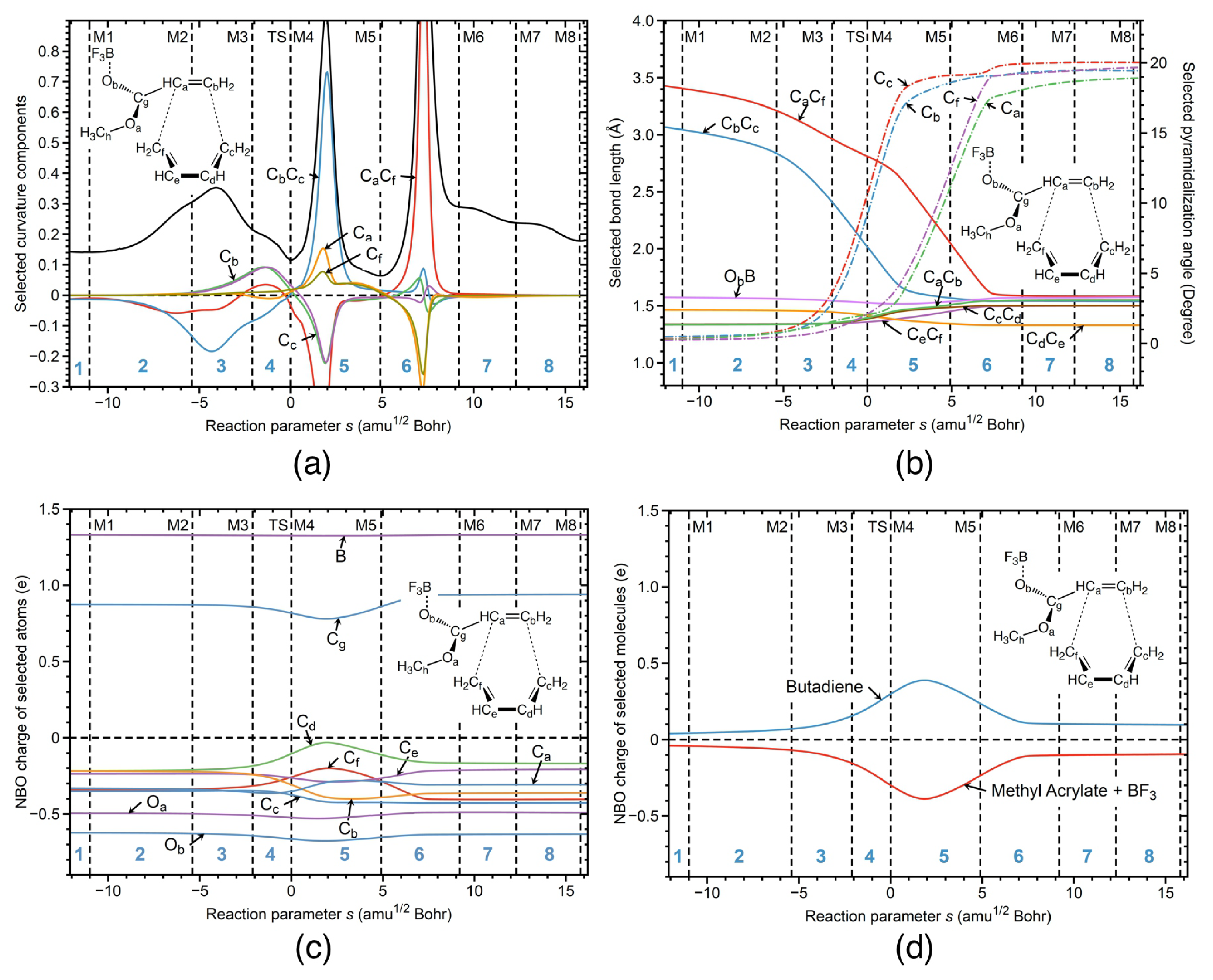
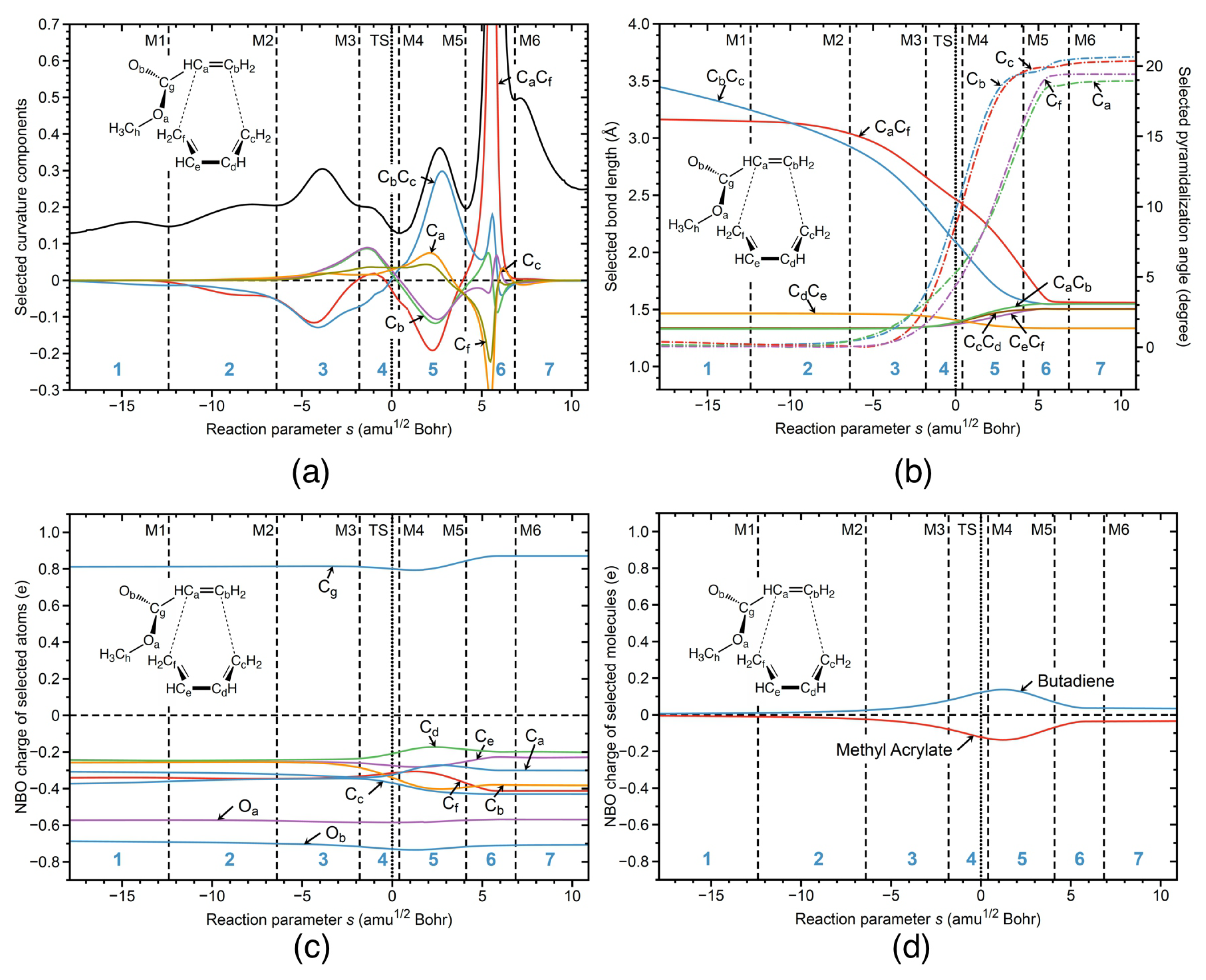
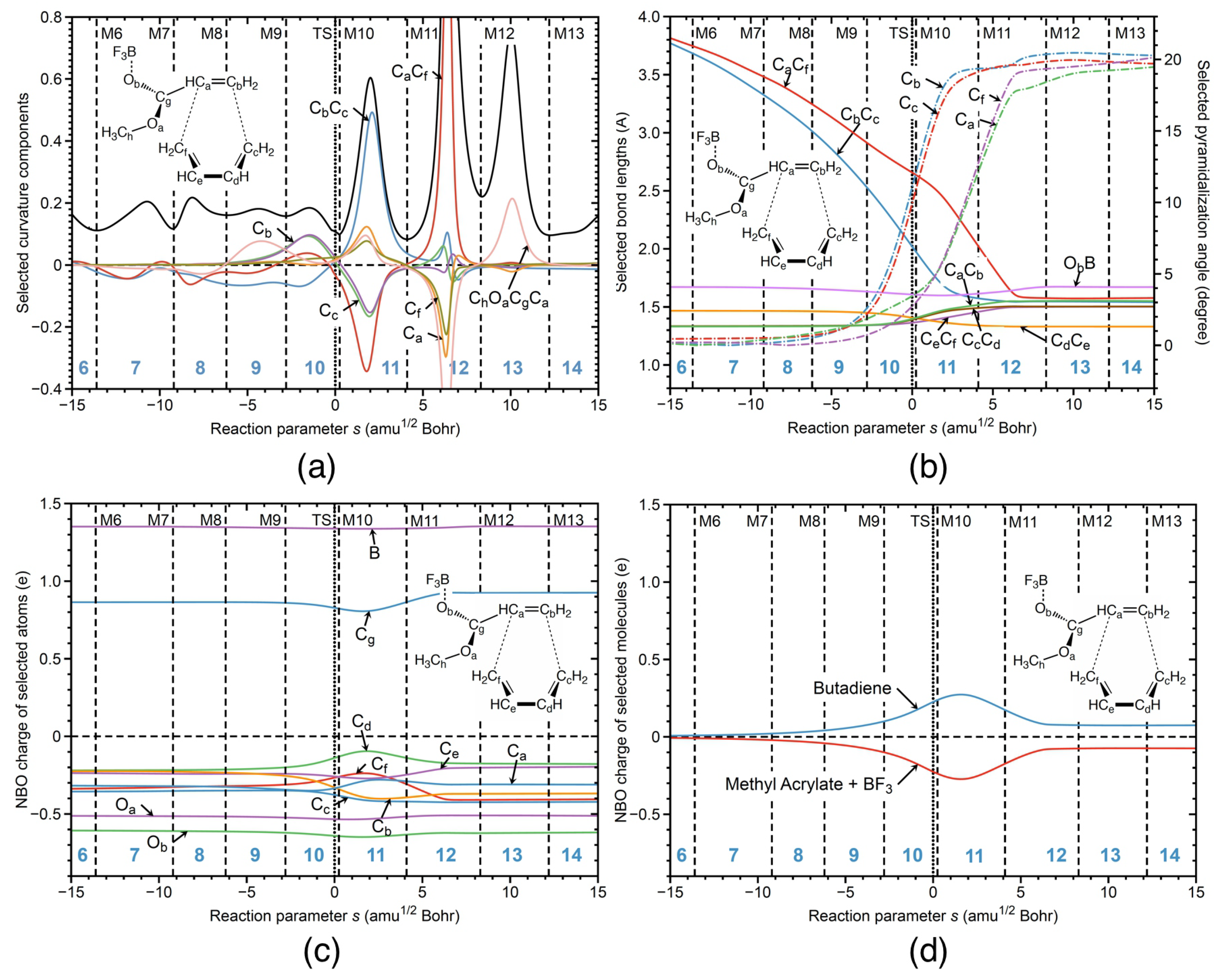
3.1. Energetics
3.2. Reaction Mechanism
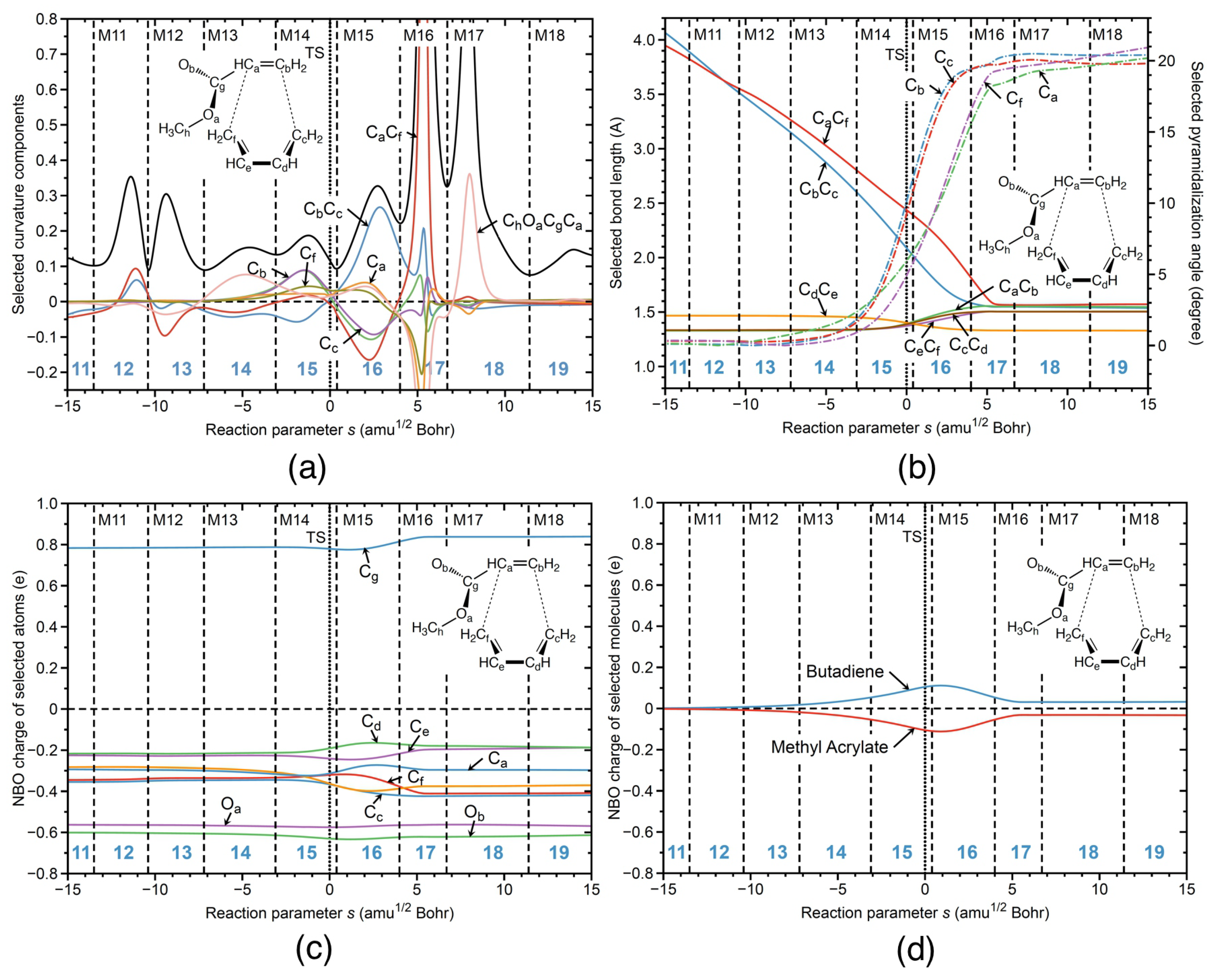
3.3. Chemical Bond Analysis
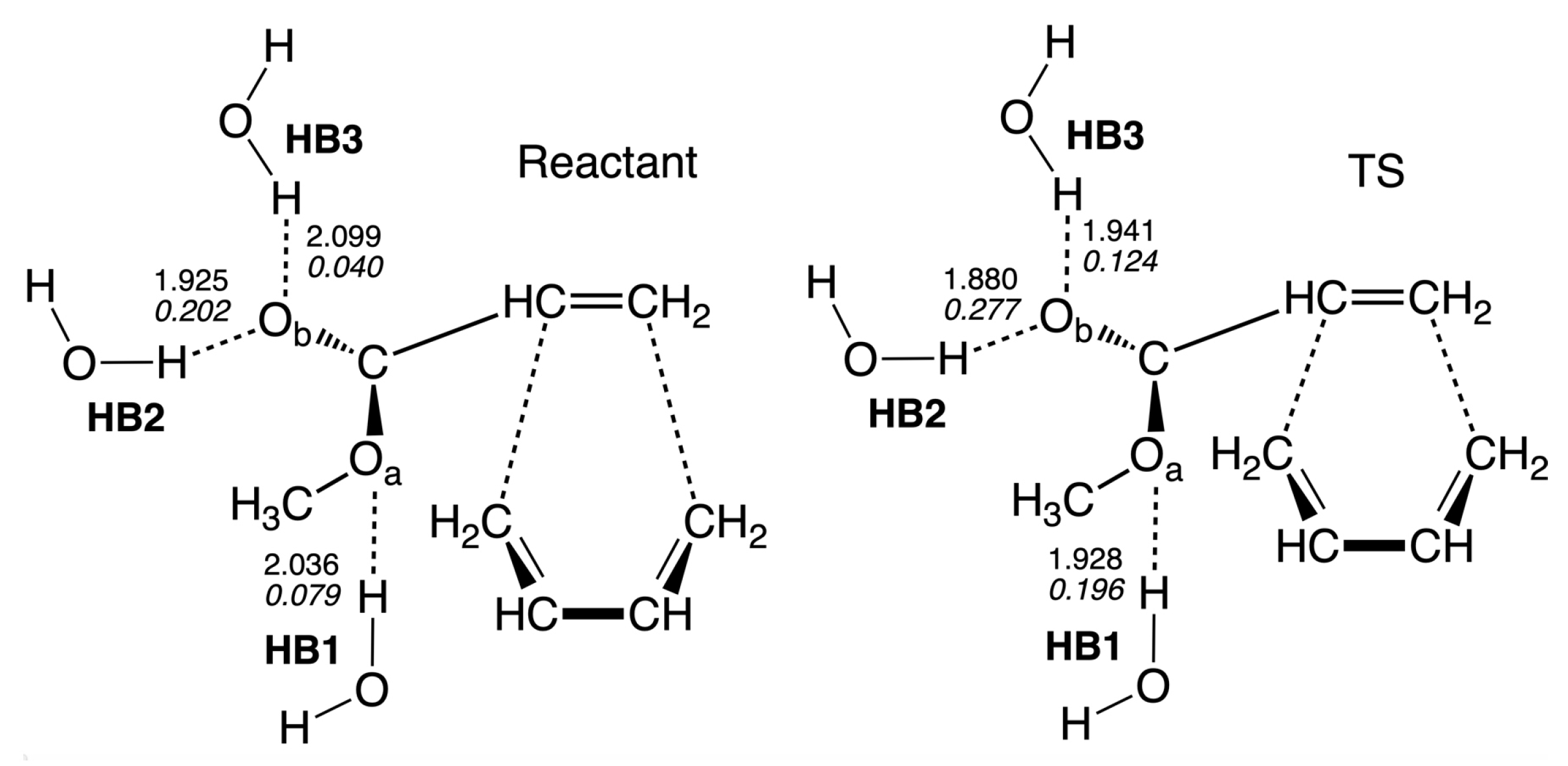
3.4. Hydrogen Bonds
3.5. Puckering Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| URVA | Unified Reaction Valley Approach |
| LMA | Local Mode Analysis |
| QM/MM | Quantum Mechanical Molecular Mechanical |
| DFT | Density Functional Theory |
| FMO | Frontier Molecular Orbitals |
| HOMO | Highest Occupied Molecular Orbital |
| LUMO | Lowest Unoccupied Molecular Orbital |
| IRC | Intrinsic Reaction Coordinate |
| CNM | Characterization of Normal Mode |
| NBO | Natural Bond Orbital |
| BSO | Bond Strength Order |
| TS | Transition State |
References
- Houk, K.N.; Liu, F.; Yang, Z.; Seeman, J.I. Evolution of the Diels–Alder Reaction Mechanism since the 1930s: Woodward, Houk with Woodward, and the Influence of Computational Chemistry on Understanding Cycloadditions. Angew. Chem. Int. Ed. 2021, 60, 12660–12681. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The Diels–Alder Reaction in Total Synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698. [Google Scholar] [CrossRef]
- Takao, K.I.; Munakata, R.; Tadano, K.I. Recent Advances in Natural Product Synthesis by Using Intramolecular Diels–Alder Reactions. Chem. Rev. 2005, 105, 4779–4807. [Google Scholar] [CrossRef] [PubMed]
- Juhl, M.; Tanner, D. Recent applications of intramolecular Diels–Alder reactions to natural product synthesis. Chem. Soc. Rev. 2009, 38, 2983–2992. [Google Scholar] [CrossRef] [PubMed]
- Funel, J.A.; Abele, S. Industrial Applications of the Diels–Alder Reaction. Angew. Chem. Int. Ed. 2013, 52, 3822–3863. [Google Scholar] [CrossRef] [PubMed]
- Settle, A.E.; Berstis, L.; Rorrer, N.A.; Roman-Leshkóv, Y.; Beckham, G.T.; Richards, R.M.; Vardon, D.R. Heterogeneous Diels–Alder catalysis for biomass–derived aromatic compounds. Green Chem. 2017, 19, 3468–3492. [Google Scholar] [CrossRef]
- Minami, A.; Oikawa, H. Recent advances of Diels–Alderases involved in natural product biosynthesis. J. Antibiot. 2016, 69, 500–506. [Google Scholar] [CrossRef]
- Oluwasanmi, A.; Hoskins, C. Potential use of the Diels–Alder reaction in biomedical and nanomedicine applications. Int. J. Pharm. 2021, 604, 120727. [Google Scholar] [CrossRef]
- Tanaka, K.; Nagase, S.; Anami, T.; Wierzbicki, M.; Urbanczyk-Lipkowska, Z. Enantioselective Diels–Alder reaction in the confined space of homochiral metal–organic frameworks. RSC Adv. 2016, 6, 111436–111439. [Google Scholar] [CrossRef] [Green Version]
- Salavati-fard, T.; Caratzoulas, S.; Lobo, R.F.; Doren, D.J. Catalysis of the Diels–Alder Reaction of Furan and Methyl Acrylate in Lewis Acidic Zeolites. ACS Catal. 2017, 7, 2240–2246. [Google Scholar] [CrossRef]
- Nayab, S.; Trouillet, V.; Gliemann, H.; Hurrle, S.; Weidler, P.G.; Rashid Tariq, S.; Goldmann, A.S.; Barner-Kowollik, C.; Yameen, B. Chemically reprogrammable metal organic frameworks (MOFs) based on Diels–Alder chemistry. Chem. Commun. 2017, 53, 11461–11464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohling, R.Y.; Tranca, I.C.; Hensen, E.J.M.; Pidko, E.A. Mechanistic Insight into the 4 + 2 Diels–Alder Cycloaddition over First Row d-Block Cation–Exchanged Faujasites. ACS Catal. 2019, 9, 376–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.L.; Huang, B.; Wu, M.X.; Zhu, Y.K.; Zhao, X.L.; Shi, X.; Yang, H.B. Post-Synthetic Modification of Metal-Organic Frameworks Bearing Phenazine Radical Cations for aza–Diels–Alder Reactions. Chem. Asian J. 2021, 16, 3985–3992. [Google Scholar] [CrossRef]
- Nayab, S.; Trouillet, V.; Gliemann, H.; Weidler, P.G.; Azeem, I.; Tariq, S.R.; Goldmann, A.S.; Barner-Kowollik, C.; Yameen, B. Reversible Diels–Alder and Michael Addition Reactions Enable the Facile Postsynthetic Modification of Metal–Organic Frameworks. Inorg. Chem. 2021, 60, 4397–4409. [Google Scholar] [CrossRef] [PubMed]
- Laschat, S. Pericyclic Reactions in Biological Systems–Does Nature Know About the Diels–Alder Reaction? Angew. Chem. Int. Ed. 1996, 35, 289–291. [Google Scholar] [CrossRef]
- Kim, H.J.; Ruszczycky, M.W.; Liu, H.w. Current developments and challenges in the search for a naturally selected Diels–Alderase. Curr. Opin. Chem. Biol. 2012, 16, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Klas, K.; Tsukamoto, S.; Sherman, D.H.; Williams, R.M. Natural Diels–Alderases: Elusive and Irresistable. J. Org. Chem. 2015, 80, 11672–11685. [Google Scholar] [CrossRef] [Green Version]
- Byrne, M.J.; Lees, N.R.; Han, L.C.; van der Kamp, M.W.; Mulholland, A.J.; Stach, J.E.M.; Willis, C.L.; Race, P.R. The Catalytic Mechanism of a Natural Diels–Alderase Revealed in Molecular Detail. J. Am. Chem. Soc. 2016, 138, 6095–6098. [Google Scholar] [CrossRef] [Green Version]
- Gagnon, K.T.; Ju, S.Y.; Goshe, M.B.; Maxwell, E.S.; Franzen, S. A role for hydrophobicity in a Diels–Alder reaction catalyzed by pyridyl–modified RNA. Nucl. Acids Res. 2009, 37, 3074–3082. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Su, C.; Du, X.; Wang, R.; Chen, S.; Zhou, Y.; Liu, C.; Liu, X.; Tian, R.; Zhang, L.; et al. FAD–dependent enzyme–catalysed intermolecular 4+2 cycloaddition in natural product biosynthesis. Nat. Chem. 2020, 12, 620–628. [Google Scholar] [CrossRef]
- Ghattas, W.; Mahy, J.P.; Réglier, M.; Simaan, A.J. Artificial Enzymes for Diels–Alder Reactions. ChemBioChem 2021, 22, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.; Woodward, R.B. Selection Rules for Concerted Cycloaddition Reactions. J. Am. Chem. Soc. 1965, 87, 2046–02048. [Google Scholar] [CrossRef]
- Woodward, R.B.; Katz, T.J. The mechanism of the Diels–Alder reaction. Tetrahedron 1959, 5, 70–89. [Google Scholar] [CrossRef]
- Kraka, E.; Wu, A.; Cremer, D. Mechanism of the Diels-Alder Reaction Studied with the United Reaction Valley Approach: Mechanistic Differences between Symmetry-Allowed and Symmetry-Forbidden Reactions. J. Phys. Chem. A 2003, 107, 9008–9021. [Google Scholar] [CrossRef]
- Hancock, R.A.; Wood, B.F. Evidence for non-biradicaloid transition states in Diels–Alder reactions. J. Chem. Soc. Chem. Comm. 1988, 351–353. [Google Scholar] [CrossRef]
- Loncharich, R.J.; Brown, F.K.; Houk, K.N. Transition structures of the Diels–Alder reaction of butadiene with acrolein. J. Org. Chem. 1989, 54, 1129–1134. [Google Scholar] [CrossRef]
- Gajewski, J.J.; Peterson, K.B.; Kagel, J.R.; Huang, Y.C.J. Transition–state structure variation in the Diels–Alder reaction from secondary deuterium kinetic isotope effects. The reaction of nearly symmetrical dienes and dienophiles is nearly synchronous. J. Am. Chem. Soc. 1989, 111, 9078–9081. [Google Scholar] [CrossRef]
- Li, Y.; Houk, K.N. Diels–Alder dimerization of 1,3-butadiene: An ab initio CASSCF study of the concerted and stepwise mechanisms and butadiene–ethylene revisited. J. Am. Chem. Soc. 1993, 115, 7478–7485. [Google Scholar] [CrossRef]
- Froese, R.D.J.; Coxon, J.M.; West, S.C.; Morokuma, K. Theoretical Studies of Diels–Alder Reactions of Acetylenic Compounds. J. Org. Chem. 1997, 62, 6991–6996. [Google Scholar] [CrossRef]
- Chen, J.S.; Houk, K.N.; Foote, C.S. Theoretical Study of the Concerted and Stepwise Mechanisms of Triazolinedione Diels–Alder Reactions. J. Am. Chem. Soc. 1998, 120, 12303–12309. [Google Scholar] [CrossRef]
- Beno, B.R.; Wilsey, S.; Houk, K.N. The C7H10 Potential Energy Landscape: Concerted Transition States and Diradical Intermediates for the Retro-Diels–Alder Reaction and 1,3 Sigmatropic Shifts of Norbornene. J. Am. Chem. Soc. 1999, 121, 4816–4826. [Google Scholar] [CrossRef]
- Houk, K.N. Generalized frontier orbitals of alkenes and dienes. Regioselectivity in Diels–Alder reactions. J. Am. Chem. Soc. 1973, 95, 4092–4094. [Google Scholar] [CrossRef]
- Houk, K.N.; Strozier, R.W. Lewis acid catalysis of Diels–Alder reactions. J. Am. Chem. Soc. 1973, 95, 4094–4096. [Google Scholar] [CrossRef]
- Houk, K.N. Frontier molecular orbital theory of cycloaddition reactions. Acc. Chem. Res. 1975, 8, 361–369. [Google Scholar] [CrossRef]
- Black, K.; Liu, P.; Xu, L.; Doubleday, C.; Houk, K.N. Dynamics, transition states, and timing of bond formation in Diels–Alder reactions. Proc. Natl. Acad. Sci. USA 2012, 109, 12860. [Google Scholar] [CrossRef] [Green Version]
- Inukai, T.; Kojima, T. Aluminum chloride catalyzed diene condensation. IV. Kinetic study of butadiene–methyl acrylate reaction. J. Org. Chem. 1967, 32, 872–875. [Google Scholar] [CrossRef]
- Pindur, U.; Lutz, G.; Otto, C. Acceleration and selectivity enhancement of Diels–Alder reactions by special and catalytic methods. Chem. Rev. 1993, 93, 741–761. [Google Scholar] [CrossRef]
- Bakos, M.; Dobi, Z.; Fegyverneki, D.; Gyömöre, Á.; Fernández, I.; Soós, T. Janus Face of the Steric Effect in a Lewis Acid Catalyst with Size-Exclusion Design: Steric Repulsion and Steric Attraction in the Catalytic Exo–Selective Diels–Alder Reaction. ACS Sustain. Chem. Eng. 2018, 6, 10869–10875. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Lim, D.; Blake, J.F. Ab initio study of Diels–Alder reactions of cyclopentadiene with ethylene, isoprene, cyclopentadiene, acrylonitrile, and methyl vinyl ketone. J. Am. Chem. Soc. 1993, 115, 2936–2942. [Google Scholar] [CrossRef]
- García, J.; Mayoral, J.; Salvatella, L. Is It 4 + 2 or 2 + 4? A New Look at Lewis Acid Catalyzed Diels–Alder Reactions. J. Am. Chem. Soc. 1996, 118, 11680–11681. [Google Scholar] [CrossRef]
- García, J.I.; Martínez-Merino, V.; Mayoral, J.A.; Salvatella, L. Density Functional Theory Study of a Lewis Acid Catalyzed Diels–Alder Reaction. The Butadiene + Acrolein Paradigm. J. Am. Chem. Soc. 1998, 120, 2415–2420. [Google Scholar] [CrossRef]
- Levandowski, B.J.; Houk, K.N. Theoretical Analysis of Reactivity Patterns in Diels–Alder Reactions of Cyclopentadiene, Cyclohexadiene, and Cycloheptadiene with Symmetrical and Unsymmetrical Dienophiles. J. Org. Chem. 2015, 80, 3530–3537. [Google Scholar] [CrossRef] [PubMed]
- Sakata, K.; Fujimoto, H. Roles of Lewis Acid Catalysts in Diels–Alder Reactions between Cyclopentadiene and Methyl Acrylate. ChemistryOpen 2020, 9, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Vermeeren, P.; Hamlin, T.A.; Fernández, I.; Bickelhaupt, F.M. How Lewis Acids Catalyze Diels–Alder Reactions. Angew. Chem. In. Ed. 2020, 59, 6201–6206. [Google Scholar] [CrossRef]
- Rideout, D.C.; Breslow, R. Hydrophobic acceleration of Diels–Alder reactions. J. Am. Chem. Soc. 1980, 102, 7816–7817. [Google Scholar] [CrossRef]
- Blokzijl, W.; Blandamer, M.J.; Engberts, J.B.F.N. Diels–Alder reactions in aqueous solutions. Enforced hydrophobic interactions between diene and dienophile. J. Am. Chem. Soc. 1991, 113, 4241–4246. [Google Scholar] [CrossRef]
- Breslow, R. Hydrophobic effects on simple organic reactions in water. Acc. Chem. Res. 1991, 24, 159–164. [Google Scholar] [CrossRef]
- Cativiela, C.; García, J.; Gil, J.; Martínez, R.; Mayoral, J.; Salvatella, L.; Urieta, J.; Mainar, A.; Abraham, M. Solvent effects on Diels–Alder reactions. The use of aqueous mixtures of fluorinated alcohols and the study of reactions of acrylonitrile. J. Chem. Soc. Perkin Trans. 1997, 4, 653–660. [Google Scholar] [CrossRef]
- Wijnen, J.W.; Engberts, J.B.F.N. Retro-Diels–Alder Reaction in Aqueous Solution: Toward a Better Understanding of Organic Reactivity in Water. J. Org. Chem. 1997, 62, 2039–2044. [Google Scholar] [CrossRef]
- Hayashi, Y.; Samanta, S.; Gotoh, H.; Ishikawa, H. Asymmetric Diels–Alder Reactions of α,β–Unsaturated Aldehydes Catalyzed by a Diarylprolinol Silyl Ether Salt in the Presence of Water. Angew. Chem. Int. Ed. 2008, 47, 6634–6637. [Google Scholar] [CrossRef]
- Kiselev, V.D.; Kashaeva, H.A.; Potapova, L.N.; Kornilov, D.A.; Latypova, L.I.; Konovalov, A.I. Hydrophobic acceleration in the Diels–Alder reaction of 9–hydroxymethylanthracene with N–phenylmaleimide. Russ. Chem. Bull. 2016, 65, 2202–2205. [Google Scholar] [CrossRef]
- Narayan, S.; Muldoon, J.; Finn, M.G.; Fokin, V.V.; Kolb, H.C.; Sharpless, K.B. “On Water”: Unique Reactivity of Organic Compounds in Aqueous Suspension. Angew. Chem. Int. Ed. 2005, 44, 3275–3279. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Engberts, J.B.F.N. Diels–Alder reactions in water. Pure Appl. Chem. 2000, 72, 1365–1372. [Google Scholar] [CrossRef] [Green Version]
- Otto, S.; Engberts, J.B.F.N. Hydrophobic interactions and chemical reactivity. Org. Biomol. Chem. 2003, 1, 2809–2820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Windmon, N.; Dragojlovic, V. Diels–Alder reactions in the presence of a minimal amount of water. Green Chem. Lett. Rev. 2008, 1, 155–163. [Google Scholar] [CrossRef]
- Shrinidhi, A. Diels–Alder Reaction with Hydrophilic Dienes and Dienophiles. ChemistrySelect 2016, 1, 3016–3021. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, X.; Han, Y.; Yan, P.; Bie, F.; Cao, H. Diels–Alder reactions between cyclopentadiene analogs and benzoquinone in water and their application in the synthesis of polycyclic cage compounds. RSC Adv. 2020, 10, 739–745. [Google Scholar] [CrossRef] [Green Version]
- Blake, J.F.; Lim, D.; Jorgensen, W.L. Enhanced Hydrogen Bonding of Water to Diels–Alder Transition States. Ab Initio Evidence. J. Org. Chem. 1994, 59, 803–805. [Google Scholar] [CrossRef]
- Furlani, T.R.; Gao, J. Hydrophobic and Hydrogen–Bonding Effects on the Rate of Diels–Alder Reactions in Aqueous Solution. J. Org. Chem. 1996, 61, 5492–5497. [Google Scholar] [CrossRef]
- Harano, Y.; Sato, H.; Hirata, F. A theoretical study on a Diels–Alder reaction in ambient and supercritical water: Viewing solvent effects through frontier orbitals. Chem. Phys. 2000, 258, 151–161. [Google Scholar] [CrossRef]
- Harano, Y.; Sato, H.; Hirata, F. Solvent Effects on a Diels–Alder Reaction in Supercritical Water: RISM-SCF Study. J. Am. Chem. Soc. 2000, 122, 2289–2293. [Google Scholar] [CrossRef]
- Kong, S.; Evanseck, J.D. Density Functional Theory Study of Aqueous–Phase Rate Acceleration and Endo/Exo Selectivity of the Butadiene and Acrolein Diels–Alder Reaction. J. Am. Chem. Soc. 2000, 122, 10418–10427. [Google Scholar] [CrossRef]
- Chandrasekhar, J.; Shariffskul, S.; Jorgensen, W.L. QM/MM Simulations for Diels–Alder Reactions in Water: Contribution of Enhanced Hydrogen Bonding at the Transition State to the Solvent Effect. J. Phys. Chem. B 2002, 106, 8078–8085. [Google Scholar] [CrossRef]
- Acevedo, O.; Jorgensen, W.L. Understanding Rate Accelerations for Diels–Alder Reactions in Solution Using Enhanced QM/MM Methodology. J. Chem. Theory Comput. 2007, 3, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.L.; Tirado-Rives, J.; Jorgensen, W.L. Quantum Mechanical/Molecular Mechanical Modeling Finds Diels–Alder Reactions Are Accelerated Less on the Surface of Water Than in Water. J. Am. Chem. Soc. 2010, 132, 3097–3104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Doubleday, C.; Houk, K.N. QM/MM Protocol for Direct Molecular Dynamics of Chemical Reactions in Solution: The Water–Accelerated Diels–Alder Reaction. J. Chem. Theory Comput. 2015, 11, 5606–5612. [Google Scholar] [CrossRef]
- Theilacker, K.; Buhrke, D.; Kaupp, M. Validation of the Direct–COSMO–RS Solvent Model for Diels–Alder Reactions in Aqueous Solution. J. Chem. Theory Comput. 2015, 11, 111–121. [Google Scholar] [CrossRef]
- Soto-Delgado, J.; Tapia, R.A.; Torras, J. Multiscale Treatment for the Molecular Mechanism of a Diels–Alder Reaction in Solution: A QM/MM–MD Study. J. Chem. Theory Comput. 2016, 12, 4735–4742. [Google Scholar] [CrossRef]
- Liu, F.; Yang, Z.; Mei, Y.; Houk, K.N. QM/QM’Direct Molecular Dynamics of Water–Accelerated Diels–Alder Reaction. J. Phys. Chem. B 2016, 120, 6250–6254. [Google Scholar] [CrossRef]
- Li, P.; Liu, F.; Jia, X.; Shao, Y.; Hu, W.; Zheng, J.; Mei, Y. Efficient Computation of Free Energy Surfaces of Diels–Alder Reactions in Explicit Solvent at Ab Initio QM/MM Level. Molecules 2018, 23, 2487. [Google Scholar] [CrossRef]
- Banerjee, S.; Gnanamani, E.; Yan, X.; Zare, R.N. Can all bulk-phase reactions be accelerated in microdroplets? Analyst 2017, 142, 1399–1402. [Google Scholar] [CrossRef] [PubMed]
- Pestana, L.R.; Hao, H.; Head-Gordon, T. Diels–Alder Reactions in Water Are Determined by Microsolvation. Nano Lett. 2020, 20, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Kraka, E.; Zou, W.; Tao, Y.; Freindorf, M. Exploring the Mechanism of Catalysis with the Unified Reaction Valley Approach (URVA)—A Review. Catalysts 2020, 10, 691. [Google Scholar] [CrossRef]
- Kraka, E.; Zou, W.; Tao, Y. Decoding Chemical Information from Vibrational Spectroscopy Data: Local Vibrational Mode Theory. WIREs Comput. Mol. Sci. 2020, 10, 1480. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular Interactions from a Natural Bond Orbital, Donor-Acceptor Viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Bader, R.F.W. The Quantum Mechanical Basis of Conceptual Chemistry. Monatshefte Chem. 2005, 136, 819–854. [Google Scholar] [CrossRef]
- Cremer, D.; Pople, J.A. General Definition of Ring Puckering Coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- Zou, W.; Tao, Y.; Cremer, D.; Kraka, E. Systematic description of molecular deformations with Cremer-Pople puckering and deformation coordinates utilizing analytic derivatives: Applied to cycloheptane, cyclooctane, and cyclo[18]carbon. J. Chem. Phys. 2020, 152, 154107. [Google Scholar] [CrossRef] [Green Version]
- Zou, W.; Sexton, T.; Kraka, E.; Freindorf, M.; Cremer, D. A New Method for Describing the Mechanism of a Chemical Reaction Based on the Unified Reaction Valley Approach. J. Chem. Theory Comput. 2016, 12, 650–663. [Google Scholar] [CrossRef]
- Kraka, E. Reaction Path Hamiltonian and the Unified Reaction Valley Approach. WIREs Comput. Mol. Sci. 2011, 1, 531–556. [Google Scholar] [CrossRef]
- Joo, H.; Kraka, E.; Quapp, W.; Cremer, D. The Mechanism of a Barrierless Reaction: Hidden Transition State and Hidden Intermediates in the Reaction of Methylene with Ethene. Mol. Phys. 2007, 105, 2697–2717. [Google Scholar] [CrossRef]
- Makoś, M.Z.; Freindorf, M.; Tao, Y.; Kraka, E. Theoretical Insights into [NHC]Au(I) Catalyzed Hydroalkoxylation of Allenes: A Unified Reaction Valley Approach Study. J. Org. Chem. 2021, 86, 5714–5726. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, S.; Freindorf, M.; Tao, Y.; Kraka, E. Modeling Hydrogen release from water with Borane and Alane catalysts: A Unified Reaction Valley Approach. J. Phys. Chem. A 2020, 124, 8978–8993. [Google Scholar] [CrossRef]
- Tao, Y.; Zou, W.; Nanayakkara, S.; Kraka, E. PyVibMS: A PyMOL plugin for visualizing vibrations in molecules and solids. J. Mol. Model. 2020, 26, 290-1–290-12. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, S.; Kraka, E. A New Way of Studying Chemical Reactions: A Hand-in-hand URVA and QTAIM Approach. Phys. Chem. Chem. Phys. 2019, 21, 15007–15018. [Google Scholar] [CrossRef]
- Freindorf, M.; Tao, Y.; Sethio, D.; Cremer, D.; Kraka, E. New Mechanistic Insights into the Claisen Rearrangement of Chorismate—A Unified Reaction Valley Approach Study. Mol. Phys. 2018, 117, 1172–1192. [Google Scholar] [CrossRef]
- Freindorf, M.; Cremer, D.; Kraka, E. Gold(I)-Assisted Catalysis—A Comprehensive View on the [3,3]-Sigmatropic Rearrangement of Allyl Acetate. Mol. Phys. 2017, 116, 611–630. [Google Scholar] [CrossRef]
- Reis, M.C.; López, C.S.; Kraka, E.; Cremer, D.; Faza, O.N. Rational Design in Catalysis: A Mechanistic Study of β-Hydride Eliminations in Gold(I) and Gold(III) Complexes Based on Features of the Reaction Valley. Inorg. Chem. 2016, 55, 8636–8645. [Google Scholar] [CrossRef]
- Sexton, T.; Kraka, E.; Cremer, D. Extraordinary Mechanism of the Diels-Alder Reaction: Investigation of Stereochemistry, Charge Transfer, Charge Polarization, and Biradicaloid Formation. J. Phys. Chem. A 2016, 120, 1097–1111. [Google Scholar] [CrossRef]
- Cremer, D.; Kraka, E. From Molecular Vibrations to Bonding, Chemical Reactions, and Reaction Mechanism. Curr. Org. Chem. 2010, 14, 1524–1560. [Google Scholar] [CrossRef]
- Kraka, E.; Cremer, D. Computational Analysis of the Mechanism of Chemical Reactions in Terms of Reaction Phases: Hidden Intermediates and Hidden Transition States. Acc. Chem. Res. 2010, 43, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K. The Path of Chemical Reactions—The IRC Approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Marcus, R. On analytical mechanics of chemical reactions. Quantum mechanics of linear collisions. J. Chem. Phys. 1966, 45, 4493–4499. [Google Scholar] [CrossRef] [Green Version]
- Marcus, R. On analytical mechanics of chemical reactions. Classical mechanics of linear collisions. J. Chem. Phys. 1966, 45, 4500–4504. [Google Scholar] [CrossRef] [Green Version]
- Marcus, R. Analytical mechanics of chemical reactions. 3. Natural collision coordinates. J. Chem. Phys. 1968, 49, 2610–2616. [Google Scholar] [CrossRef]
- Miller, W.H.; Handy, N.C.; Adams, J.E. Reaction Path Hamiltonian for Polyatomic Molecules. J. Chem. Phys. 1980, 72, 99–112. [Google Scholar] [CrossRef] [Green Version]
- Konkoli, Z.; Cremer, D. A New Way of Analyzing Vibrational Spectra. I. Derivation of Adiabatic Internal Modes. Int. J. Quantum Chem. 1998, 67, 1–9. [Google Scholar] [CrossRef]
- Konkoli, Z.; Larsson, J.A.; Cremer, D. A New Way of Analyzing Vibrational Spectra. II. Comparison of Internal Mode Frequencies. Int. J. Quantum Chem. 1998, 67, 11–27. [Google Scholar] [CrossRef]
- Konkoli, Z.; Cremer, D. A New Way of Analyzing Vibrational Spectra. III. Characterization of Normal Vibrational Modes in terms of Internal Vibrational Modes. Int. J. Quantum Chem. 1998, 67, 29–40. [Google Scholar] [CrossRef]
- Konkoli, Z.; Larsson, J.A.; Cremer, D. A New Way of Analyzing Vibrational Spectra. IV. Application and Testing of Adiabatic Modes within the Concept of the Characterization of Normal Modes. Int. J. Quantum Chem. 1998, 67, 41–55. [Google Scholar] [CrossRef]
- Cremer, D.; Larsson, J.A.; Kraka, E. New Developments in the Analysis of Vibrational Spectra on the Use of Adiabatic Internal Vibrational Modes. In Theoretical and Computational Chemistry; Parkanyi, C., Ed.; Elsevier: Amsterdam, The Netherlands, 1998; pp. 259–327. [Google Scholar]
- Wilson, E.; Decius, J.; Cross, P. Molecular Vibrations. The Theory of Infrared and Raman Vibrational Spectra; McGraw-Hill: New York, NY, USA, 1955. [Google Scholar]
- Zou, W.; Cremer, D. C2 in a Box: Determining its Intrinsic Bond Strength for the X1Σ+g Ground State. Chem. Eur. J. 2016, 22, 4087–4097. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.A.A.; Humason, A.; Kalescky, R.; Freindorf, M.; Kraka, E. Exceptionally Long Covalent CC Bonds—A Local Vibrational Mode Study. Molecules 2021, 26, 950. [Google Scholar] [CrossRef] [PubMed]
- Kraka, E.; Larsson, J.A.; Cremer, D. Generalization of the Badger Rule Based on the Use of Adiabatic Vibrational Modes. In Computational Spectroscopy; Grunenberg, J., Ed.; Wiley: New York, NY, USA, 2010; pp. 105–149. [Google Scholar]
- Kalescky, R.; Kraka, E.; Cremer, D. Identification of the Strongest Bonds in Chemistry. J. Phys. Chem. A 2013, 117, 8981–8995. [Google Scholar] [CrossRef] [PubMed]
- Kraka, E.; Cremer, D. Characterization of CF Bonds with Multiple-Bond Character: Bond Lengths, Stretching Force Constants, and Bond Dissociation Energies. ChemPhysChem 2009, 10, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Kraka, E.; Setiawan, D.; Cremer, D. Re-Evaluation of the Bond Length-Bond Strength Rule: The Stronger Bond Is not Always the Shorter Bond. J. Comput. Chem. 2015, 37, 130–142. [Google Scholar] [CrossRef]
- Setiawan, D.; Sethio, D.; Cremer, D.; Kraka, E. From Strong to Weak NF Bonds: On the Design of a New Class of Fluorinating Agents. Phys. Chem. Chem. Phys. 2018, 20, 23913–23927. [Google Scholar] [CrossRef]
- Sethio, D.; Lawson Daku, L.M.; Hagemann, H.; Kraka, E. Quantitative Assessment of B−B−B, B−Hb−B, and B−Ht Bonds: From BH3 to B12H122−. ChemPhysChem 2019, 20, 1967–1977. [Google Scholar] [CrossRef]
- Freindorf, M.; Yannacone, S.; Oliveira, V.; Verma, N.; Kraka, E. Halogen Bonding Involving I2 and d8 Transition-Metal Pincer Complexes. Crystals 2021, 11, 373. [Google Scholar] [CrossRef]
- Oliveira, V.; Kraka, E.; Cremer, D. The Intrinsic Strength of the Halogen Bond: Electrostatic and Covalent Contributions Described by Coupled Cluster Theory. Phys. Chem. Chem. Phys. 2016, 18, 33031–33046. [Google Scholar] [CrossRef]
- Oliveira, V.; Kraka, E.; Cremer, D. Quantitative Assessment of Halogen Bonding Utilizing Vibrational Spectroscopy. Inorg. Chem. 2016, 56, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.; Cremer, D. Transition from Metal-Ligand Bonding to Halogen Bonding Involving a Metal as Halogen Acceptor: A Study of Cu, Ag, Au, Pt, and Hg Complexes. Chem. Phys. Lett. 2017, 681, 56–63. [Google Scholar] [CrossRef]
- Yannacone, S.; Oliveira, V.; Verma, N.; Kraka, E. A Continuum from Halogen Bonds to Covalent Bonds: Where Do λ3 Iodanes Fit? Inorganics 2019, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, V.P.; Marcial, B.L.; Machado, F.B.C.; Kraka, E. Metal-Halogen Bonding Seen through the Eyes of Vibrational Spectroscopy. Materials 2020, 13, 55. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, V.; Cremer, D.; Kraka, E. The Many Facets of Chalcogen Bonding: Described by Vibrational Spectroscopy. J. Phys. Chem. A 2017, 121, 6845–6862. [Google Scholar] [CrossRef]
- Oliveira, V.; Kraka, E. Systematic Coupled Cluster Study of Noncovalent Interactions Involving Halogens, Chalcogens, and Pnicogens. J. Phys. Chem. A 2017, 121, 9544–9556. [Google Scholar] [CrossRef]
- Setiawan, D.; Kraka, E.; Cremer, D. Hidden Bond Anomalies: The Peculiar Case of the Fluorinated Amine Chalcogenides. J. Phys. Chem. A 2015, 119, 9541–9556. [Google Scholar] [CrossRef]
- Setiawan, D.; Kraka, E.; Cremer, D. Strength of the Pnicogen Bond in Complexes Involving Group VA Elements N, P, and As. J. Phys. Chem. A 2014, 119, 1642–1656. [Google Scholar] [CrossRef]
- Setiawan, D.; Kraka, E.; Cremer, D. Description of Pnicogen Bonding with the help of Vibrational Spectroscopy-The Missing Link Between Theory and Experiment. Chem. Phys. Lett. 2014, 614, 136–142. [Google Scholar] [CrossRef]
- Setiawan, D.; Cremer, D. Super-Pnicogen Bonding in the Radical Anion of the Fluorophosphine Dimer. Chem. Phys. Lett. 2016, 662, 182–187. [Google Scholar] [CrossRef] [Green Version]
- Sethio, D.; Oliveira, V.; Kraka, E. Quantitative Assessment of Tetrel Bonding Utilizing Vibrational Spectroscopy. Molecules 2018, 23, 2763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freindorf, M.; Kraka, E.; Cremer, D. A Comprehensive Analysis of Hydrogen Bond Interactions Based on Local Vibrational Modes. Int. J. Quantum Chem. 2012, 112, 3174–3187. [Google Scholar] [CrossRef]
- Kalescky, R.; Zou, W.; Kraka, E.; Cremer, D. Local Vibrational Modes of the Water Dimer—Comparison of Theory and Experiment. Chem. Phys. Lett. 2012, 554, 243–247. [Google Scholar] [CrossRef]
- Kalescky, R.; Kraka, E.; Cremer, D. Local Vibrational Modes of the Formic Acid Dimer—The Strength of the Double H-Bond. Mol. Phys. 2013, 111, 1497–1510. [Google Scholar] [CrossRef]
- Tao, Y.; Zou, W.; Jia, J.; Li, W.; Cremer, D. Different Ways of Hydrogen Bonding in Water—Why Does Warm Water Freeze Faster than Cold Water? J. Chem. Theory Comput. 2017, 13, 55–76. [Google Scholar] [CrossRef]
- Tao, Y.; Zou, W.; Kraka, E. Strengthening of Hydrogen Bonding With the Push-Pull Effect. Chem. Phys. Lett. 2017, 685, 251–258. [Google Scholar] [CrossRef]
- Makoś, M.Z.; Freindorf, M.; Sethio, D.; Kraka, E. New Insights into Fe–H2 and Fe–H− Bonding of a [NiFe] Hydrogenase Mimic—A Local Vibrational Mode Study. Theoretical Chem. Acc. 2019, 138, 76. [Google Scholar] [CrossRef]
- Lyu, S.; Beiranvand, N.; Freindorf, M.; Kraka, E. Interplay of Ring Puckering and Hydrogen Bonding in Deoxyribonucleosides. J. Phys. Chem. A 2019, 123, 7087–7103. [Google Scholar] [CrossRef]
- Verma, N.; Tao, Y.; Kraka, E. Systematic Detection and Characterization of Hydrogen Bonding in Proteins via Local Vibrational Modes. J. Phys. Chem. B 2021, 125, 2551–2565. [Google Scholar] [CrossRef]
- Beiranvand, N.; Freindorf, M.; Kraka, E. Hydrogen Bonding in Natural and Unnatural Base Pairs—Explored with Vibrational Spectroscopy. Molecules 2021, 26, 2268. [Google Scholar] [CrossRef]
- Yannacone, S.; Freindorf, M.; Tao, Y.; Zou, W.; Kraka, E. Local Vibrational Mode Analysis of π-Hole Interactions between Aryl Donors and Small Molecule Acceptors. Crystals 2020, 10, 556. [Google Scholar] [CrossRef]
- Zou, W.; Kalescky, R.; Kraka, E.; Cremer, D. Relating Normal Vibrational Modes to Local Vibrational Modes with the Help of an Adiabatic Connection Scheme. J. Chem. Phys. 2012, 137, 084114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, N.; Tao, Y.; Zou, W.; Chen, X.; Chen, X.; Freindorf, M.; Kraka, E. A Critical Evaluation of Vibrational Stark Effect (VSE) Probes with the Local Vibrational Mode Theory. Sensors 2020, 20, 2358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory (International Series of Monographs on Chemistry); Clarendon Press: Oxford, UK, 1994. [Google Scholar]
- Popelier, P.L. Atoms in Molecules: An Introduction; Prentice Hall: Hoboken, NJ, USA, 2000. [Google Scholar]
- Cremer, D.; Kraka, E. Chemical Bonds without Bonding Electron Density? Does the Difference Electron-Density Analysis Suffice for a Description of the Chemical Bond? Angew. Chem. Int. Ed. 1984, 23, 627–628. [Google Scholar] [CrossRef]
- Cremer, D.; Kraka, E. A Description of the Chemical Bond in Terms of Local Properties of Electron Density and Energy. Croat. Chem. Acta 1984, 57, 1259–1281. [Google Scholar]
- Kraka, E.; Cremer, D. Chemical Implication of Local Features of the Electron Density Distribution. In Theoretical Models of Chemical Bonding. The Concept of the Chemical Bond; Maksic, Z.B., Ed.; Springer: Berlin/Heidelberg, Germany, 1990; Volume 2, pp. 453–542. [Google Scholar]
- Cremer, D. A General Definition of Ring Substituent Positions. Isr. J. Chem. 1980, 20, 12–19. [Google Scholar] [CrossRef]
- Cremer, D. Theoretical Determination of Molecular Structure and Conformation. II. Hydrogen Trioxide—A Model Compound for Studying the Conformational Modes of Geminal Double Rotors and Five Membered Rings. J. Chem. Phys. 1978, 69, 4456–4471. [Google Scholar] [CrossRef] [Green Version]
- Cremer, D.; Dorofeeva, O.; Mastryukov, V. Structure and Puckering Potential of Azetidine, (CH2)3NH, Studied by Electron Diffraction and Ab Initio Calculations. J. Mol. Struct. 1981, 75, 225–240. [Google Scholar] [CrossRef]
- Cremer, D. Conformational Analysis of Ring Compounds. Fresenius Z. Anal. Chem. 1980, 304, 275–276. [Google Scholar] [CrossRef]
- Cremer, D. Theoretical Determination of Molecular Structure and Conformation. XI. The Puckering of Oxolanes. Isr. J. Chem. 1983, 23, 72–84. [Google Scholar] [CrossRef]
- Essén, H.; Cremer, D. On the Relationship Between the Mean Plane and the Least-Squares Plane of an N-Membered Puckered Ring. Acta Crystall. B 1984, 40, 418–420. [Google Scholar] [CrossRef] [Green Version]
- Cremer, D. On the Correct Usage of the Cremer-Pople Puckering Parameters as Quantitative Descriptors of Ring Shapes—A Reply to recent Criticism By Petit, Dillen And Geise. Acta Crystall. B 1984, 40, 498–500. [Google Scholar] [CrossRef]
- Cremer, D.; Kraka, E. Theoretical Determination of Molecular Structure and Conformation. 16. Substituted Cyclopropanes—An Electron Density Model of Substituent-Ring Interactions. J. Am. Chem. Soc. 1985, 107, 3811–3819. [Google Scholar] [CrossRef]
- Cremer, D. Calculation of Puckered Rings with Analytical Gradients. J. Phys. Chem. 1990, 94, 5502–5509. [Google Scholar] [CrossRef]
- Cremer, D.; Szabo, K.J. Ab Initio Studies of Six-Membered Rings: Present Status and Future Developments. In Conformational Behavior of Six-Membered Rings; Juaristi, E., Ed.; Wiley-VCH: Weinheim, Germany, 1995; pp. 59–135. [Google Scholar]
- Wu, A.; Cremer, D.; Auer, A.A.; Gauss, J. Extension of the Karplus Relationship for NMR Spin-Spin Coupling Constants to Nonplanar Ring Systems: Pseudorotation of Cyclopentane. J. Phys. Chem. A 2002, 106, 657–667. [Google Scholar] [CrossRef]
- Wu, A.; Cremer, D. Extension of the Karplus Relationship for NMR Spin, Spin Coupling Constants to Nonplanar Ring Systems—Pseudorotation of Tetrahydrofurane. Int. J. Mol. Sci. 2003, 4, 158–192. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.; Cremer, D. New Approach for Determining the Conformational Features of Pseudorotating Ring Molecules Utilizing Calculated and Measured NMR Spin-Spin Coupling Constants. J. Phys. Chem. A 2003, 107, 1797–1810. [Google Scholar] [CrossRef]
- Jahn, M.; Dewald, D.; Vallejo-López, M.; Cocinero, E.; Lesarri, A.; Zou, W.; Cremer, D.; Grabow, J.U. Pseudorotational Landscape of Seven-Membered Rings: The Most Stable Chair and Twist-Boat Conformers of ϵ-Caprolactone. Chem. Eur. J. 2014, 20, 14084–14089. [Google Scholar] [CrossRef]
- Zou, W.; Tao, Y.; Cremer, D.; Kraka, E. Describing Polytopal Rearrangements of Fluxional Molecules with Curvilinear Coordinates Derived from Normal Vibrational Modes—A Conceptual Extension of Cremer-Pople Puckering Coordinates. J. Chem. Theory Comput. 2020, 16, 3162–3192. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vosko, S.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef] [Green Version]
- Stephens, P.; Devlin, F.; Chabalowski, C.; Frisch, M. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+G basis set for first-row elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Riplinger, C.; Neese, F. An efficient and near linear scaling pair natural orbital based local coupled cluster method. J. Chem. Phys. 2013, 138, 034106. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Case, D.A.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E.; Cruzeiro, V.W.D.; Darden, T.A.; Duke, R.E.; Ghoreishi, D.; Gilson, M.K.; et al. AMBER; University of California: San Francisco, CA, USA, 2018. [Google Scholar]
- Chung, L.W.; Sameera, W.M.C.; Ramozzi, R.; Page, A.J.; Hatanaka, M.; Petrova, G.P.; Harris, T.V.; Li, X.; Ke, Z.; Liu, F.; et al. The ONIOM Method and Its Applications. Chem. Rev. 2015, 115, 5678–5796. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. GAUSSIAN 09; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Zou, W.; Tao, Y.; Freindorf, M.; Makoś, M.Z.; Verma, N.; Cremer, D.; Kraka, E. Local Vibrational Mode Analysis (LModeA). Computational and Theoretical Chemistry Group (CATCO); Southern Methodist University: Dallas, TX, USA, 2021. [Google Scholar]
- Keith, T.A. AIMALL; TK Gristmill Software: Overland Park, KS, USA, 2017. [Google Scholar]
- Dimitry Izotov, W.Z.; Cremer, D.; Kraka, E. Local Vibrational Mode Analysis (RING). Computational and Theoretical Chemistry Group (CATCO); Southern Methodist University: Dallas, TX, USA, 2021. [Google Scholar]
- Cremer, D.; Wu, A.; Kraka, E. The Mechanism of the Reaction FH + H2C=CH2→ H2C-CFH3. Investigation of Hidden Intermediates with the Unified Reaction Valley Approach. Phys. Chem. Chem. Phys. 2001, 3, 674–687. [Google Scholar] [CrossRef]
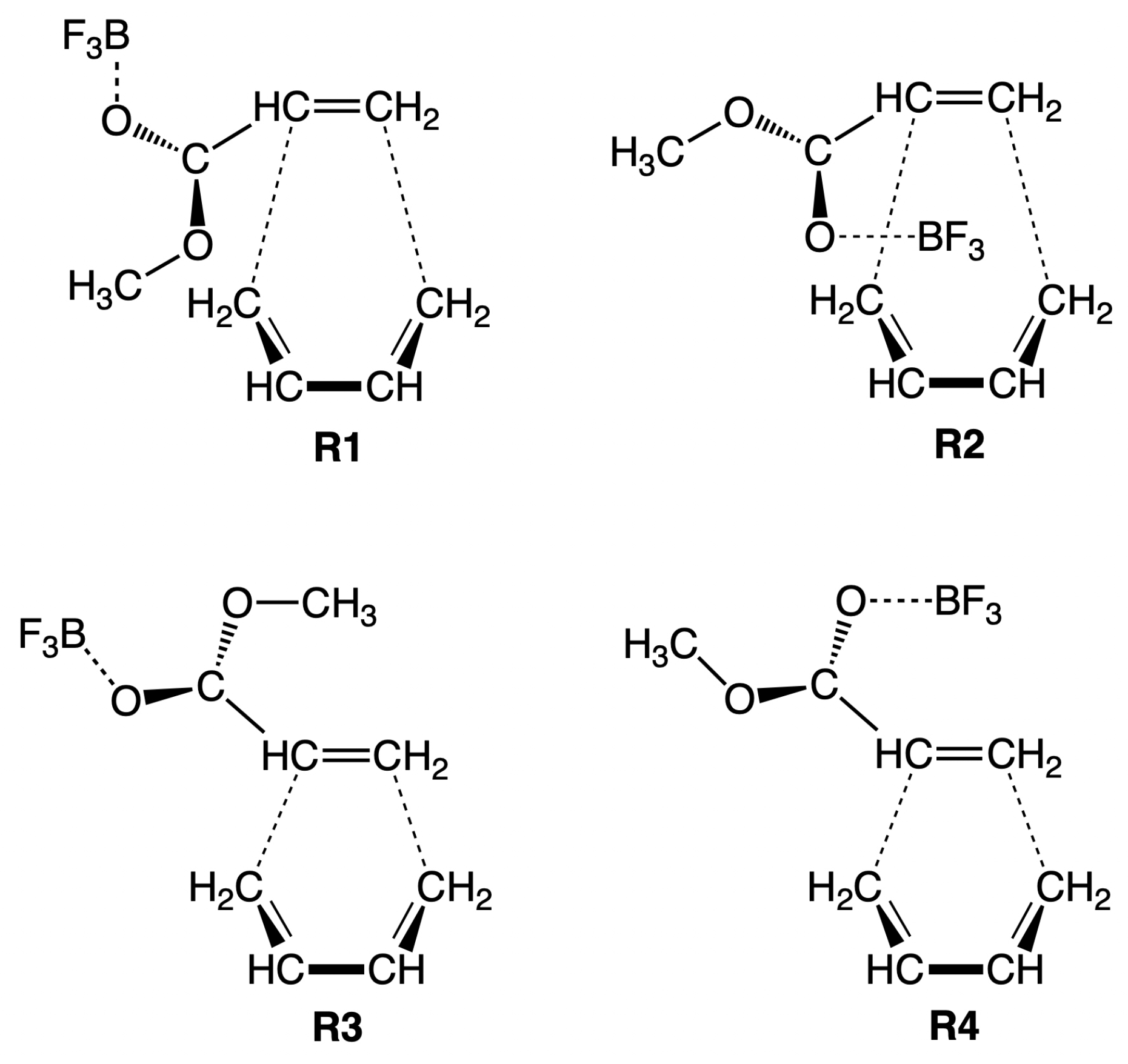



| Energy | Enthalpy | Energy | Enthalpy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reaction | E | E | H | H | Reaction | E | E | H | H | ||
| DFT | |||||||||||
| R1cw | 9.06 | −30.56 | 9.03 | −27.39 | R1nw | 15.71 | −33.24 | 15.83 | −30.31 | ||
| R2cw | 12.65 | −29.94 | 12.40 | −28.03 | R2nw | 15.42 | −30.46 | 15.22 | −27.75 | ||
| R3cw | 15.40 | −27.46 | 14.92 | −24.82 | R3nw | 14.74 | −33.39 | 14.45 | −30.93 | ||
| R4cw | 12.08 | −29.60 | 11.92 | −27.49 | R4nw | 15.96 | −31.28 | 15.69 | −28.02 | ||
| R1cg | 15.61 | −34.26 | 15.41 | −31.38 | R1ng | 20.68 | −34.61 | 20.43 | −31.73 | ||
| R2cg | 16.35 | −32.77 | 16.17 | −29.94 | R2ng | 20.27 | −34.24 | 20.02 | −31.43 | ||
| R3cg | 16.12 | −29.19 | 15.96 | −26.25 | R3ng | 20.59 | −28.82 | 20.33 | −25.96 | ||
| R4cg | 13.97 | −29.69 | 13.95 | −26.71 | R4ng | 19.91 | −29.16 | 19.67 | −26.31 | ||
| CCSD(T) | |||||||||||
| R1cg | 15.96 | −43.32 | 15.76 | −40.44 | R1ng | 18.28 | −44.89 | 18.03 | −42.01 | ||
| R2cg | 16.40 | −41.56 | 16.22 | −38.73 | R2ng | 17.83 | −44.69 | 17.57 | −41.88 | ||
| R3cg | 16.74 | −38.60 | 16.58 | −35.66 | R3ng | 19.20 | −38.49 | 18.94 | −35.63 | ||
| R4cg | 15.82 | −38.98 | 15.80 | −35.99 | R4ng | 18.89 | −38.43 | 18.65 | −35.58 | ||
| Reaction | Bond | Molecule | d | |||||
|---|---|---|---|---|---|---|---|---|
| Å | mDyn/Å | cm | e/Bohr | Hr/Bohr | ||||
| R1cw | CC | Re | 3.431 | 0.048 | 0.029 | 116.7 | 0.0061 | 0.0008 |
| TS | 2.810 | 0.113 | 0.058 | 179.1 | 0.0187 | 0.0011 | ||
| Pr | 1.584 | 2.715 | 0.733 | 876.4 | 0.2103 | −0.1407 | ||
| CC | Re | 3.069 | 0.071 | 0.040 | 141.3 | 0.0093 | 0.0013 | |
| TS | 2.014 | 0.061 | 0.035 | 131.6 | 0.0776 | −0.0225 | ||
| Pr | 1.540 | 3.592 | 0.916 | 1008.0 | 0.2331 | −0.1718 | ||
| R1nw | CC | Re | 3.164 | 0.117 | 0.060 | 182.0 | 0.0078 | 0.0012 |
| TS | 2.465 | 0.120 | 0.061 | 184.1 | 0.0339 | −0.0026 | ||
| Pr | 1.560 | 3.165 | 0.828 | 946.2 | 0.2220 | −0.1563 | ||
| CC | Re | 3.449 | 0.030 | 0.020 | 92.6 | - | - | |
| TS | 2.088 | 0.082 | 0.045 | 152.5 | 0.0677 | −0.0173 | ||
| Pr | 1.550 | 3.450 | 0.887 | 987.8 | 0.2287 | −0.1644 | ||
| R1cg | CC | Re | 6.114 | - | - | - | - | - |
| TS | 2.657 | 0.127 | 0.064 | 189.8 | 0.0242 | 0.0001 | ||
| Pr | 1.548 | 3.323 | 0.861 | 969.6 | 0.2287 | −0.1659 | ||
| CC | Re | 5.565 | - | - | - | - | - | |
| TS | 2.020 | 0.084 | 0.046 | 154.2 | 0.0780 | −0.0226 | ||
| Pr | 1.532 | 3.894 | 0.977 | 1049.5 | 0.2388 | −0.1793 | ||
| R1ng | CC | Re | 5.495 | - | - | - | - | - |
| TS | 2.438 | 0.119 | 0.060 | 183.1 | 0.0364 | −0.0035 | ||
| Pr | 1.541 | 3.550 | 0.908 | 1002.1 | 0.2342 | −0.1726 | ||
| CC | Re | 9.456 | - | - | - | - | - | |
| TS | 2.088 | 0.092 | 0.049 | 161.7 | 0.0691 | −0.0179 | ||
| Pr | 1.533 | 3.873 | 0.973 | 1046.6 | 0.2385 | −0.1788 |
| Reactant | TS | |||||||
|---|---|---|---|---|---|---|---|---|
| H–Bond | d | d | ||||||
| Å | mDyn/Å | e | Å | mDyn/Å | e | |||
| HB1 | 2.0363 | 0.079 | 0.237 | −0.572 | 1.9283 | 0.196 | 0.312 | −0.585 |
| HB2 | 1.9251 | 0.202 | 0.315 | −0.688 | 1.8800 | 0.277 | 0.346 | −0.730 |
| HB3 | 2.0988 | 0.040 | 0.193 | −0.688 | 1.9415 | 0.124 | 0.272 | −0.730 |
| Reaction | Molecule | Chair | Boat | Tboat |
|---|---|---|---|---|
| R1cw | Re | 1.2 | 74.7 | 24.1 |
| TS | 0.6 | 88.2 | 11.2 | |
| Pr | 1.4 | 69.4 | 29.2 | |
| R1nw | Re | 1.9 | 93.0 | 5.1 |
| TS | 0.1 | 99.3 | 0.6 | |
| Pr | 0.4 | 96.1 | 3.5 | |
| R1cg | Re | - | - | - |
| TS | 0.0 | 99.1 | 0.8 | |
| Pr | 39.8 | 0.1 | 60.1 | |
| R1ng | Re | - | - | - |
| TS | 0.0 | 100.0 | 0.0 | |
| Pr | 39.8 | 0.7 | 59.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freindorf, M.; Kraka, E. BF3–Catalyzed Diels–Alder Reaction between Butadiene and Methyl Acrylate in Aqueous Solution—An URVA and Local Vibrational Mode Study. Catalysts 2022, 12, 415. https://doi.org/10.3390/catal12040415
Freindorf M, Kraka E. BF3–Catalyzed Diels–Alder Reaction between Butadiene and Methyl Acrylate in Aqueous Solution—An URVA and Local Vibrational Mode Study. Catalysts. 2022; 12(4):415. https://doi.org/10.3390/catal12040415
Chicago/Turabian StyleFreindorf, Marek, and Elfi Kraka. 2022. "BF3–Catalyzed Diels–Alder Reaction between Butadiene and Methyl Acrylate in Aqueous Solution—An URVA and Local Vibrational Mode Study" Catalysts 12, no. 4: 415. https://doi.org/10.3390/catal12040415





