Cu-Y2O3 Catalyst Derived from Cu2Y2O5 Perovskite for Water Gas Shift Reaction: The Effect of Reduction Temperature
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Samples
2.2. Catalytic Performance Test
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Catalyst Characterization
4.3. Catalyst Performance Test
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Poggio-Fraccari, E.; Abele, A.; Zitta, N.; Francesconi, J.; Marino, F. CO removal for hydrogen purification via Water Gas Shift and COPROX reactions with monolithic catalysts. Fuel 2022, 310, 122419. [Google Scholar] [CrossRef]
- Wang, Y.; Diaz, D.F.R.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells—A review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Palma, V.; Goodall, R.; Thompson, A.; Ruocco, C.; Renda, S.; Leach, R.; Martino, M. Ceria-coated replicated aluminium sponges as catalysts for the CO-water gas shift process. Int. J. Hydrogen Energy 2021, 46, 12158–12168. [Google Scholar] [CrossRef]
- Trimm, D.L. Minimisation of carbon monoxide in a hydrogen stream for fuel cell application. Appl. Catal. A Gen. 2005, 296, 1–11. [Google Scholar] [CrossRef]
- Plama, V.; Ruocco, C.; Cortses, M.; Renda, S.; Meloni, E.; Festa, G.; Martino, M. Platinum Based Catalysts in the Water Gas Shift Reaction: Recent Advances. Metals 2020, 10, 866. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhu, B.; Zhu, A. Boosting low-temperature water gas shift reaction over Au/TiO2 nanocatalyst activated by oxygen plasma. Chem. Eng. J. 2021, 430, 133013. [Google Scholar] [CrossRef]
- Mandapaka, R.; Madras, G. Zinc and platinum co-doped ceria for WGS and CO oxidation. Appl. Catal. B Environ. 2017, 211, 137–147. [Google Scholar] [CrossRef]
- Ma, L.; Han, D.; Ma, H.; Liu, L.; Guo, H. Characterization of Highly Dispersed Rod- and Particle-Shaped CuFe19Ox Catalysts and Their Shape Effects on WGS. Catalysts 2019, 8, 635. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Wang, J.; Zhang, N.; An, Y.; Yang, Z. Effect of Cu doping on the catalytic activity of Fe3O4 in water-gas shift reactions. Int. J. Hydrogen Energy 2015, 40, 2193–2198. [Google Scholar] [CrossRef]
- Jeong, D.W.; Na, H.S.; Shim, J.O.; Jang, W.J.; Roh, H.S.; Jung, U.H.; Yoon, W.L. Hydrogen production from low temperature WGS reaction on co-precipitated Cu-CeO2 catalysts: An optimization of Cu loading. Int. J. Hydrogen Energy 2014, 39, 9135–9142. [Google Scholar] [CrossRef]
- Yan, H.; Shao, W.P.; Cai, L.H.; Wang, W.W.; Jin, Z.; Jia, C.J. Construction of stabilized bulk-nano interfaces for highly promoted inverse CeO2/Cu catalyst. Nat. Commun. 2019, 10, 3470. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, G.; Cai, Z.; Pan, Y.; Zhao, X. Promotion of the water-gas shift reaction by pre-adsorbed oxygen on Cu(hkl) surfaces: A theoretical study. Theochem J. Mol. Struct. 2004, 710, 97–104. [Google Scholar] [CrossRef]
- Mudiyanselage, K.; Senanayake, S.D.; Ramirez, P.J.; Kundu, S.; Baber, A.; Yang, F.; Agnoli, S.; Axnanda, S.; Liu, Z.; Hrbek, J. Intermediates Arising from the Water-Gas Shift Reaction over Cu Surfaces: From UHV to Near Atmospheric Pressures. Top. Catal. 2015, 58, 4–6. [Google Scholar] [CrossRef]
- Muller, A.; Comas-Vives, A.; Coperet, C. Shape and Surface Morphology of Copper Nanoparticles under CO2 Hydrogenation Conditions from First Principles. J. Phys. Chem. C 2021, 125, 396–409. [Google Scholar] [CrossRef]
- Li, Z.; Li, N.; Wang, N.; Zhou, B.; Yin, P.; Song, B.; Yu, J.; Yang, Y. Mechanism Investigations on Water Gas Shift Reaction over Cu (111), Cu (100), and Cu (211) Surfaces. ACS Omega 2022, 7, 3514–3521. [Google Scholar] [CrossRef] [PubMed]
- Krupa, B.R.V.; Dasgupta, A.; Ghosh, C.; Sinha, S.K. Analysis of structural transformation in nanocrystalline Y2O3 during high energy ball milling. J. Alloy. Compd. 2022, 900, 163550. [Google Scholar]
- Wang, Y.; Zhang, M.; Cao, F.; Liu, Y.; Shao, L. Interficial stability of Cu/Cu(Ru)/Si contact system for barrier-free copper metallization. J. Alloy. Compd. 2011, 509, L180–L182. [Google Scholar] [CrossRef]
- Reddy, C.V.; Koutavarapu, R.; Shim, J.; Cheloho, B.; Reddy, K.R. Novel g-C3N4/Cu-doped ZrO2 hybrid heterostructures for efficient photocatalytic Cr(VI) photoreduction and electrochemical energy storage applications. Chemosphere 2022, 295, 133851. [Google Scholar] [CrossRef]
- Haider, S.; Shar, S.S.; Shakir, I.; Agboola, P.O. Visible light active Cu-doped iron oxide for photocatalytic treatment of methylene blue. Cerma. Int. 2022, 48, 7605–7612. [Google Scholar] [CrossRef]
- Shu, H.; Liu, Y.; Jia, Y. Construction of Cu-BTC by carboxylic acid organic ligand and its application in low temperature SCR denitration. Sci. Total Environ. 2022, 820, 152984. [Google Scholar]
- Zhao, R.; Wei, X.; Chu, B.; Chen, K.; Qin, Q.; Liu, H.; Zhou, Y.; Li, B.; Dong, L. Multi-phase coexisting metal oxide derived by MOFs for the CO-SCR reaction at low temperature and in situ DRIFTS study on reaction mechanism. Appl. Surf. Sci. 2022, 580, 152277. [Google Scholar] [CrossRef]
- Garler, V.O.; Darie, C.; Isnard, O.; Bordet, P. Synthesis and neutron powder diffraction structural analysis of oxidized delafossite YCuO2.5. Solid State Sci. 2006, 8, 457–461. [Google Scholar] [CrossRef]
- Wang, F.; Cheng, Z.; Deng, L.; Xie, H.; Xu, B.; Jiao, Z.; Zhou, G.; Zhang, X. Role of metal Cu species on methyl laurate catalytic hydrogenation to oxygen-containing compounds. J. Saudi Chem. Soc. 2020, 24, 733–741. [Google Scholar] [CrossRef]
- Sun, M.; Cao, Y.; Lan, L.; Zou, S.; Fang, Z.; Chen, Y. Selective Catalytic Oxidation of Ammonia to Nitrogen over Iron and Copper Bimetallic Catalysts. Acta Phys. Chim. Sin. 2015, 30, 2300–2306. [Google Scholar]
- Shan, Y.; Shi, X.; Yan, Z.; Liu, J.; Yu, Y.; He, H. Deactivation of Cu-SSZ-13 in the presence of SO2 during hydrothermal aging. Catal. Today 2018, 320, 84–90. [Google Scholar] [CrossRef]
- Baeza, P.; Bassi, R.; Villarroel, M.; Ojeda, J.; Araya, P.; Aguila, G. Adsrption of 4,6-Dimethyldibenzothyldibenzothiophene over Cu/ZrO2. J. Chil. Chem. Soc. 2015, 60, 2817–2821. [Google Scholar] [CrossRef] [Green Version]
- Sivakumar, M.; Muthukutty, B.; Chen, T.W.; Chen, S.M.; Vivekanandan, A.K.; Chen, S.H.; Hatshan, M.R.; Kumar, M. Electrocatalytic detection of noxious antioxidant diphenylamine in fruit samples with support of Cu@nanoporous carbon modified sensor. Chemosphere 2022, 292, 133400. [Google Scholar] [CrossRef]
- Cao, F.; Xu, Y.; Sui, J.; Li, X.; Yang, J.; Wang, Y. Low temperature Cu/Ti/Al Ohmic contacts to p-type 4H-SiC. J. Alloy. Compd. 2022, 901, 163580. [Google Scholar] [CrossRef]
- Swathi, S.; Yuvakkumar, R.; Kumar, P.S.; Ravi, G.; Velauthapillai, D. Polyvinylpyrrolidone-assisted novel copper antimony sulfide nanorods for highly efficient hydrogen evolution reaction. Fuel 2022, 314, 123096. [Google Scholar] [CrossRef]
- Ge, S.; Liu, X.; Liu, J.; Liu, H.; Liu, H.; Chen, X.; Wang, G.; Chen, J.; Zhang, G.; Zhang, Y.; et al. Synthesis of TixSn1−xO2 mixed metal oxide for copper catalysts as high-efficiency NH3 selective catalytic oxidation. Fuel 2022, 314, 123061. [Google Scholar] [CrossRef]
- Elbakkay, M.H.; El-Dek, S.I.; Farghali, A.A.; Rouby, W.M.A.E. Highly active atomic Cu catalyst anchored on superlattice CoFe layered double hydroxide for efficient oxygen evolution electrocatalysis. Int. J. Hydrogen Energy 2022, 47, 9876–9894. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Geng, X.; Dai, G.; Chu, Z.; Luo, F.; Zhang, F.; Wang, Q. Sandwich-Type Electrochemical Sensor for Mucin-1 Detection Based on a Cysteine-Histidine-Cu@Cuprous Oxide Nanozyme. ACS Appl. Nano Mater. 2022, 5, 2204–2213. [Google Scholar] [CrossRef]
- Hansson, P.; Ahadi, A.; Melin, S. Molecular dynamics modeling of metric scaling effects in nanosized Cu beams holding a grain boundary. Theor. Appl. Fract. Mec. 2020, 107, 102509. [Google Scholar] [CrossRef]
- Zhang, C.; Ruan, D.; Tang, Z.; Lu, K.; Wang, X.; Qi, J.; Liao, Z.; Lu, T. Fabrication and luminescent properties of uranium doped Y2O3 transparent ceramics by sintering aid combinations. Opt. Mater. 2022, 125, 112064. [Google Scholar] [CrossRef]
- Asaithambi, S.; Balaji, V.; Karuppaiah, M.; Sakthivel, P.; Eswari, K.M.; Yuvakkumar, R.; Selvakumar, P.; Velauthapillai, D.; Ravi, G. The electrochemical energy storage and photocatalytic performances analysis of rare earth metal (Tb and Y) doped SnO2@CuS composites. Adv. Powder Technol. 2022, 33, 103442. [Google Scholar] [CrossRef]
- Fox-Rabinovitch, G.; Dosbaeva, G.; Kovalev, A.; Gershman, I.; Yamamoto, K.; Locks, E.; Paiva, J.; Konovalov, E.; Veldhuis, S. Enhancement of Multi-Scale Self-Organization Processes during Inconel DA 718 Machining through the Optimization of TiAlCrSiN/TiAlCrN Bi-Nano-Multilayer Coating Characteristics. Materials 2022, 15, 1329. [Google Scholar] [CrossRef]
- Ilieva, L.; Ivanov, I.; Petrova, P.; Munteanu, G.; Karajirova, Y.; Sobczak, J.W.; Lisowski, W.; Anghel, E.M.; Kaszkur, Z.; Tabakova, T. Effect of Y-doping on the catalytic properties of CuO/CeO2 catalysts for water-gas shift reaction. Int. J. Hydrogen Energy 2020, 45, 26286. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Chen, X.; Fu, H.; Chen, Y.; Ning, S.; Fujita, T.; Wei, Y.; Wang, X. Layered ammonium vanadate nanobelt as efficient adsorbents for removal of Sr2+ and Cs+ from contaminated water. J. Colloid Interface Sci. 2022, 615, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zeng, G.; Lin, Q.; Gu, Y.; Wang, X.; Feng, Z.; Sengupta, A. Application of 3D magnetic nanocomposites: MXene-supported Fe3O4@CS nanospheres for highly efficient adsorption and separation of dyes. Sci. Total Environ. 2022, 822, 153544. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, S.I.; Wang, H.; He, Y.; Yan, L.; Jiang, Y.; Wu, B.; Wang, T.; Gang, H.; Huang, L.; Jin, L.; et al. Fluoride remediation from on-site wastewater using optimized bauxite nanocomposite (Bx-Ce-La@500): Synthesis maximization, and mechanism of F- removal. J. Hazard. Mater. 2022, 430, 128401. [Google Scholar] [CrossRef] [PubMed]
- Teiaira, M.M.; Santos, L.C.; Tello, A.C.M.; Almeida, P.B.; Da Silva, J.S.; Laier, L.; Gracia, L.; Teodoro, M.D.; Da Silva, L.F.; Andres, J.; et al. alpha-Ag2WO4 under microwave, electron beam and femtosecond laser irradiations: Unveiling the relationship between morphology and photoluminescence emissions. J. Alloy. Compd. 2022, 903, 163840. [Google Scholar]
- Zhang, X.; Shi, Y.; Ouyang, Q.; Zhang, X.; Zhu, C.; Zhang, X.; Chen, Y. Identification of the Intrinsic Dielectric Properties of Metal Single Atoms for Electromagnetic Wave Absorption. Nano-Micro Lett. 2022, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; He, J.; Xie, T.; Xu, Q.; Li, G.; Du, L.; Huang, J.; Yang, J.; Li, W.; Wang, J. Peroxymonosulfate activation using a composite of copper and nickel oxide coated on SBA-15 for the removal of sulfonamide antibiotics. Environ. Res. 2022, 206, 112301. [Google Scholar] [CrossRef] [PubMed]
- Lykaki, M.; Stefa, S.; Carabineiro, S.A.C.; Soria, M.A.; Madeira, L.M.; Konsolakis, M. Shape Effects of Ceria Nanoparticles on the Water-Gas Shift Performance of CuOx/CeO2 Catalysts. Catalysts 2021, 11, 753. [Google Scholar] [CrossRef]
- Lee, W.J.; Lee, Y.S.; Rha, S.K.; Lee, Y.J.; Lim, K.Y.; Chung, Y.D.; Whang, C.N. Adhesion and interface chemical reactions of Cu/polyimide and Cu/TiN by XPS. Appl. Surf. Sci. 2003, 205, 128–136. [Google Scholar] [CrossRef]
- Wen, Y.; Zhao, S.; Yi, H.; Gao, F.; Yu, Q.; Liu, J.; Tang, T.; Tang, X. Efficient catalytic oxidation of methyl mercaptan to sulfur dioxide with NiCuFe mixed metal oxides. Environ. Technol. Innov. 2022, 26, 102252. [Google Scholar] [CrossRef]
- Pandi, K.; Preeyanghaa, M.; Vinesh, V.; Madhavan, J.; Neppolian, B. Complete photocatalytic degradation of tetracycline by carbon doped TiO2 supported with stable metal nitrate hydroxide. Environ. Res. 2022, 207, 112188. [Google Scholar] [CrossRef]
- Jurado, L.; Garcia-Moncada, N.; Bobadilla, L.F.; Romero-Sarria, F.; Odriozola, J.A. Elucidation of Water Promoter Effect of Proton Conductor in WGS Reaction over Pt-Based Catalyst: An Operando DRIFTS Study. Catalysts 2020, 10, 841. [Google Scholar] [CrossRef]
- Liu, P. Water-gas shift reaction on oxide/Cu(111): Rational catalyst screening from density functional theory. J. Chem. Phys. 2010, 133, 204705. [Google Scholar] [CrossRef]
- Gunawardana, P.V.D.S.; Lee, H.C.; Kim, D.H. Performance of copper-ceria catalysts for water gas shift reaction in medium temperature range. Int. J. Hydrogen Energy 2009, 34, 1336–1341. [Google Scholar] [CrossRef]
- Ilieva, L.; Ivanov, I.; Sobczak, J.W.; Lisowski, W.; Karashanova, D.; Kaszkur, Z.; Petrova, P.; Tabakova, T. Effect of support preparation method on water-gas shift activity of copper-based catalysts. Int. J. Hydrogen Energy 2021. [Google Scholar] [CrossRef]
- Petroski, J.M.; Wang, Z.L.; Green, T.C.; El-Sayed, M.A. Kinetically controlled growth and shape formation mechanism of platinum nanoparticles. J. Phys. Chem. B 1998, 102, 3316–3320. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.L. Transmission electron microscopy of shape-controlled nanocrystals and their assemblies. J. Phys. Chem. B 2000, 104, 1153–1175. [Google Scholar] [CrossRef]
- Shido, T.; Iwasawa, Y. Regulation of Reaction Intermediate by Reactant in the Water Gas Shift Reaction on CeO2, in Relation to Reactant-Promoted Mechanism. J. Catal. 1992, 136, 493–503. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Jia, A.P.; Lu, J.Q.; Huang, W.X. Morphology-Dependent CO Reduction Kinetics and Surface Copper Species Evolution of Cu2O Nanocrystals. J. Phys. Chem. C 2020, 124, 21568–21576. [Google Scholar] [CrossRef]
- Gokhale, A.A.; Dumesic, J.A.; Mavrikakis, M. On the mechanism of low-temperature water gas shift reaction on copper. J. Am. Chem. Soc. 2008, 130, 1402–1414. [Google Scholar] [CrossRef]
- Koryabkina, N.A.; Phatak, A.A.; Ruettinger, W.F.; Farrauto, R.J.; Ribeiro, F.H. Determination of kinetic parameters for the water-gas shift reaction on copper catalysts under realistic conditions for fuel cell applications. J. Catal. 2003, 217, 233–239. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.; Song, R.; Cao, T.; Luo, L.; Chen, X.; Gao, Y.; Lu, J.; Li, W.; Huang, W. The most active Cu facet for low-temperature water gas shift reaction. Nat. Commun. 2017, 8, 488. [Google Scholar] [CrossRef]
- Baraj, E.; Ciahotny, K.; Hlincik, T. The water gas shift reaction: Catalysts and reaction mechanism. Fuel 2021, 288, 119817. [Google Scholar] [CrossRef]
- Chen, W.H.; Chen, C.Y. Water gas shift reaction for hydrogen production and carbon dioxide capture: A review. Appl. Energy 2020, 258, 114078. [Google Scholar] [CrossRef]
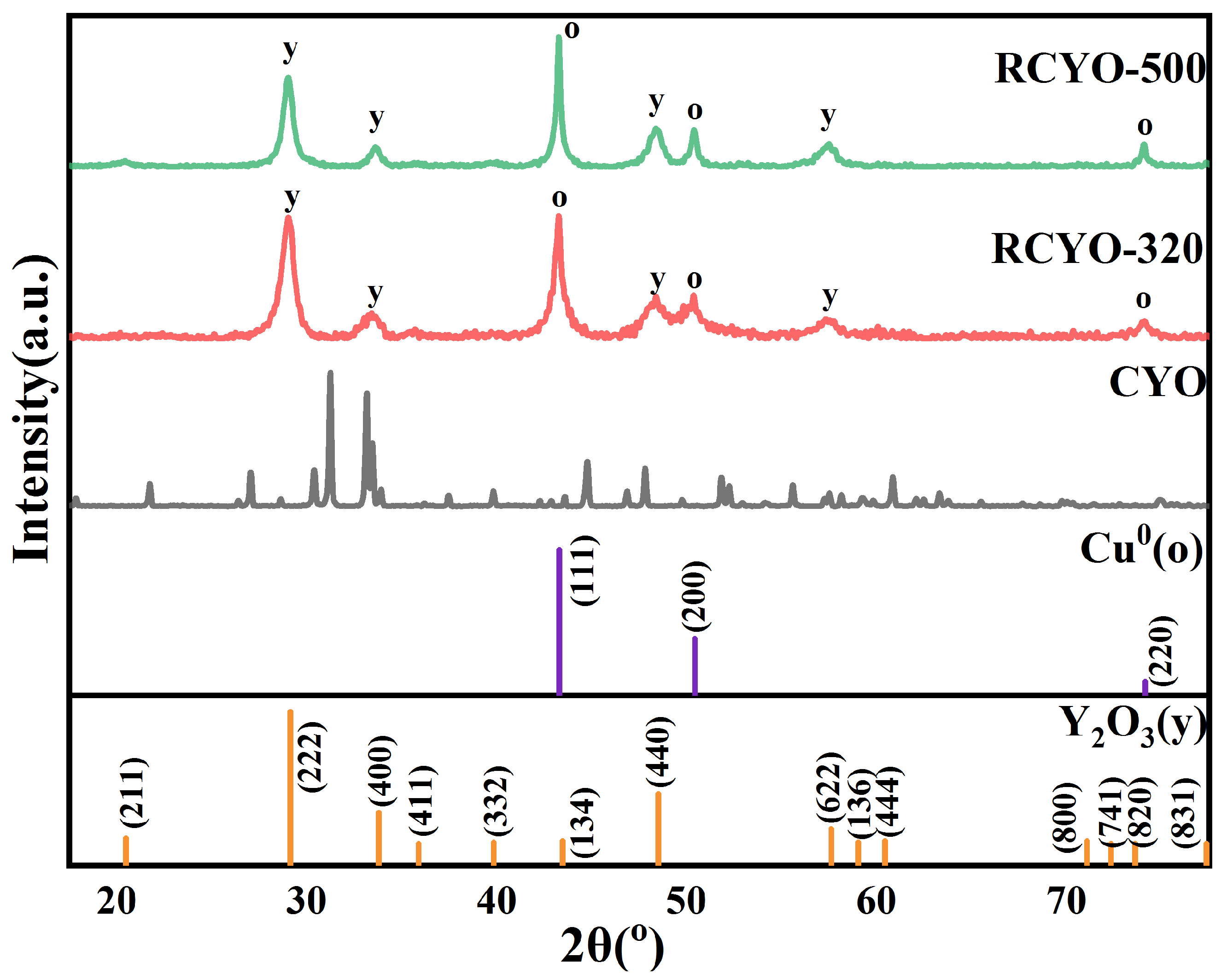

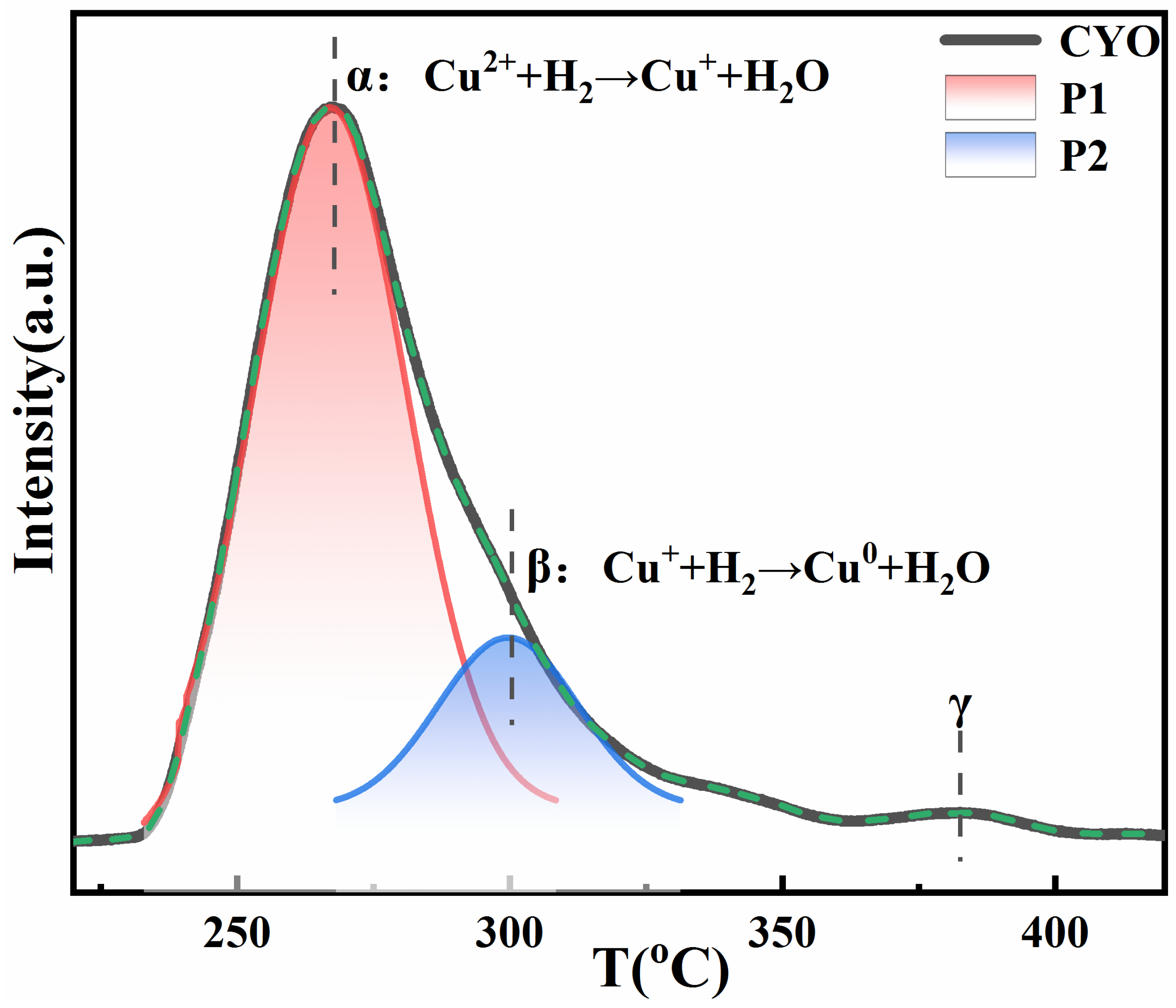

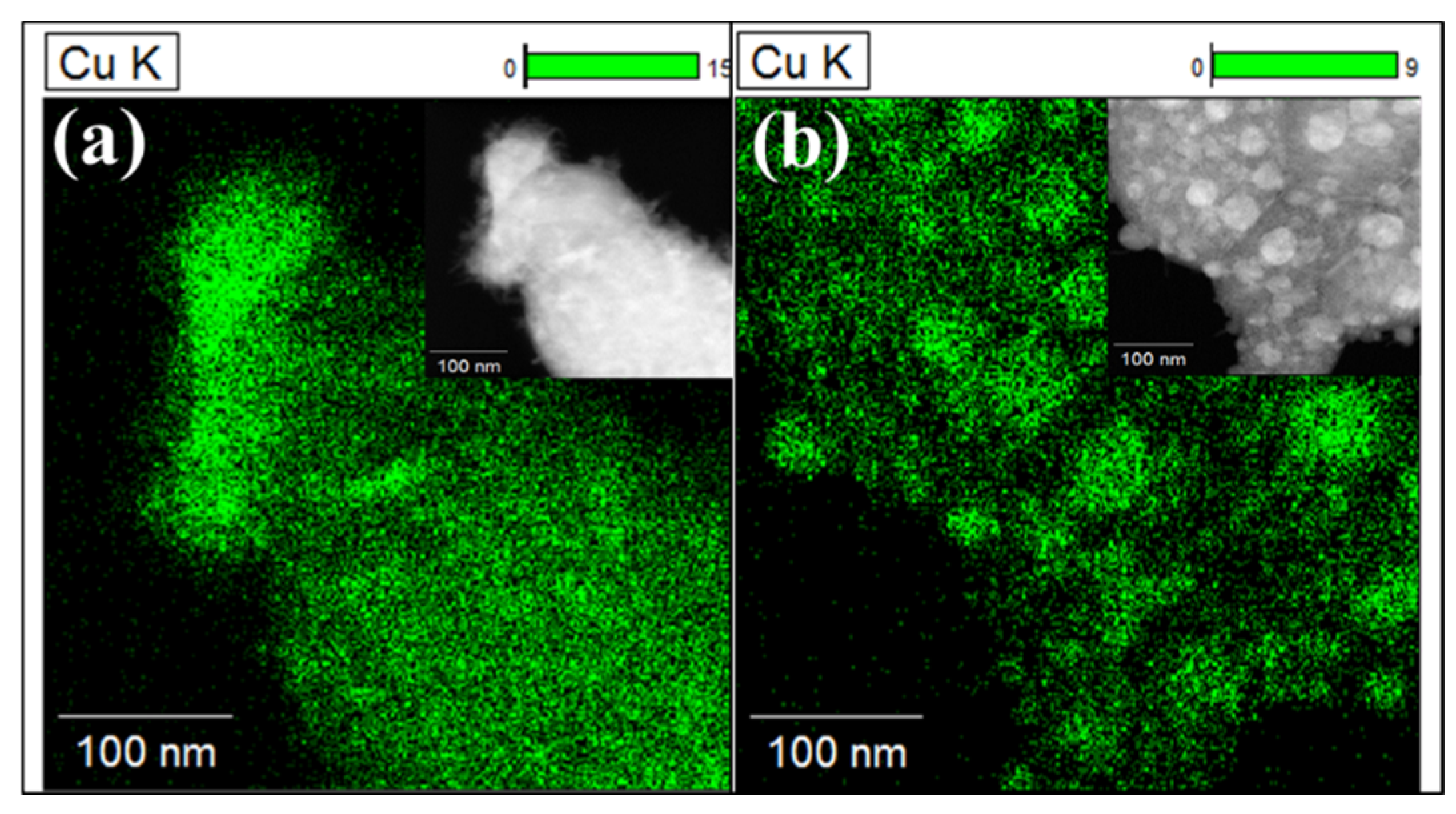





| Sample | Cu Content (%) | Perovskite Calcinated | Reduction Condition |
|---|---|---|---|
| CYO | 33.02 | 650 °C-3 h + 850 °C-5 h | - |
| RCYO-320 | 33.02 | 650 °C-3 h + 850 °C-5 h | 320 °C-4 h(8% H2/Ar) |
| RCYO-500 | 33.02 | 650 °C-3 h + 850 °C-5 h | 500 °C-4 h(8% H2/Ar) |
| Sample | Total Pore Volume (cc·g−1) | Surface Area (m2·g−1) |
|---|---|---|
| RCYO-320 | 5.786 × 10−2 | 87.316 |
| RCYO-500 | 8.221 × 10−2 | 97.614 |
| Catalysts | Surface Cu Species | |||||
|---|---|---|---|---|---|---|
| Cu° | Cu+ | Cu2+ | ||||
| B.E. (eV) | % Area | B.E. (eV) | % Area | B.E. (eV) | % Area | |
| RCYO-320 | 932.30 | 34.3 | 933.87 | 55.1 | 935.80 | 10.6 |
| RCYO-500 | 932.84 | 35.8 | 934.10 | 58.0 | 936.57 | 6.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Luo, F.; Wang, N.; Li, X. Cu-Y2O3 Catalyst Derived from Cu2Y2O5 Perovskite for Water Gas Shift Reaction: The Effect of Reduction Temperature. Catalysts 2022, 12, 481. https://doi.org/10.3390/catal12050481
Wang Z, Luo F, Wang N, Li X. Cu-Y2O3 Catalyst Derived from Cu2Y2O5 Perovskite for Water Gas Shift Reaction: The Effect of Reduction Temperature. Catalysts. 2022; 12(5):481. https://doi.org/10.3390/catal12050481
Chicago/Turabian StyleWang, Zeyu, Fengying Luo, Nan Wang, and Xinjun Li. 2022. "Cu-Y2O3 Catalyst Derived from Cu2Y2O5 Perovskite for Water Gas Shift Reaction: The Effect of Reduction Temperature" Catalysts 12, no. 5: 481. https://doi.org/10.3390/catal12050481





