2.1. Bulk-Phase and Electronic Structure Characterization
Nitrogen adsorption–desorption isotherms of all catalysts are displayed in
Figure 1a. The isotherms show that all catalysts possessed the typical IV type isotherms with obvious hysteresis loops at high relative pressures (0.6 < P/P
0 < 1), indicating the presence of mesopores. Meanwhile, there were almost no microspores in the samples, as there was little N
2 adsorption for all catalysts at low relative pressures (P/P
0 < 0.1), which can also be clearly observed from the pore size distribution curves (
Figure 1b). In addition, as shown in
Table 1, the specific surface area of the catalysts decreased with increased synthesis temperature, to 65 °C, yet it exhibited slightly increasing return when the synthesis temperature reached 75 °C and 85 °C. A larger specific surface area usually provides more active sites, yet it had little relationship with the catalytic activity in this case, as Co–Cu
t=65°C exhibited the highest RhB removal with the smallest surface area. In addition, the catalysts had a higher specific surface area, which often exists in more active sites, and the specific surface area of the catalyst synthesized at 65 °C was the smallest. The result indicates that the structure of the catalyst is sensitive to the synthesis temperature. Furthermore, according to the experimental results of ICP and XPS (
Table 1), the molar ratio of copper and cobalt elements in the catalyst is about 4.15 and 4.2, respectively, which were very close to the theoretical value of 4 for the molar ratio of copper and cobalt elements.
The bulk-phase structure of catalysts was further studied by XRD analysis. As shown in
Figure 2a, when the synthesis temperature was in the range of 45–75 °C, all catalysts exhibited a group of characteristic diffractions corresponding to (003), (006), (012), and (015) crystalline planes of the hydrotalcite-like (JCPDS#35-0965) structure at 2θ of 11.7, 23.4, 33.7, and 39.1, respectively [
35]. The basal spacing d(003) around 0.74 nm was in line with the presence of carbonate in the interlayer space, as in previous reports [
25]. Moreover, a second phase was Cu(OH)
2 (JCPDS#80-0656) owing to the excessive Cu existence besides the necessary composition for LDH or the Jahn-Teller effect. This is because Cu
2+ could be situated in near-lying octahedra with the formation of the copper compound with distorted octahedra, which is energetically preferred to the compound of LDH [
25]. A higher synthesis temperature would enhance the crystallinity of the LDH structure, with an improved intensity of (003) reflection in the range of 45–65 °C. This may be highly related to the promoted oxidation of Co
2+, which resulted in the improved LDH structure with increasing temperature. However, the condition for Co–Cu
t=75°C inversed for the decomposition of LDH framework under the circumstances. When the synthesis temperature was 85 °C, the LDH structure of the catalyst disappeared. Thus, the Co–Cu
t=65°C catalyst had a better LDH structure. As shown in
Figure 3, the catalyst presented sheet-like structures, as is typical for LDH materials.
FT-IR characterization was then carried out to investigate the special Co–Cu interaction, LDH property, and their influence on catalytic performance. The patterns in
Figure 2b show some vibrational information of different catalyst structures. There are three types of O–H stretching modes in the catalysts. One is the ν
O–H vibration of the metal hydroxide located at about 3570 cm
−1 and 3623 cm
−1 for Cu(OH)
2 and Co(OH)
2, respectively [
36]. Another broad band in the region of 3300–3500 cm
−1 can be attributed to the O–H stretching of the adsorbed and interlayer water [
25]. Furthermore, the weak band at 1634 cm
−1 was associated with the hydroxyl deformation mode of the water molecules in the interlayer [
37]. In addition, the peaks at c.a. 1361 cm
−1, 992 cm
−1, and 683 cm
−1 can be attributed to different stretching modes of carbonate [
25], indicating carbonates as the primary compensating anions in the LDH interlayer, which is in line with XRD results. Moreover, the peaks located below 683 cm
−1 were related to M–O vibrations (M = Co and/or Cu) [
36], which varied across samples, indicating that the different M–O bonds may determine their corresponding catalytic performance.
Raman spectra analysis was further conducted to investigate these M–O bonds’ properties. As shown in
Figure 4a, the four vibrational modes exhibited at ca. 189, 461, 527, and 688 cm
−1 all belonged to cobalt oxides corresponding to
,
,
, and
species, respectively [
38,
39,
40], and no Cu-related peaks were detected in all catalysts. In particular, the shifts for
suggested that this is probably related to the incorporation of Cu in the Co-related lattice. Specifically, the bands at 688 cm
−1 (A1g) and 189 cm
−1 (
) could be assigned to the Raman vibration of Co
3+–O
2− at octahedral sites and Co
2+–O
2− at tetrahedral sites, respectively [
41]. The bands at 461 cm
−1 (
) and 527 cm
−1 (
) can be attributed to the combined vibrations in tetrahedral sites and octahedral oxygen motions [
42]. Particularly, the band of 527 cm
−1 can be ascribed to Co-related doubly occupied Ovs bound with donor defects [
43]. According to our previous study, the band’s peak intensity was positive to the concentration of surface Ovs. With the improved LDH crystallinity and peak intensity, shown in
Figure 4a,
modes exhibited the same enhanced tendency, indicating an increasing Ov density.
As shown in
Figure 5, the convolutions of O1s XPS spectra were carried out for detailed information, especially for oxygen defects. The core level spectra were fitted into three identified peaks. Peaks at 529.8 and 530.6 eV were attributed to oxygen atoms bound to lattice oxygen for metal oxides and hydroxyl species, respectively [
31]. It is noted that the peak at ca. 533.4 eV can be ascribed to the presence of defect sites in the low oxygen-coordination [
44]. In addition, Co–Cu
t=65°C presented the highest defect density (
Table 2), with its changing agreeing well with the Raman analysis.
UV-Vis DRS spectra (
Figure 4b) shed more light on the special electronic property of Ov and the LDH structure. The absorption peak observed at 230 nm can be attributed to ligand-to-metal charge-transfer excitations occurring in the MO
6 coordination [
45]. Moreover, the peaks at c.a. 323–364 nm and 642 nm can be associated with O
2−→Co
2+ and O
2−→Co
3+, respectively [
41,
46,
47]. The shifts of the peaks also depicted the different doping conditions of Cu in the LDH structure for different catalysts. In addition, the distinctive peak at 532 nm is related to the special metal–metal charge transfer of the Co–O–Cu oxo-bridge in the MO
6 environment for LDH, as it included the transitions of d
z2→d
x2−y
2 for Cu
2+,
1A
1g→
1T
1g for Co
3+, and
4T
1g(F)→
4T
1g(P) for Co
2+ with weak-field ligands [
48,
49,
50,
51], which is also related to the generation of surface Ov, according to previous publications [
52]. A stronger peak intensity suggested a higher Ov concentration, with its changing agreeing well with the Raman analysis. Thus, it can be concluded from the above discussion that improved LDH structure is likely to possess more Co–O–Cu oxo–bridge interaction with more lattice disorder resulting from the incorporation of Cu in Co sites. This special Co–O–Cu structure can promote the generation of surface Ov, which is positively related to the catalytic performance [
35,
41].
2.2. Catalytic Performance
The catalytic activity for all catalysts with different synthesis temperatures are shown in
Figure 6a. The RhB removal was raised from 82.5% to 99.3% as the fabricated temperature increased from 45 °C to 65 °C, respectively. However, a further increment in the synthesis temperature to 75 °C and 85 °C decreased the RhB degradation efficiency to 85.10% and 65.99%, respectively. Moreover, the adsorption only counted for less than 10% of the total removal without the addition of H
2O
2, indicating that self-decomposition and/or adsorption of RhB by catalysts can be excluded in this case, and the Fenton oxidation is the key factor for total organic elimination. The homogeneous Fenton test showed that the degradation resulting from metal leaching only contributed 9.3% of the total removal from the homogeneous part (
Figure 6b). Furthermore, the quenching experiment showed that ·OH was the main reactive oxygen species of the reaction, as the RhB removal declined to 9.8% with the existence of a scavenger (
Figure 6c). Thus, the RhB decomposition can be mostly ascribed to the ·OH generation over the catalyst surface rather than ·OH produced by leaching metals.
The results of the catalytic performance tests reveal that the synthesis temperature had a great impact on catalytic activity, as the optimum synthesis temperature of the catalyst was 65 °C. However, the Co–Cu
t=65°C LDH catalyst had the smallest specific surface areas in the BET analysis, indicating that the catalyst performance is more structurally dependent than the surface area in the Fenton-like reaction. In addition, among other catalysts, the Co–Cu
t=65°C LDH catalyst had the best performance, which can be attributed to its excellent LDH structure and high density of Ov based on the XRD and Raman analyses. According to a previous study [
35], Ov is the main active site of the LDH structure, and it promotes H
2O
2 adsorption, electron transfer, and H
2O
2 dissociation during reaction, which benefit the generation of effective ·OH. In addition, the Co–Cu
t=65°C LDH catalyst exhibited better RhB removal efficiency compared with other catalysts (
Table 3). Therefore, we selected the Co–Cu
t=65°C LDH catalyst to carry out the following experiments.
2.4. Plausible Mechanism
According to the above experimental results, the possible heterogeneous Fenton-like reaction mechanisms on RhB degradation by the Cu–Co LDH/H
2O
2 were proposed in Equations (1)–(7). Irrespective of the existence of Oxygen vacancy (Ov), the events of Equations (1) and (2) occurred [
62]. Surface active centers of Co and/or Cu are involved in one-electron oxidation to catalyze H
2O
2 to ·OH [
30]. Concurrently, M
(n+1)+ (M = Co and Cu) was reduced by H
2O
2 according to the Haber-Weiss mechanism [
63]. When oxygen vacancy co-existed, Equations (1) and (2) accelerated and the steps of Equations (3)–(6) were in effect [
62]. Firstly, H
2O absorbed on the surface vacancy of Vo≡Mn
+ defect sites (Equation (3)) to form the surface hydroxyl group [
64]. Secondly, through ligand exchange of the previous surface hydroxyl group which was prior to electron transfer, H
2O
2 adsorbed on the same defect sites [
64] (Equation (4)). Thirdly, the existence of Ov stretched of the O–O bond, which facilitated activation of H
2O
2 to produce ·OH (Equation (5)) [
21,
65,
66]. Finally, the reactive radicals (·OH) readily attacked RHB that adsorbed nearby the active sites (Equation (7)):
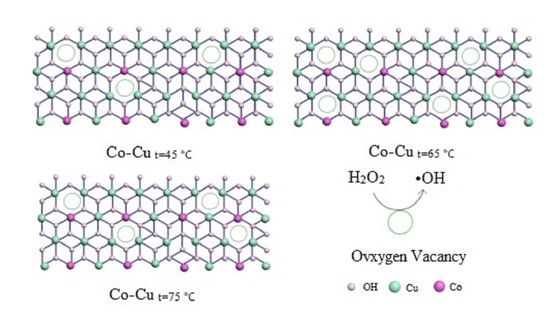
Therefore, Ov plays a key role in the heterogeneous Fenton-like reaction. The higher the content of Ov in the catalyst, the faster the ·OH is generated, which in turn increases the degradation rate of RhB. It can be seen intuitively from
Figure 11 that the Co–Cu
t=65°C catalyst contains more Vo, and this is also the reason for the better performance of the Co–Cu
t=65°C catalyst.