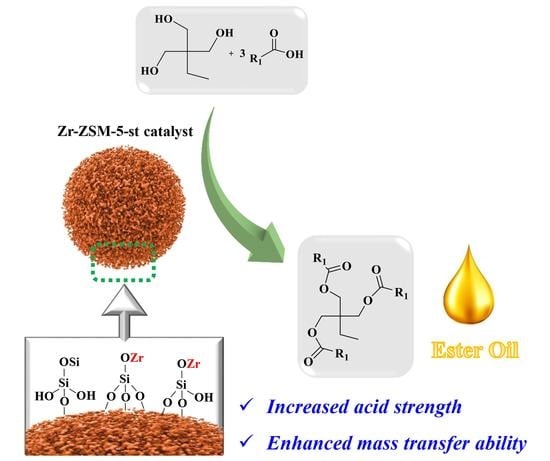
An Efficient Zr-ZSM-5-st Solid Acid Catalyst for the Polyol Esterification Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Catalytic Activity Test
2.3. Discussion
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Activity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, H.G.; Zhao, Q.; Wang, Y.N.; Zhao, Q.L.; Jang, C.; Niu, Y.F.; Lou, W.J.; Qi, Y.X. SnO nanoparticles on graphene oxide as an effective catalyst for synthesis of lubricating ester oils. Cataly. Commun. 2022, 162, 106370–106374. [Google Scholar] [CrossRef]
- Wang, Y.N.; Ma, R.; Jiang, C.; Lou, W.; Wang, X.B. An efficient catalyst COK-15b for the catalytic synthesis of lubricating ester oils. Catal. Commun. 2019, 122, 28–32. [Google Scholar] [CrossRef]
- Yu, S.T.; Wu, S.S.; Li, L.; Ge, X.P. Upgrading bio-oil from waste cooking oil by esterification using SO42−/ZrO2 as catalyst. Fuel 2020, 276, 118019–118024. [Google Scholar] [CrossRef]
- Yu, G.X.; Zhou, X.L.; Li, C.L.; Chen, L.F.; Wang, J.A. Esterification over rare earth oxide and alumina promoted SO42−/ZrO2. Catal. Today 2009, 148, 169–173. [Google Scholar] [CrossRef]
- Zaccheria, F.; Mariani, M.; Psaro, R.; Bondioli, P.; Ravasio, N. Environmentally friendly lubricants through a zero waste process. Appl. Catal. B-Environ. 2016, 181, 581–586. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef]
- Forde, M.M.; Armstrong, R.D.; Hammond, C.; He, Q.; Jenkins, R.L.; Kondrat, S.A.; Dimitratos, N.; Lopez-Sanchez, J.A.; Taylor, S.H.; Willock, D.; et al. Partial oxidation of ethane to oxygenates using Fe- and Cu- containing ZSM-5. J. Am. Chem. Soc. 2013, 135, 11087–11099. [Google Scholar] [CrossRef]
- Shang, J.Y.; Fu, G.B.; Cai, Z.P.; Feng, X.; Tuo, Y.X.; Zhou, X.; Yan, H.; Peng, C.; Jin, X.; Liu, Y.B.; et al. Regulating light olefins or aromatics production in ex-situ catalytic pyrolysis of biomass by engineering the structure of tin modified ZSM-5 catalyst. Bioresource Technol. 2021, 330, 124975. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.Y.; Fan, H.H.; Wang, D.; Rui, N.; Du, Y.H.; Senanayake, S.D.; Xie, Z.H.; Nie, X.W.; Chen, J.G. CO2-assisted ethane aromatization over zinc and phosphorous modified ZSM-5 catalysts. Appl. Catal. B-Environ. 2022, 304, 120956. [Google Scholar] [CrossRef]
- Wang, H.R.; Li, X.C.; Meng, F.R.; Wang, G.Y.; Zhang, D.K. Preparation and evaluation of iron nanoparticles embedded CNTs grown on ZSM-5 as catalysts for NO decomposition. Chem. Eng. J. 2020, 392, 123798. [Google Scholar] [CrossRef]
- Mohebbi, S.; Rostamizadeh, M.; Kahforoushan, D. Efficient sulfated high silica ZSM-5 nanocatalyst for esterification of oleic acid with methanol. Micropor. Mesopor. Mat. 2020, 294, 109845. [Google Scholar] [CrossRef]
- Mahyuddin, M.H.; Staykov, A.; Shiota, Y.; Yoshizawa, K. Direct conversion of methane to methanol by metal-exchanged ZSM-5 zeolite (metal = Fe, Co, Ni, and Cu). ACS Catal. 2016, 6, 8321–8331. [Google Scholar] [CrossRef]
- Hou, X.; Qiu, Y.; Tian, Y.J.; Diao, Z.H.; Zhang, X.W.; Liu, G.Z. Reaction pathways of n-pentane cracking on the fresh and regenerated Sr, Zr and La-loaded ZSM-5 zeolites. Chem. Eng. J. 2018, 349, 297–308. [Google Scholar] [CrossRef]
- Fan, M.J.; Si, Z.K.; Sun, W.J.; Zhang, P.B. Sulfonated ZrO2-TiO2 nanorods as efficient solid acid catalysts for heterogeneous esterification of palmitic acid. Fuel 2019, 252, 254–261. [Google Scholar] [CrossRef]
- Popova, M.; Lazarova, H.; Kalvachev, Y.; Todorova, T.; Szegedi, Á.; Shestakova, P.; Mali, G.; Dasireddy, V.D.B.C.; Likozar, B. Zr-modified hierarchical mordenite as heterogeneous catalyst for glycerol esterification. Catal. Commun. 2017, 100, 10–14. [Google Scholar] [CrossRef]
- Li, G.; Gao, L.; Sheng, Z.Z.; Zhan, Y.L.; Zhang, C.Y.; Ju, J.; Zhang, Y.H.; Tang, Y. A Zr-Al-Beta zeolite with open Zr(IV) sites: An efficient bifunctional Lewis-Brønsted acid catalyst for a cascade reaction. Catal. Sci. Technol. 2019, 9, 4055–4065. [Google Scholar] [CrossRef]
- Groen, J.C.; Moulijn, J.A.; Pérez-Ramírez, J. Decoupling mesoporosity formation and acidity modification in ZSM-5 zeolites by sequential desilication-dealumination. Micropor. Mesopor. Mat. 2005, 87, 153–161. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, D.; Zhang, H.X.; Yin, B.Y.; Liu, Q.R.; Lu, X.H.; Yang, S.J.; Xia, Q.H. In-situ construction and catalytic property of highly exposed Lewis acidity on hierarchical Zr-zeolite assisted by K+ cation. Micropor. Mesopor. Mat. 2021, 324, 110898. [Google Scholar] [CrossRef]
- Li, X.C.; Yuan, X.H.; Xia, G.P.; Liang, J.; Liu, C.; Wang, Z.D.; Yang, W.M. Catalytic production of γ-valerolactone from xylose over delaminated Zr-Al-SCM-1 zeolite via a cascade process. J. Catal. 2020, 392, 175–185. [Google Scholar] [CrossRef]
- Miyake, N.; Brezicki, G.; Davis, R.J. Cascade reaction of ethanol to butadiene over multifunctional silica-supported Ag and ZrO2 Catalysts. ACS Sustain. Chem. Eng. 2022, 10, 1020–1035. [Google Scholar] [CrossRef]
- Liao, Y.H.; Zhong, R.Y.; d’Halluin, M.; Verboekend, D.; Sels, B.F. Aromatics production from lignocellulosic biomass: Shape selective dealkylation of lignin-derived phenolics over hierarchical ZSM-5. ACS Sustain. Chem. Eng. 2020, 8, 8713–8722. [Google Scholar] [CrossRef]
- Holzinger, J.; Beato, P.; Lundegaard, L.F.; Skibsted, J. Distribution of aluminum over the tetrahedral sites in ZSM-5 zeolites and their evolution after steam treatment. J. Phys. Chem. C 2018, 122, 15595–15613. [Google Scholar] [CrossRef]
- Xue, Y.F.; Niu, Y.L.; Zheng, H.Y.; Cui, X.J.; Ma, Q.G.; Tang, J.K.; Deng, L.F. Selective dealumination of ZSM-5 by steaming and its effect on ethanol to propene. J. Fuel Chem. Technol. 2021, 49, 1111–1121. [Google Scholar] [CrossRef]
- Yang, B.T.; Jiang, J.G.; Zhang, K.; Wu, P. Synthesis of novel titanosilicate catalysts by simultaneous isomorphous substitution and interlayer expansion of zeolitic layered silicates. Chem. Mater. 2016, 28, 5295–5303. [Google Scholar] [CrossRef]
- Miyake, K.; Inoue, R.; Miura, T.; Nakai, M.; Al-Jabri, H.; Hirota, Y.; Uchida, Y.; Tanaka, S.; Miyamoto, M.; Inagaki, S.; et al. Improving hydrothermal stability of acid sites in MFI type aluminosilicate zeolite (ZSM-5) by coating MFI type all silica zeolite (silicalite-1) shell layer. Micropor. Mesopor. Mat. 2019, 288, 109523. [Google Scholar] [CrossRef]
- Kis, A.K.; Omota, F.; Dimian, A.C.; Rothenberg, G. The heterogeneous advantage: Biodiesel by catalytic reactive distillation. Top. Catal. 2006, 40, 141–150. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves, Structure, Chemistry and Use; John Wiley & Sons: NewYork, NY, USA, 1974. [Google Scholar]
- Sani, Y.M.; Alaba, P.A.; Raji-Yahya, A.O.; Aziz, A.R.A.; Daud, W.M.A.W. Facile synthesis of sulfated mesoporous Zr/ZSM-5with improved Brønsted acidity and superior activity over SZr/Ag, SZr/Ti, andSZr/W in transforming UFO into biodiesel. J. Taiwan Ins. Chem. E. 2016, 60, 247–257. [Google Scholar] [CrossRef]
- Almutairi, S.M.T.; Mezari, B.; Pidko, E.A.; Magusin, P.C.M.M.; Hensen, E.J.M. Influence of steaming on the acidity and the methanol conversion reaction of HZSM-5 zeolite. J. Cata. 2013, 307, 194–203. [Google Scholar] [CrossRef]
- Kuzminska, M.; Backov, R.; Gaigneaux, E.M. Behavior of cation-exchange resins employed as heterogeneous catalysts for esterification of oleic acid with tri-methylolpropane. Appl. Catal. A: Gen. 2015, 504, 11–16. [Google Scholar] [CrossRef]
- Åkerman, C.O.; Hagström, A.E.V.; Mollaahmad, M.A.; Stefan, K.B.; Hatti-Kaul, R. Bio-lubricant synthesis using immobilisedlipase: Process optimization of tri-methylolpropane oleate production. Process Bio. Chem. 2011, 46, 2225–2231. [Google Scholar] [CrossRef]





| Catalyst a | TAN b | Yield c (%) | Zr (wt.%) d | Al (wt.%) e |
|---|---|---|---|---|
| None | 75.0 | 80.51 | 0 | 0 |
| ZSM-5 | 63.1 | 82.32 | 0 | 1.35 |
| Zr(40 f)-ZSM-5 | 42.7 | 88.33 | 4.63 | 1.34 |
| Zr(40)-ZSM-5-st(400 g) | 29.7 | 91.91 | 4.79 | 1.07 |
| Zr(40)-ZSM-5-st(500) | 20.9 | 94.41 | 4.77 | 0.95 |
| Zr(40)-ZSM-5-st(600) | 20.2 | 94.38 | 4.81 | 0.88 |
| Zr(40)-ZSM-5-st(700) | 25.9 | 92.82 | 4.74 | 0.82 |
| Zr(133)-ZSM-5-st(500) | 55.13 | 84.72 | 1.30 | 0.96 |
| Zr(67)-ZSM-5-st(500) | 31.89 | 91.39 | 2.44 | 0.94 |
| Zr(12)-ZSM-5-st(500) | 19.9 | 94.47 | 10.37 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, H.; Zong, Z.; Lou, W.; Zhao, Q.; Wang, X.; Feng, X.; Qi, Y.; Song, Z. An Efficient Zr-ZSM-5-st Solid Acid Catalyst for the Polyol Esterification Reaction. Catalysts 2022, 12, 901. https://doi.org/10.3390/catal12080901
Su H, Zong Z, Lou W, Zhao Q, Wang X, Feng X, Qi Y, Song Z. An Efficient Zr-ZSM-5-st Solid Acid Catalyst for the Polyol Esterification Reaction. Catalysts. 2022; 12(8):901. https://doi.org/10.3390/catal12080901
Chicago/Turabian StyleSu, Huaigang, Ze Zong, Wenjing Lou, Qin Zhao, Xiaobo Wang, Xiang Feng, Yanxing Qi, and Zhaoning Song. 2022. "An Efficient Zr-ZSM-5-st Solid Acid Catalyst for the Polyol Esterification Reaction" Catalysts 12, no. 8: 901. https://doi.org/10.3390/catal12080901