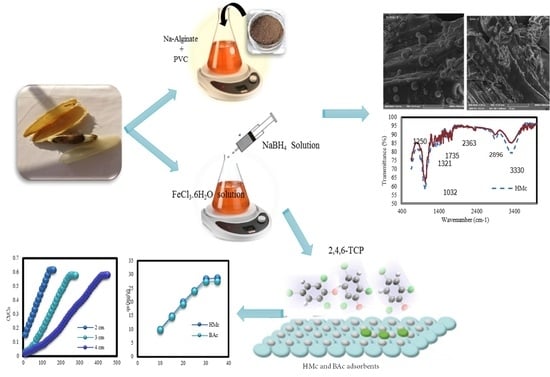
Mango Seed-Derived Hybrid Composites and Sodium Alginate Beads for the Efficient Uptake of 2,4,6-Trichlorophenol from Simulated Wastewater
Abstract
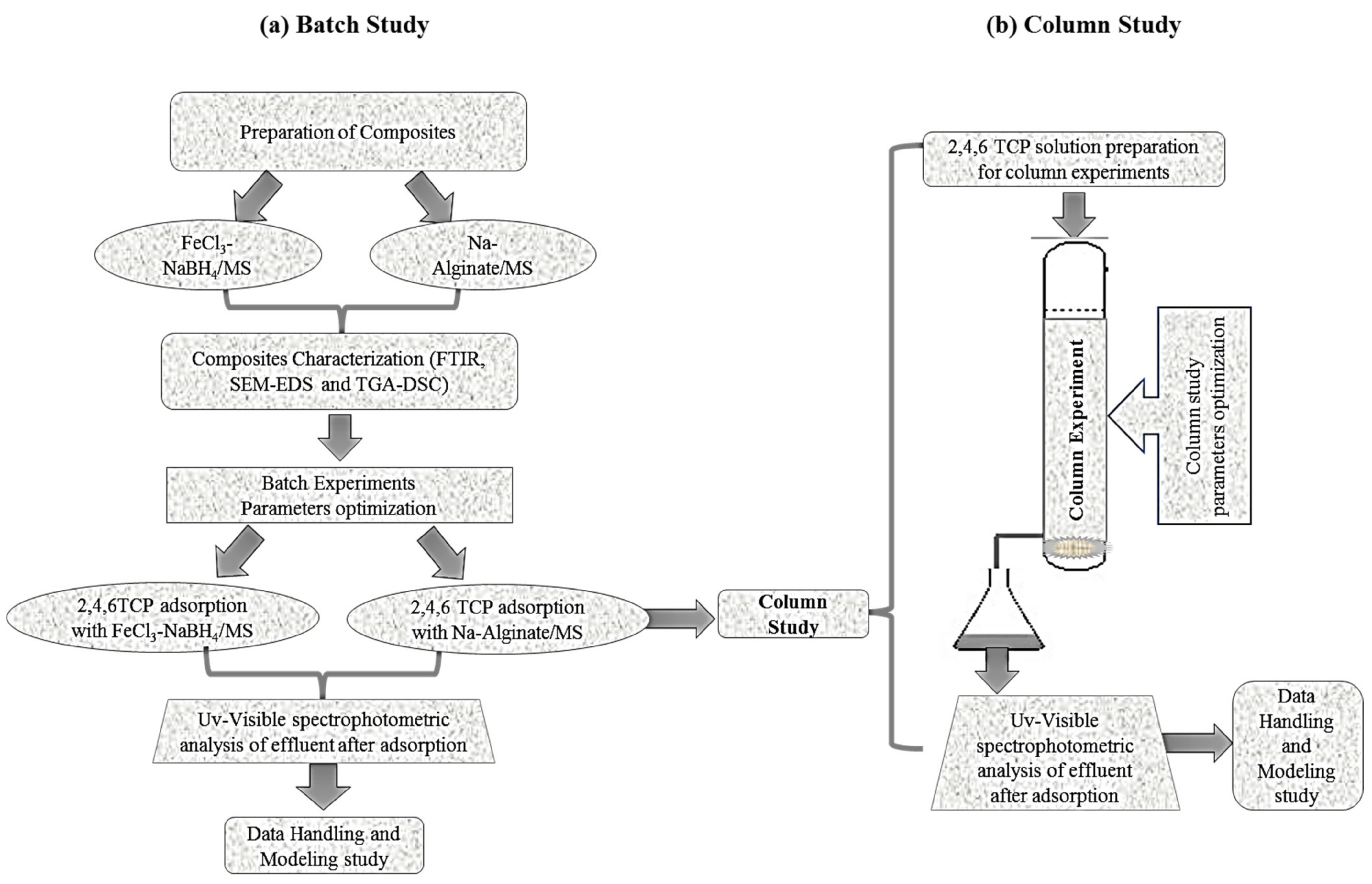
:1. Introduction
2. Results and Discussion
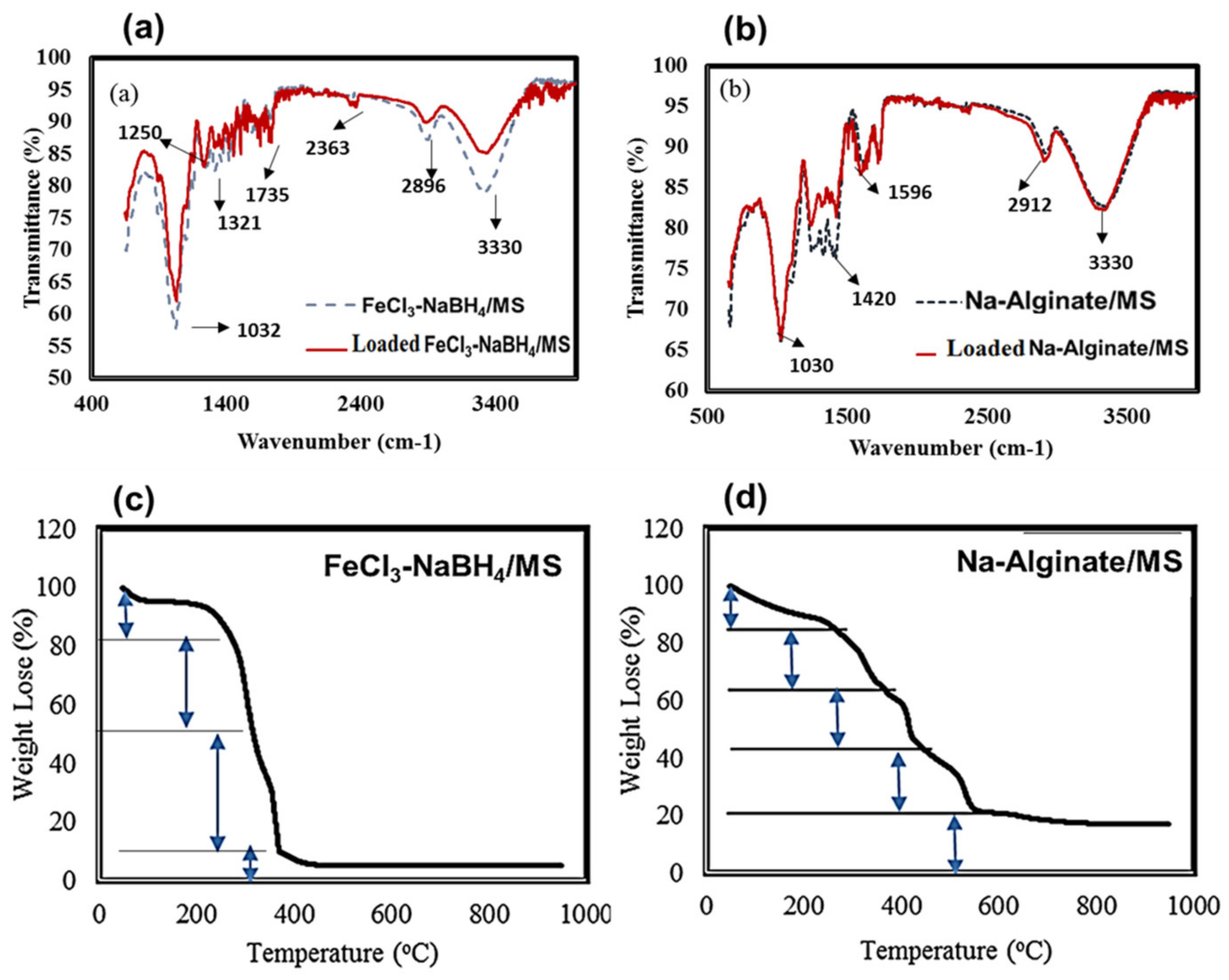
2.1. Functional, Thermal, and Morphological Analysis of Composite Materials
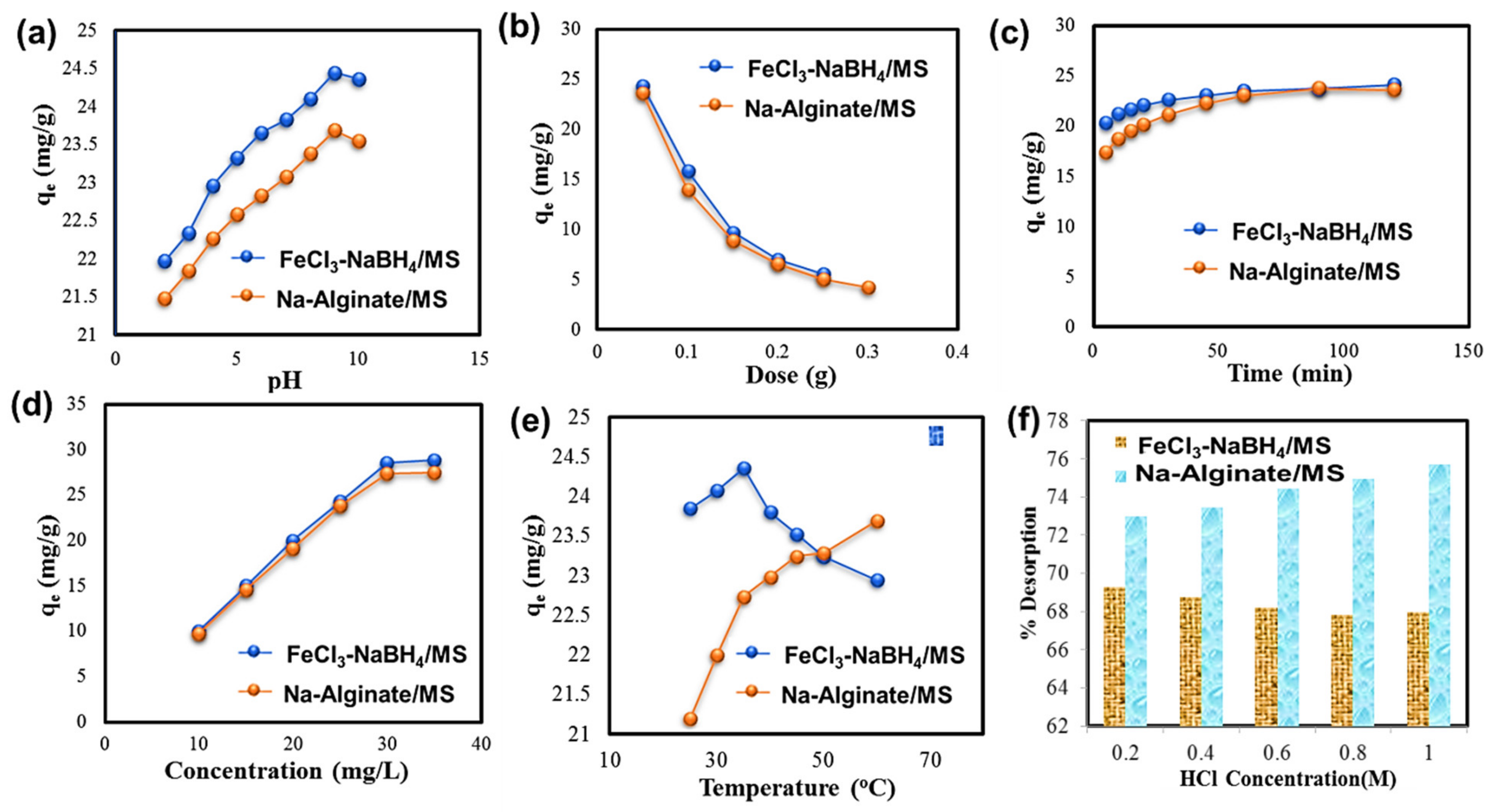
2.2. Batch Experimental Study
2.2.1. Influence of pH
2.2.2. Influence of Adsorbent Dosage
2.2.3. Influence of Contact Time and Kinetic Study
2.2.4. Influence of Initial Concentration and Equilibrium Studies
2.2.5. Influence of Temperature and Thermodynamics Studies
2.3. Desorption Study
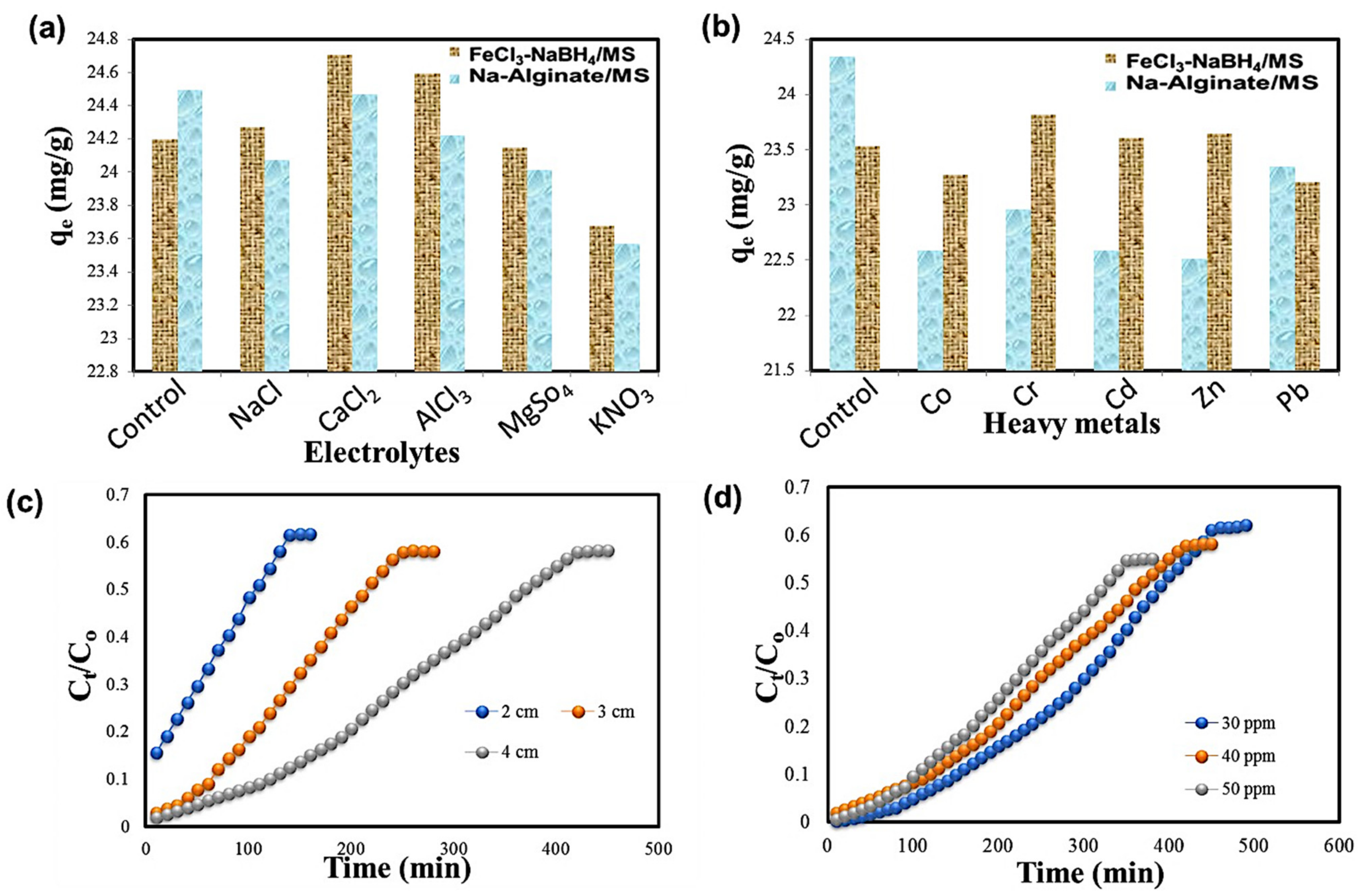
2.4. Influence of Electrolytes and Heavy Metals
2.5. Column Study
2.5.1. Influence of Bed Height
2.5.2. Influence of 2,4,6-TCP Initial Dye Concentration
2.5.3. Models for Column Study
3. Synthetic Methodologies
3.1. Chemical Reagents and Biomass
3.2. Preparation of Composite and Composite Beads
3.3. Characterization of Composites
3.4. Preparation of 2,4,6-TCP Stock Solution
3.5. Experimental Batch Study
3.6. Desorption Study
3.7. Column Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devi, P.; Saroha, A.K. Simultaneous adsorption and dechlorination of pentachlorophenol from effluent by Ni–ZVI magnetic biochar composites synthesized from paper mill sludge. Chem. Eng. J. 2015, 271, 195–203. [Google Scholar] [CrossRef]
- Wang, L.; Gan, K.; Lu, D.; Zhang, J. Hydrophilic Fe3O4 @C for High-Capacity Adsorption of 2,4-Dichlorophenol. Eur. J. Inorg. Chem. 2016, 2016, 890–896. [Google Scholar] [CrossRef]
- Shi, W.; Ren, H.; Huang, X.; Li, M.; Tang, Y.; Guo, F. Low cost red mud modified graphitic carbon nitride for the removal of organic pollutants in wastewater by the synergistic effect of adsorption and photocatalysis. Sep. Purif. Technol. 2019, 237, 116477. [Google Scholar] [CrossRef]
- Pei, Z.; Li, L.; Sun, L.; Zhang, S.; Shan, X.-Q.; Yang, S.; Wen, B. Adsorption characteristics of 1,2,4-trichlorobenzene, 2,4,6-trichlorophenol, 2-naphthol and naphthalene on graphene and graphene oxide. Carbon 2013, 51, 156–163. [Google Scholar] [CrossRef]
- Liu, H.; Ruan, X.; Zhao, D.; Fan, X.; Feng, T. Enhanced Adsorption of 2,4-Dichlorophenol by Nanoscale Zero-Valent Iron Loaded on Bentonite and Modified with a Cationic Surfactant. Ind. Eng. Chem. Res. 2016, 56, 191–197. [Google Scholar] [CrossRef]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef]
- Heo, J.; Yoon, Y.; Lee, G.; Kim, Y.; Han, J.; Park, C.M. Enhanced adsorption of bisphenol A and sulfamethoxazole by a novel magnetic CuZnFe2O4–biochar composite. Bioresour. Technol. 2019, 281, 179–187. [Google Scholar] [CrossRef]
- Ahmad, N.; Al-Fatesh, A.S.; Wahab, R.; Alam, M.; Fakeeha, A.H. Synthesis of silver nanoparticles decorated on reduced graphene oxide nanosheets and their electrochemical sensing towards hazardous 4-nitrophenol. J. Mater. Sci. Mater. Electron. 2020, 31, 11927–11937. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, B. Bisphenol A adsorption using reduced graphene oxide prepared by physical and chemical reduction methods. Chem. Eng. Res. Des. 2015, 104, 519–529. [Google Scholar] [CrossRef]
- Jin, M.-Y.; Lin, Y.; Liao, Y.; Tan, C.-H.; Wang, R. Development of highly-efficient ZIF-8@PDMS/PVDF nanofibrous composite membrane for phenol removal in aqueous-aqueous membrane extractive process. J. Membr. Sci. 2018, 568, 121–133. [Google Scholar] [CrossRef]
- Chen, X.H.; Shan, Z.J.; Zhai, H.L. QSAR models for predicting the toxicity of halogenated phenols to Tetrahymena. Toxicol. Environ. Chem. 2016, 99, 273–284. [Google Scholar] [CrossRef]
- Gomri, M.; Abderrazak, H.; Chabbah, T.; Souissi, R.; Saint-Martin, P.; Casabianca, H.; Chatti, S.; Mercier, R.; Errachid, A.; Hammami, M.; et al. Adsorption characteristics of aromatic pollutants and their halogenated derivatives on bio-based poly (ether-pyridine)s. J. Environ. Chem. Eng. 2020, 8, 104333. [Google Scholar] [CrossRef]
- Pham, T.D.; Bui, T.T.; Truong, T.T.T.; Hoang, T.H.; Le, T.S.; Duong, V.D.; Yamaguchi, A.; Kobayashi, M.; Adachi, Y. Adsorption characteristics of beta-lactam cefixime onto nanosilica fabricated from rice HUSK with surface modification by polyelectrolyte. J. Mol. Liq. 2019, 298, 111981. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.-J. Microwave-assisted acid functionalized carbon nanofibers decorated with Mn doped TNTs nanocomposites: Efficient contenders for lithium adsorption and recovery from aqueous media. J. Ind. Eng. Chem. 2020, 92, 263–277. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.-J. Functionalized titanate nanotubes for efficient lithium adsorption and recovery from aqueous media. J. Solid State Chem. 2019, 283, 121157. [Google Scholar] [CrossRef]
- Shankar, A.; Kongot, M.; Saini, V.K.; Kumar, A. Removal of pentachlorophenol pesticide from aqueous solutions using modified chitosan. Arab. J. Chem. 2020, 13, 1821–1830. [Google Scholar] [CrossRef]
- Naganathan, K.K.; Faizal, A.N.M.; Zaini, M.A.A.; Ali, A. Adsorptive removal of Bisphenol a from aqueous solution using activated carbon from coffee residue. Mater. Today: Proc. 2021, 47, 1307–1312. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Z.; Yang, J. A novel composite hydrogel for adsorption and photocatalytic degradation of bisphenol A by visible light irradiation. Chem. Eng. J. 2018, 334, 1679–1690. [Google Scholar] [CrossRef]
- Batra, S.; Datta, D.; Beesabathuni, N.S.; Kanjolia, N.; Saha, S. Adsorption of Bisphenol-A from aqueous solution using amberlite XAD-7 impregnated with aliquat 336: Batch, column, and design studies. Process Saf. Environ. Prot. 2018, 122, 232–246. [Google Scholar] [CrossRef]
- Men, X.; Guo, Q.; Meng, B.; Ren, S.; Shen, B. Adsorption of bisphenol A in aqueous solution by composite bentonite with organic moity. Microporous Mesoporous Mater. 2020, 308, 110450. [Google Scholar] [CrossRef]
- Kamran, U.; Heo, Y.-J.; Lee, J.W.; Park, S.-J. Chemically modified activated carbon decorated with MnO2 nanocomposites for improving lithium adsorption and recovery from aqueous media. J. Alloy. Compd. 2019, 794, 425–434. [Google Scholar] [CrossRef]
- Duan, F.; Chen, C.; Zhao, X.; Yang, Y.; Liu, X.; Qin, Y. Water-compatible surface molecularly imprinted polymers with synergy of bi-functional monomers for enhanced selective adsorption of bisphenol A from aqueous solution. Environ. Sci. Nano 2016, 3, 213–222. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Isiuku, B.O. 2,4,6-Trichlorophenol (TCP) removal from aqueous solution using Canna indica L.: Kinetic, isotherm and Thermodynamic studies. Chem. Ecol. 2020, 37, 64–82. [Google Scholar] [CrossRef]
- Sahnoun, S.; Boutahala, M.; Zaghouane-Boudiaf, H.; Zerroual, L. Trichlorophenol removal from aqueous solutions by modified halloysite: Kinetic and equilibrium studies. DESALINATION Water Treat. 2015, 57, 15941–15951. [Google Scholar] [CrossRef]
- Obinna, I.B.; Ebere, E.C. A review: Water pollution by heavy metal and organic pollutants: Brief review of sources, effects and progress on remediation with aquatic plants. Anal. Methods Environ. Chem. J. 2019, 2, 5–38. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.-J. MnO2-decorated biochar composites of coconut shell and rice husk: An efficient lithium ions adsorption-desorption performance in aqueous media. Chemosphere 2020, 260, 127500. [Google Scholar] [CrossRef]
- Kamran, U.; Bhatti, H.N.; Noreen, S.; Tahir, M.A.; Park, S.-J. Chemically modified sugarcane bagasse-based biocomposites for efficient removal of acid red 1 dye: Kinetics, isotherms, thermodynamics, and desorption studies. Chemosphere 2021, 291, 132796. [Google Scholar] [CrossRef]
- Kamran, U.; Bhatti, H.N.; Iqbal, M.; Jamil, S.; Zahid, M. Biogenic synthesis, characterization and investigation of photocatalytic and antimicrobial activity of manganese nanoparticles synthesized from Cinnamomum verum bark extract. J. Mol. Struct. 2018, 1179, 532–539. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption isotherms, kinetics, thermodynamics and desorption studies of 2,4,6-trichlorophenol on oil palm empty fruit bunch-based activated carbon. J. Hazard. Mater. 2009, 164, 473–482. [Google Scholar] [CrossRef]
- Mubarik, S.; Saeed, A.; Athar, M.; Iqbal, M. Characterization and mechanism of the adsorptive removal of 2,4,6-trichlorophenol by biochar prepared from sugarcane baggase. J. Ind. Eng. Chem. 2016, 33, 115–121. [Google Scholar] [CrossRef]
- Lisowski, P.; Colmenares, J.C.; Mašek, O.; Lisowski, W.; Lisovytskiy, D.; Kamińska, A.; Łomot, D. Dual Functionality of TiO2/Biochar Hybrid Materials: Photocatalytic Phenol Degradation in the Liquid Phase and Selective Oxidation of Methanol in the Gas Phase. ACS Sustain. Chem. Eng. 2017, 5, 6274–6287. [Google Scholar] [CrossRef]
- Gundogdu, A.; Duran, C.; Senturk, H.B.; Soylak, M.; Ozdes, D.; Serencam, H.; Imamoglu, M. Adsorption of Phenol from Aqueous Solution on a Low-Cost Activated Carbon Produced from Tea Industry Waste: Equilibrium, Kinetic, and Thermodynamic Study. J. Chem. Eng. Data 2012, 57, 2733–2743. [Google Scholar] [CrossRef]
- Tao, X.; Zhou, G.; Zhuang, X.; Cheng, B.; Li, X.; Li, H. Solution blowing of activated carbon nanofibers for phenol adsorption. RSC Adv. 2014, 5, 5801–5808. [Google Scholar] [CrossRef]
- Diao, Z.-H.; Xu, X.-R.; Chen, H.; Jiang, D.; Yang, Y.-X.; Kong, L.-J.; Sun, Y.-X.; Hu, Y.-X.; Hao, Q.-W.; Liu, L. Simultaneous removal of Cr (VI) and phenol by persulfate activated with bentonite-supported nanoscale zero-valent iron: Reactivity and mechanism. J. Hazard. Mater. 2016, 316, 186–193. [Google Scholar] [CrossRef]
- Lunagariya, J.; Chabhadiya, K.; Pathak, P.; Mashru, D. Application of Taguchi method in activated carbon adsorption process of phenol removal from ceramic gasifier wastewater. Environ. Challenges 2022, 6, 100450. [Google Scholar] [CrossRef]
- Kamran, U.; Bhatti, H.N.; Iqbal, M.; Nazir, A. Green synthesis of metal nanoparticles and their applications in different fields: A review. Z. Phys. Chem. 2019, 233, 1325–1349. [Google Scholar] [CrossRef]
- Giraldo, L.; Moreno-Piraján, J.C. Study of adsorption of phenol on activated carbons obtained from eggshells. J. Anal. Appl. Pyrolysis 2014, 106, 41–47. [Google Scholar] [CrossRef]
- Sagbas, S.; Kantar, C.; Sahiner, N. Preparation of Poly(Humic Acid) Particles and Their Use in Toxic Organo-Phenolic Compound Removal from Aqueous Environments. Water, Air, Soil Pollut. 2013, 225, 1–10. [Google Scholar] [CrossRef]
- Tamang, M.; Paul, K.K. Adsorptive treatment of phenol from aqueous solution using chitosan/calcined eggshell adsorbent: Optimization of preparation process using Taguchi statistical analysis. J. Indian Chem. Soc. 2022, 99, 100251. [Google Scholar] [CrossRef]
- Issabayeva, G.; Hang, S.Y.; Wong, M.C.; Aroua, M.K. A review on the adsorption of phenols from wastewater onto diverse groups of adsorbents. Rev. Chem. Eng. 2017, 34, 855–873. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Vasilieva, N.Y.; Fetisova, O.Y.; Sychev, V.V.; Elsuf’Ev, E.V.; Malyar, Y.N.; Issaoui, N.; Miroshnikova, A.V.; Borovkova, V.S.; Kazachenko, A.S.; et al. New reactions of betulin with sulfamic acid and ammonium sulfamate in the presence of solid catalysts. Biomass Convers. Biorefinery 2022, 1–12. [Google Scholar] [CrossRef]
- Fathy, M.; Selim, H.; Shahawy, A.E.L. Chitosan/MCM-48 nanocomposite as a potential adsorbent for removing phenol from aqueous solution. RSC Adv. 2020, 10, 23417–23430. [Google Scholar] [CrossRef]
- Salari, M.; Dehghani, M.H.; Azari, A.; Motevalli, M.D.; Shabanloo, A.; Ali, I. High performance removal of phenol from aqueous solution by magnetic chitosan based on response surface methodology and genetic algorithm. J. Mol. Liq. 2019, 285, 146–157. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.-J. Hybrid biochar supported transition metal doped MnO2 composites: Efficient contenders for lithium adsorption and recovery from aqueous solutions. Desalination 2021, 522, 115387. [Google Scholar] [CrossRef]
- Soni, U.; Bajpai, J.; Singh, S.K.; Bajpai, A. Evaluation of chitosan-carbon based biocomposite for efficient removal of phenols from aqueous solutions. J. Water Process Eng. 2017, 16, 56–63. [Google Scholar] [CrossRef]
- Anirudhan, T.; Ramachandran, M. Removal of 2,4,6-trichlorophenol from water and petroleum refinery industry effluents by surfactant-modified bentonite. J. Water Process Eng. 2014, 1, 46–53. [Google Scholar] [CrossRef]
- Hussain, A.; Dubey, S.K.; Kumar, V. Kinetic study for aerobic treatment of phenolic wastewater. Water Resour. Ind. 2015, 11, 81–90. [Google Scholar] [CrossRef]
- Olu-Owolabi, B.I.; Alabi, A.H.; Diagboya, P.N.; Unuabonah, E.I.; Düring, R.-A. Adsorptive removal of 2,4,6-trichlorophenol in aqueous solution using calcined kaolinite-biomass composites. J. Environ. Manag. 2017, 192, 94–99. [Google Scholar] [CrossRef]
- Loh, C.H.; Zhang, Y.; Goh, S.; Wang, R.; Fane, A.G. Composite hollow fiber membranes with different poly(dimethylsiloxane) intrusions into substrate for phenol removal via extractive membrane bioreactor. J. Membr. Sci. 2015, 500, 236–244. [Google Scholar] [CrossRef]
- Sierra, J.D.M.; Wang, W.; Cerqueda-Garcia, D.; Oosterkamp, M.J.; Spanjers, H.; van Lier, J.B. Temperature susceptibility of a mesophilic anaerobic membrane bioreactor treating saline phenol-containing wastewater. Chemosphere 2018, 213, 92–102. [Google Scholar] [CrossRef]
- Daramola, M.; Sadare, O.; Oluwasina, O.; Iyuke, S. Synthesis and Application of Functionalized Carbon Nanotube Infused Polymer Membrane (fCNT/PSF/PVA) for Treatment of Phenol-Containing Wastewater. J. Membr. Sci. Res. 2019, 5, 310–316. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Gayatri, S.L. Activated Tea Waste as a Potential Low-Cost Adsorbent for the Removal of p-Nitrophenol from Wastewater. J. Chem. Eng. Data 2010, 55, 4614–4623. [Google Scholar] [CrossRef]
- El-Naas, M.; Al-Zuhair, S.; Abu-Alhaija, M. Removal of phenol from petroleum refinery wastewater through adsorption on date-pit activated carbon. Chem. Eng. J. 2010, 162, 997–1005. [Google Scholar] [CrossRef]
- Agarry, S.E.; Owabor, C.N.; Ajani, A.O. Modified plantain peel as cellulose-based low-cost adsorbent for the removal of 2,6-dichlorophenol from aqueous solution: Adsorption isotherms, kinetic modeling, and thermodynamic studies. Chem. Eng. Commun. 2013, 200, 1121–1147. [Google Scholar] [CrossRef]
- Jabeen, A.; Bhatti, H.N.; Noreen, S.; Gaffar, A. Adsorptive removal of 2,4,6-trichloro-phenol from wastewater by mango seed shell and its magnetic composites: Batch and column study. Int. J. Environ. Anal. Chem. 2021, 1–21. [Google Scholar] [CrossRef]
- Hai, L.; Zhang, T.; Zhang, X.; Zhang, G.; Li, B.; Jiang, S.; Ma, X. Catalytic hydroxylation of phenol to dihydroxybenzene by Fe(II) complex in aqueous phase at ambient temperature. Catal. Commun. 2017, 101, 93–97. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Lee, B.; Choo, K.-H.; Choi, S.-J. Adsorption characteristics of phenolic and amino organic compounds on nano-structured silicas functionalized with phenyl groups. Microporous Mesoporous Mater. 2014, 185, 121–129. [Google Scholar] [CrossRef]
- Kesavan, G.; Nataraj, N.; Chen, S.-M.; Lin, L.-H. Hydrothermal synthesis of NiFe2O4 nanoparticles as an efficient electrocatalyst for the electrochemical detection of bisphenol A. New J. Chem. 2020, 44, 7698–7707. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, P.; Yang, L.; Zhang, J.; Wang, X. Rapid Method for the Separation and Recovery of Endocrine-Disrupting Compound Bisphenol AP from Wastewater. Langmuir 2013, 29, 3968–3975. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die adsorption in lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Temkin, M.J.; Pyzhev, V. Recent modifications to Langmuir isotherms. Acta Phys.Chim. Sin. 1940, 12, 217–222. [Google Scholar]
- Rahman, M.M.; Marwani, H.M.; Algethami, F.K.; Asiri, A.M.; Hameed, S.A.; Alhogbi, B. Ultra-sensitive p-nitrophenol sensing performances based on various Ag 2 O conjugated carbon material composites. Environ. Nanotechnology, Monit. Manag. 2017, 8, 73–82. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Raduskhevich, L.V. Proceedings of the academy of sciences of the USSR. Phys. Chem. 1947, 55, 327–329. [Google Scholar]
- Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon: Part I. Two-parameter models and equations allowing determination of thermodynamic parameters. J. Hazard. Mater. 2007, 147, 381–394. [Google Scholar] [CrossRef]
- López-Luna, J.; Ramírez-Montes, L.E.; Martinez-Vargas, S.; Martínez, A.I.; Mijangos-Ricardez, O.F.; González-Chávez, M.D.C.A.; Carrillo-González, R.; Solís-Domínguez, F.A.; Cuevas-Díaz, M.D.C.; Vázquez-Hipólito, V. Linear and nonlinear kinetic and isotherm adsorption models for arsenic removal by manganese ferrite nanoparticles. SN Appl. Sci. 2019, 1, 1–19. [Google Scholar] [CrossRef]
- Goel, J.; Kadirvelu, K.; Rajagopal, C.; Garg, V.K. Removal of lead(II) by adsorption using treated granular activated carbon: Batch and column studies. J. Hazard. Mater. 2005, 125, 211–220. [Google Scholar] [CrossRef]
- Kamran, U.; Heo, Y.-J.; Min, B.-G.; In, I.; Park, S.-J. Effect of nickel ion doping in MnO2/reduced graphene oxide nanocomposites for lithium adsorption and recovery from aqueous media. RSC Adv. 2020, 10, 9245–9257. [Google Scholar] [CrossRef]
- Juang, R.-S.; Lin, S.-H.; Tsao, K.-H. Sorption of phenols from water in column systems using surfactant-modified montmorillonite. J. Colloid Interface Sci. 2003, 269, 46–52. [Google Scholar] [CrossRef]
- Baek, K.W.; Song, S.H.; Kang, S.H.; Rhee, Y.W.; Lee, C.S.; Lee, B.J.; Hudson, S.; Hwang, T.S. Adsorption kinetics of boron by anion exchange resin in packed column bed. J. Ind. Eng. Chem. 2007, 13, 452–456. [Google Scholar]
- Yousef, R.I.; El-Eswed, B. The effect of pH on the adsorption of phenol and chlorophenols onto natural zeolite. Colloids Surfaces A: Physicochem. Eng. Asp. 2009, 334, 92–99. [Google Scholar] [CrossRef]
- Karunarathne, H.; Amarasinghe, B. Fixed Bed Adsorption Column Studies for the Removal of Aqueous Phenol from Activated Carbon Prepared from Sugarcane Bagasse. Energy Procedia 2013, 34, 83–90. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Jegan, J.; Palanivelu, K.; Velan, M. Removal of nickel(II) ions from aqueous solution using crab shell particles in a packed bed up-flow column. J. Hazard. Mater. 2004, 113, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.C. Heterogeneous Ion Exchange in a Flowing System. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Rao, K.; Anand, S.; Venkateswarlu, P. Modeling the kinetics of Cd(II) adsorption on Syzygium cumini L leaf powder in a fixed bed mini column. J. Ind. Eng. Chem. 2011, 17, 174–181. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Gazi, M. Fixed-bed column sorption of borate onto pomegranate seed powder-PVA beads: A response surface methodology approach. Toxicol. Environ. Chem. 2014, 96, 837–848. [Google Scholar] [CrossRef]
- Cruz-Olivares, J.; Perez-Alonso, C.; Barrera-Díaz, C.; Ureña-Nuñez, F.; Chaparro-Mercado, M.; Bilyeu, B. Modeling of lead (II) biosorption by residue of allspice in a fixed-bed column. Chem. Eng. J. 2013, 228, 21–27. [Google Scholar] [CrossRef]
- Jerold, M.; Vasantharaj, K.; Joseph, D.; Sivasubramanian, V. Fabrication of hybrid biosorbent nanoscale zero-valent iron-Sargassum swartzii biocomposite for the removal of crystal violet from aqueous solution. Int. J. Phytoremediation 2016, 19, 214–224. [Google Scholar] [CrossRef]
- Kakavandi, B.; Jahangiri-Rad, M.; Rafiee, M.; Esfahani, A.R.; Babaei, A.A. Development of response surface methodology for optimization of phenol and p -chlorophenol adsorption on magnetic recoverable carbon. Microporous Mesoporous Mater. 2016, 231, 192–206. [Google Scholar] [CrossRef]
- Motsa, M.M.; Thwala, J.M.; Msagati, T.A.M.; Mamba, B.B. Adsorption of 2,4,6-Trichlorophenol and ortho-Nitrophenol from Aqueous Media Using Surfactant-Modified Clinoptilolite–Polypropylene Hollow Fibre Composites. Water, Air, Soil Pollut. 2011, 223, 1555–1569. [Google Scholar] [CrossRef]
- Pan, J.; Yao, H.; Li, X.; Wang, B.; Huo, P.; Xu, W.; Ou, H.; Yan, Y. Synthesis of chitosan/γ-Fe2O3/fly-ash-cenospheres composites for the fast removal of bisphenol A and 2,4,6-trichlorophenol from aqueous solutions. J. Hazard. Mater. 2011, 190, 276–284. [Google Scholar] [CrossRef]


| Kinetic Model | FeCl3-NaBH4/MS | Na-Alginate/MS |
|---|---|---|
| Pseudo 1st order | ||
| qe exp. (mg/g) | 24.11 | 23.75 |
| qe cal. (mg/g) | −0.363 | −0.091 |
| K1 (min−1) | 0.014 | 0.028 |
| R2 | 0.632 | 0.922 |
| Pseudo 2nd order | ||
| qe exp. (mg/g) | 24.11 | 23.75 |
| qe cal. (mg/g) | 24.39 | 24.93 |
| K2 (g mg−1 min−1) | 0.022 | 0.012 |
| R2 | 0.999 | 0.999 |
| Intraparticle diffusion | ||
| Kpi | 0.411 | 0.735 |
| R2 | 0.923 | 0.920 |
| Ci | 19.99 | 16.64 |
| Isothermal Models | Composites | |
|---|---|---|
| Langmuir | FeCl3-NaBH4/MS | Na-Alginate/MS |
| KL | 0.998 | 0.013 |
| b | 0.0005 | 2.119 |
| qmax Cal. (mg/g) | 28.99 | 29.49 |
| qmax Exp. (mg/g) | 28.77 | 27.42 |
| R2 | 0.999 | 0.996 |
| Freundlich | ||
| KF (mg/g) | 0.144 | 0.096 |
| n | 7.018 | 3.114 |
| R2 | 0.771 | 0.786 |
| D-R | ||
| qm (mg/g) | 8.1×1011 | 4.2×1011 |
| β 104(mol2/kL2) | 0.00001 | 0.0001 |
| E | 235.71 | 70.71 |
| R2 | 0.863 | 0.945 |
| Temkin | ||
| A | 9.284 | 3.23 |
| B | 2.73 | 5.85 |
| R2 | 0.852 | 0.848 |
| Harkins–Jura | ||
| A | 0.001 | 0.109 |
| B | 0.267 | 0.226 |
| R2 | 0.503 | 0.775 |
| Elovich | ||
| KE (L/mg) | 12.838 | 1.4898 |
| qmax Cal. (mg/g) | 3.911 | 11.299 |
| qmax Exp. (mg/g) | 28.77 | 27.42 |
| R2 | 0.784 | 0.647 |
| FeCl3-NaBH4/MS | Na-Alginate/MS | |||||
|---|---|---|---|---|---|---|
| Temp. (K) | ∆G° (kJ·mol−1) | ∆H° (kJ·mol−1) | ∆S° (kJ/mol·K) | ∆G° (kJ·mol−1) | ∆H° (kJ·mol−1) | ∆S° (kJ/mol·K) |
| 297 | −7.49 | −20.74 | 0.042 | −4.24 | 26.19 | 0.103 |
| 303 | −8.20 | −5.01 | ||||
| 308 | −9.31 | −5.91 | ||||
| 313 | −7.77 | −6.33 | ||||
| 318 | −7.33 | −6.83 | ||||
| 323 | −6.94 | −6.997 | ||||
| 333 | −6.68 | −8.01 | ||||
| Inlet Conc. (ppm) | Bed Height (cm) | Flow Rate (mL·min−1) | Breakthrough Point (50%) (min) | Biosorption Capacity (mg/g) |
|---|---|---|---|---|
| 30 | 4 | 1.8 | 400 | 14.4 |
| 40 | 2 | 1.8 | 100 | 14.4 |
| 40 | 3 | 1.8 | 210 | 15.12 |
| 40 | 4 | 1.8 | 360 | 17.28 |
| 50 | 4 | 1.8 | 300 | 18 |
| Inlet Conc. (mg/L) | Bed Height (cm) | Flow Rate (mL/min) | KTh (mL/min) × 103 | qe cal. (mg/g) | qe exp. (mg/g) | R2 |
|---|---|---|---|---|---|---|
| 30 | 4 | 1.8 | 0.00037 | 14.27 | 14.4 | 0.924 |
| 40 | 2 | 1.8 | 0.00035 | 14.85 | 14.4 | 0.980 |
| 40 | 3 | 1.8 | 0.00035 | 15.95 | 15.12 | 0.954 |
| 40 | 4 | 1.8 | 0.00025 | 17.14 | 17.28 | 0.982 |
| 50 | 4 | 1.8 | 0.00024 | 18.92 | 18 | 0.917 |
| Ct/Co | A | B | Ka (L·mg−1·min−1) × 104 | No (mg·L−1) × 10−4 | R2 |
|---|---|---|---|---|---|
| 0.2 | 85 | −151.6 | 0.00024 | 1938 | 0.998 |
| 0.4 | 100 | −110 | 0.00012 | 2280 | 0.970 |
| 0.6 | 145 | −168.3 | 0.00004 | 3306 | 0.990 |
| Pollutants | Adsorbent | Initial Concentration (mg/L) | Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|---|
| 2,6-dichlorophenol | Modified plantain peel adsorbents | 50–100 | 15.9 | [54] |
| Para-Chlorophenol | Magnetic powdered activated carbon | 100–150 | 66.5 | [78] |
| 2,4,6-Trichlorophenol | Modified polypropylene hollow fiber composites | 40–100 | 66.49 | [79] |
| 2,4,6-Trichlorophenol | chitosan/fly-ash-based magnetic composites | 100–150 | 68.89 | [80] |
| Bisphenol A | chitosan/fly-ash-based magnetic composites | 50–150 | 31.92 | [80] |
| 2,4,6-Trichlorophenol | Fe3+- and Fe2+-enriched magnetic composites | 35–50 | 31.27 | [55] |
| 2,4,6-Trichlorophenol | FeCl3-NaBH4/MS | 10–35 | 28.77 | This Study |
| 2,4,6-Trichlorophenol | Na-Alginate/MS | 10–35 | 27.42 | This Study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabeen, A.; Kamran, U.; Noreen, S.; Park, S.-J.; Bhatti, H.N. Mango Seed-Derived Hybrid Composites and Sodium Alginate Beads for the Efficient Uptake of 2,4,6-Trichlorophenol from Simulated Wastewater. Catalysts 2022, 12, 972. https://doi.org/10.3390/catal12090972
Jabeen A, Kamran U, Noreen S, Park S-J, Bhatti HN. Mango Seed-Derived Hybrid Composites and Sodium Alginate Beads for the Efficient Uptake of 2,4,6-Trichlorophenol from Simulated Wastewater. Catalysts. 2022; 12(9):972. https://doi.org/10.3390/catal12090972
Chicago/Turabian StyleJabeen, Asma, Urooj Kamran, Saima Noreen, Soo-Jin Park, and Haq Nawaz Bhatti. 2022. "Mango Seed-Derived Hybrid Composites and Sodium Alginate Beads for the Efficient Uptake of 2,4,6-Trichlorophenol from Simulated Wastewater" Catalysts 12, no. 9: 972. https://doi.org/10.3390/catal12090972