Solid-State Synthesis of ZnO/ZnS Photocatalyst with Efficient Organic Pollutant Degradation Performance
Abstract
:1. Introduction
2. Results
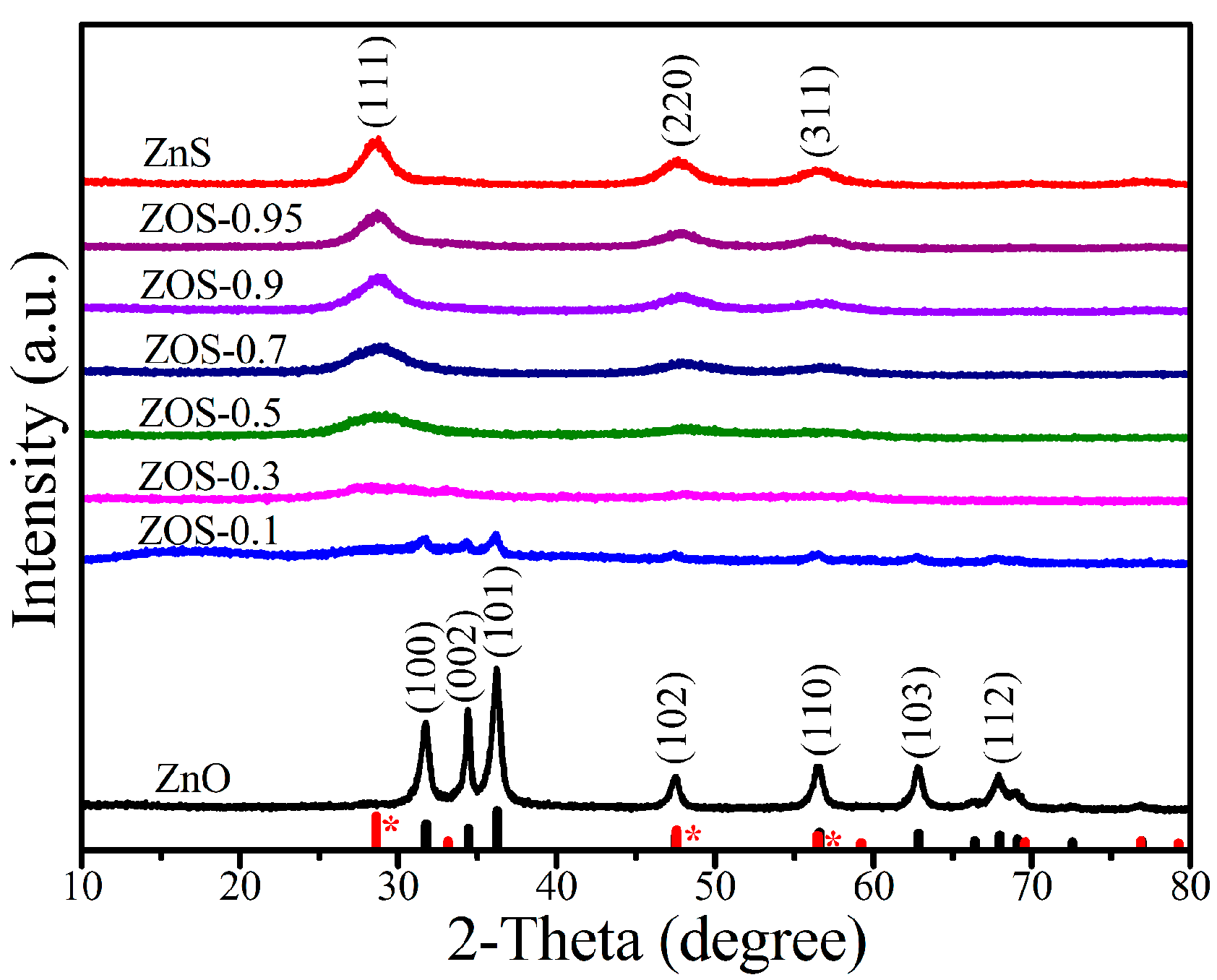
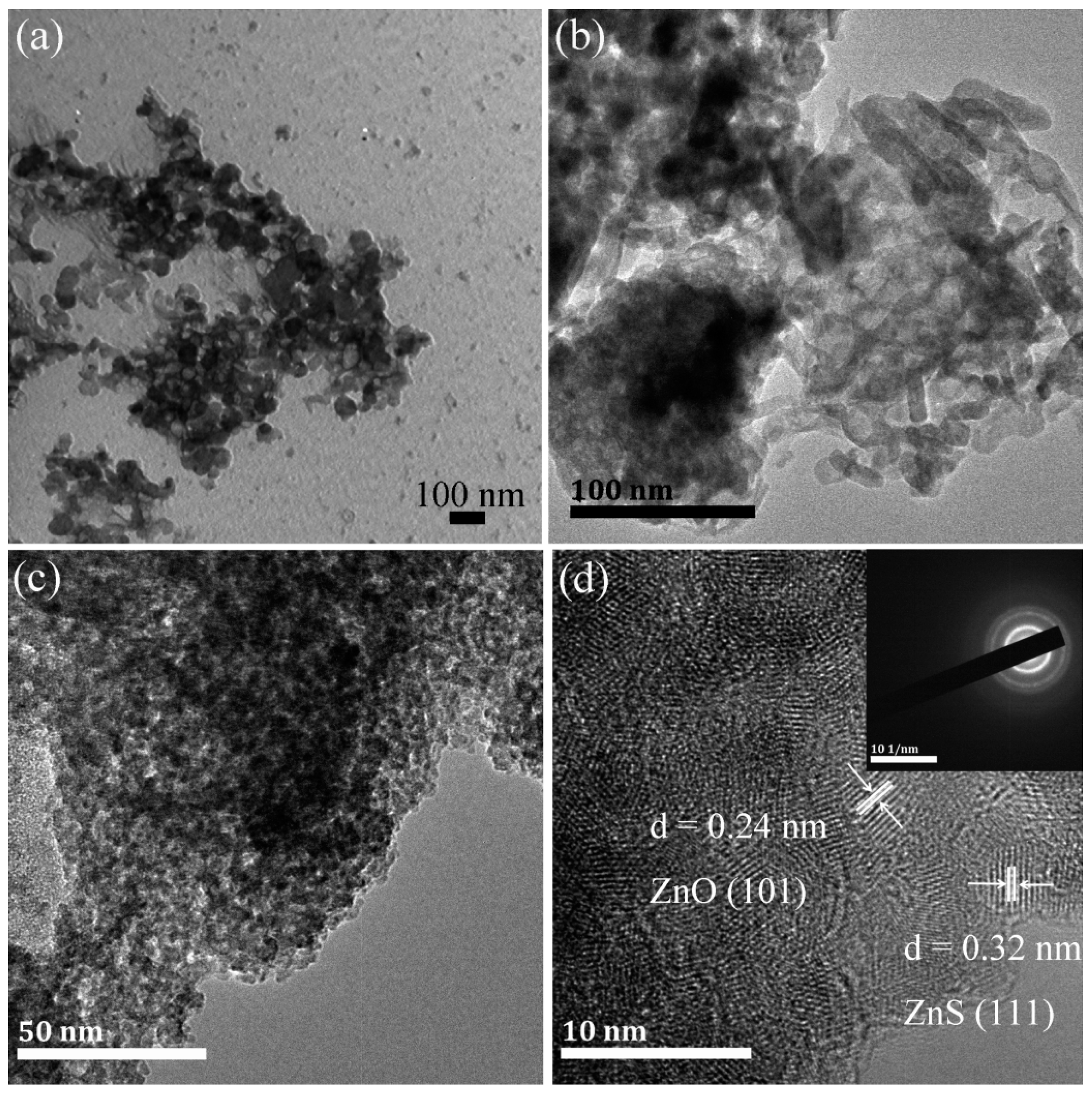
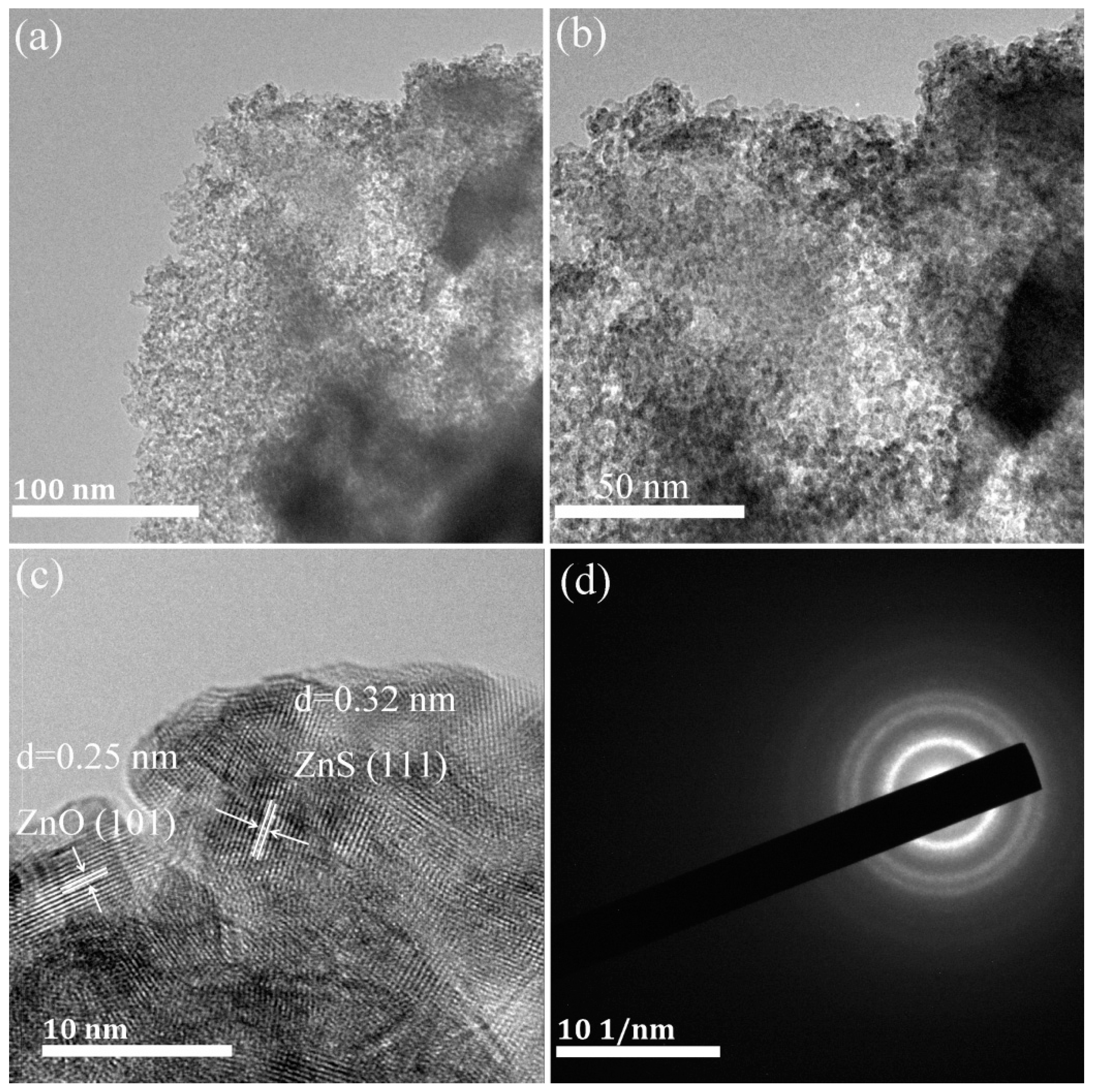
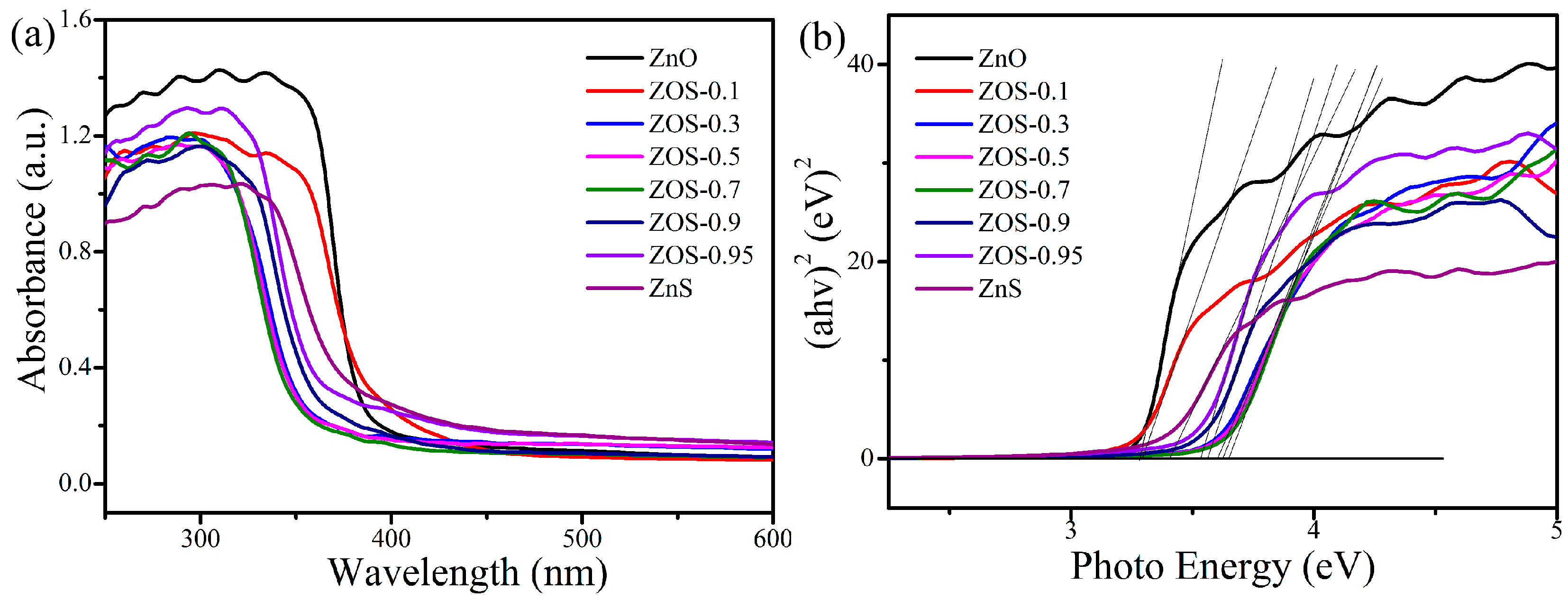
2.1. Composition, Structure and Optical Properties
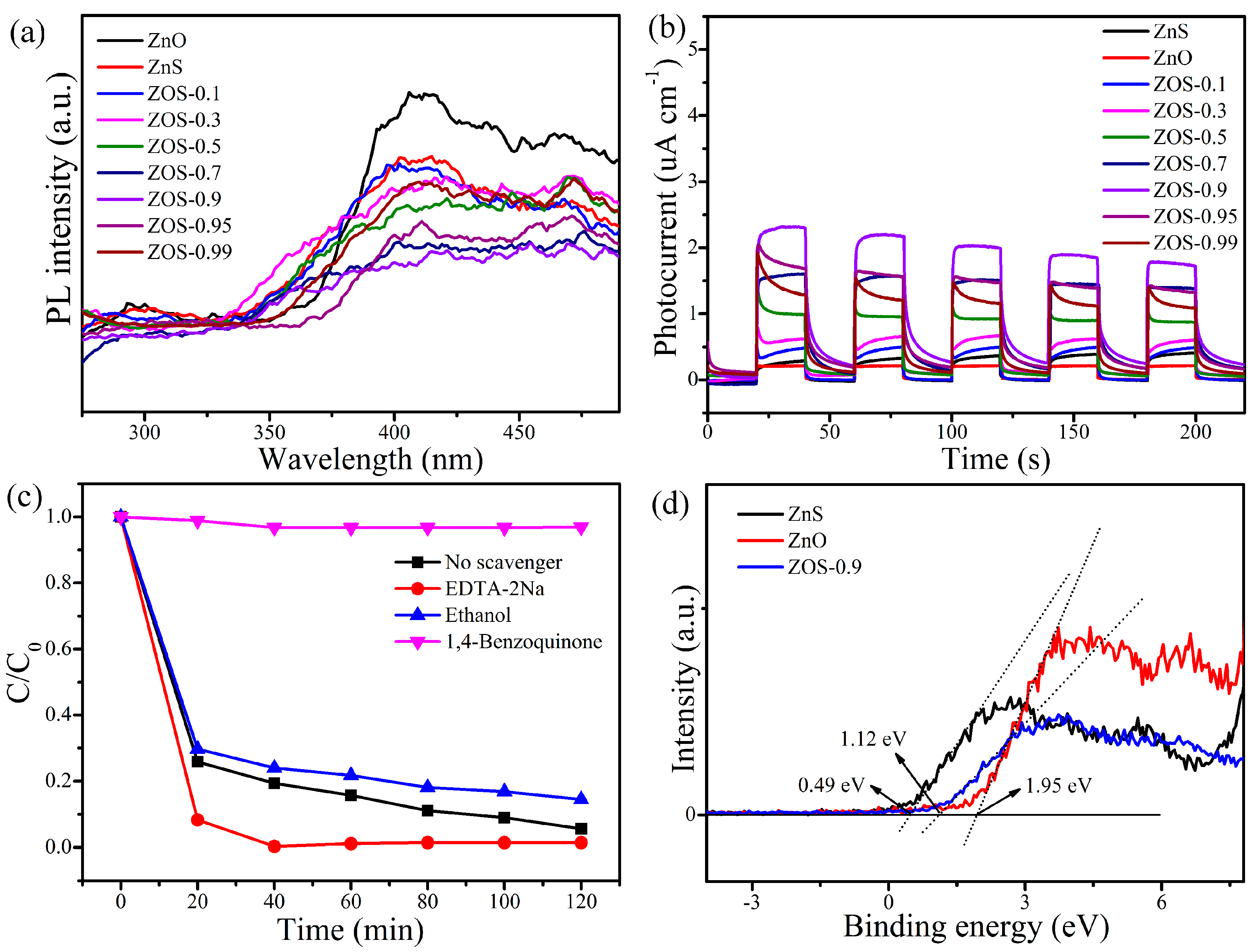
2.2. Photocatalytic Performance
2.3. Photocatalytic Mechanism
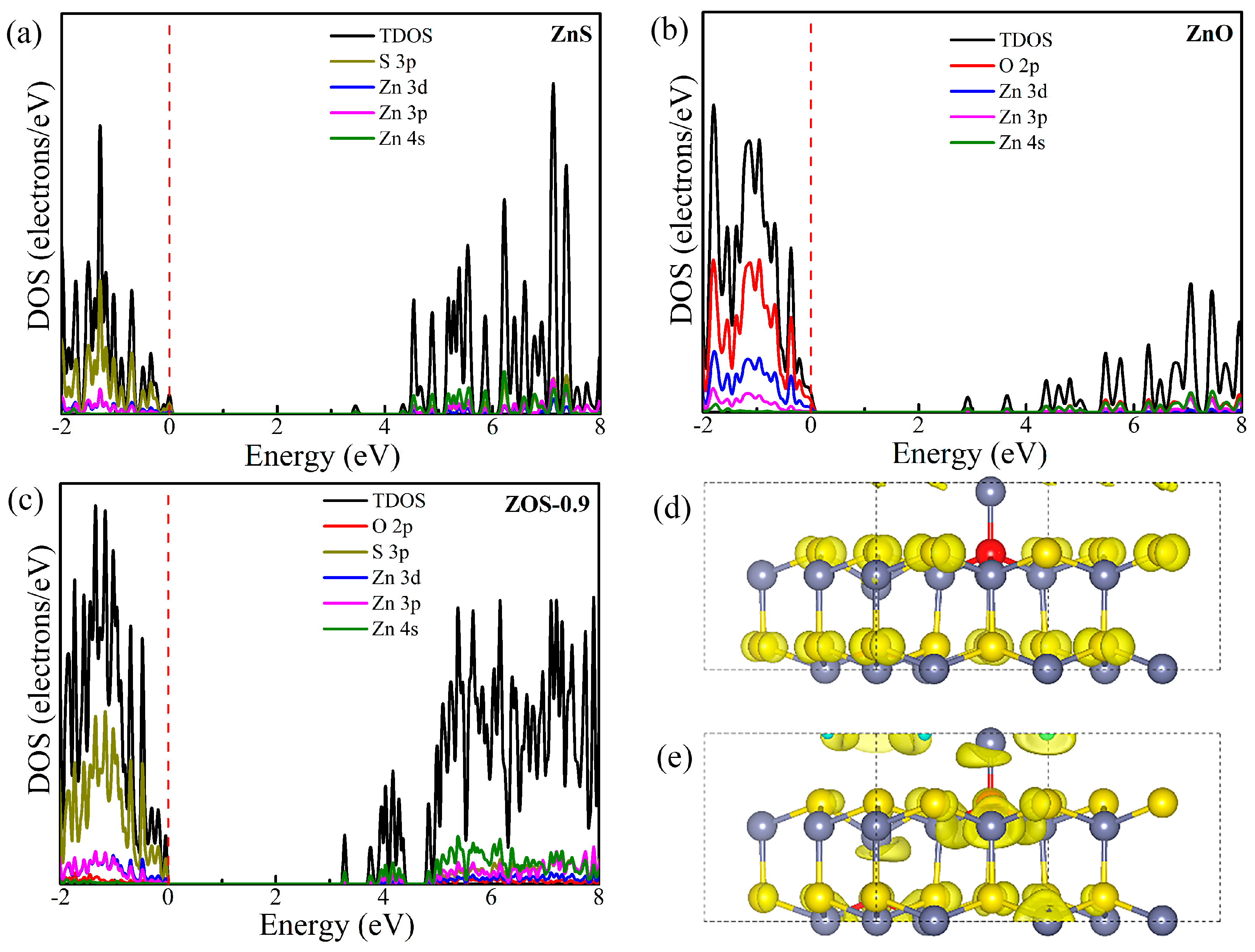
2.3.1. Theoretical Calculation
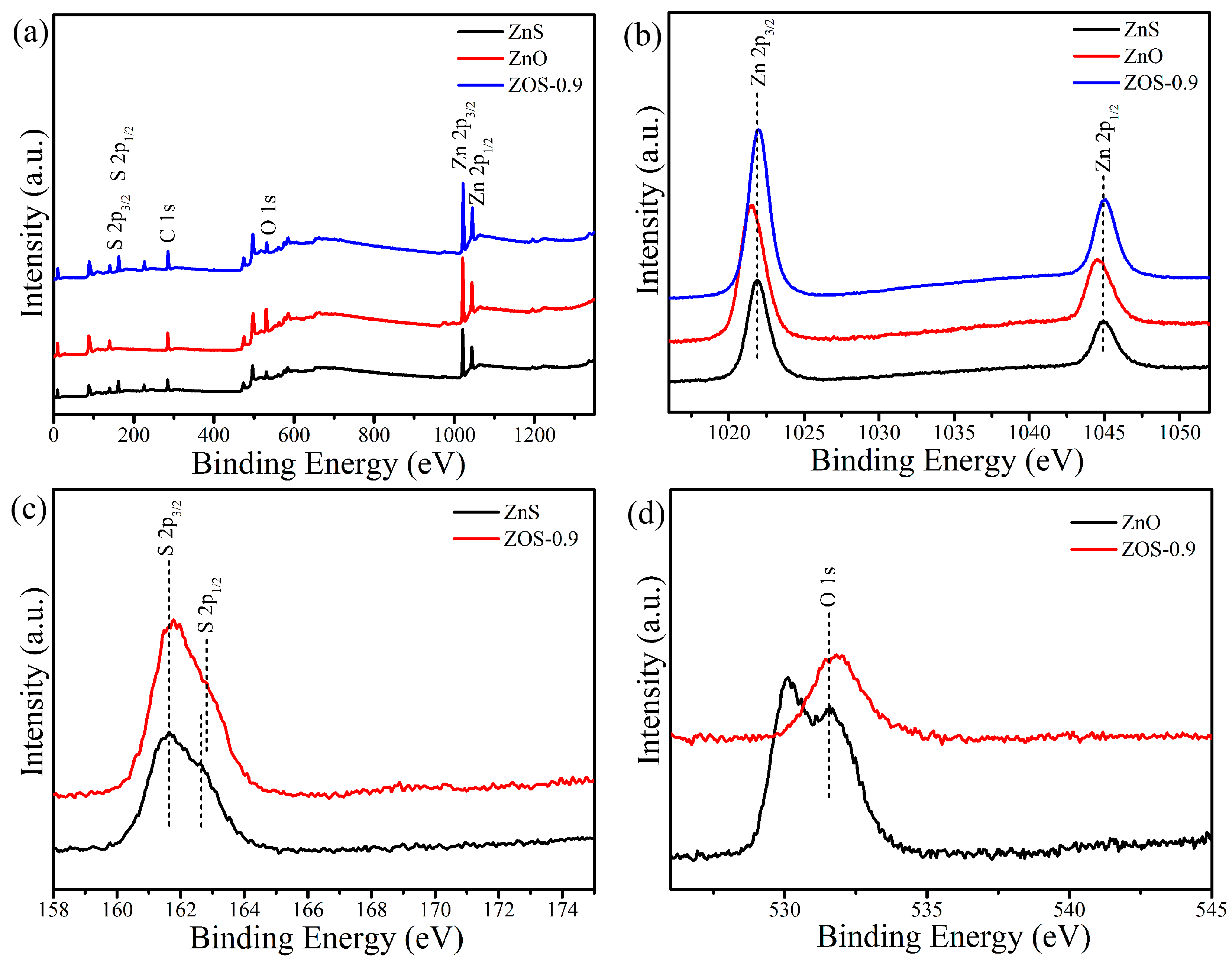
2.3.2. Mechanism Characterization
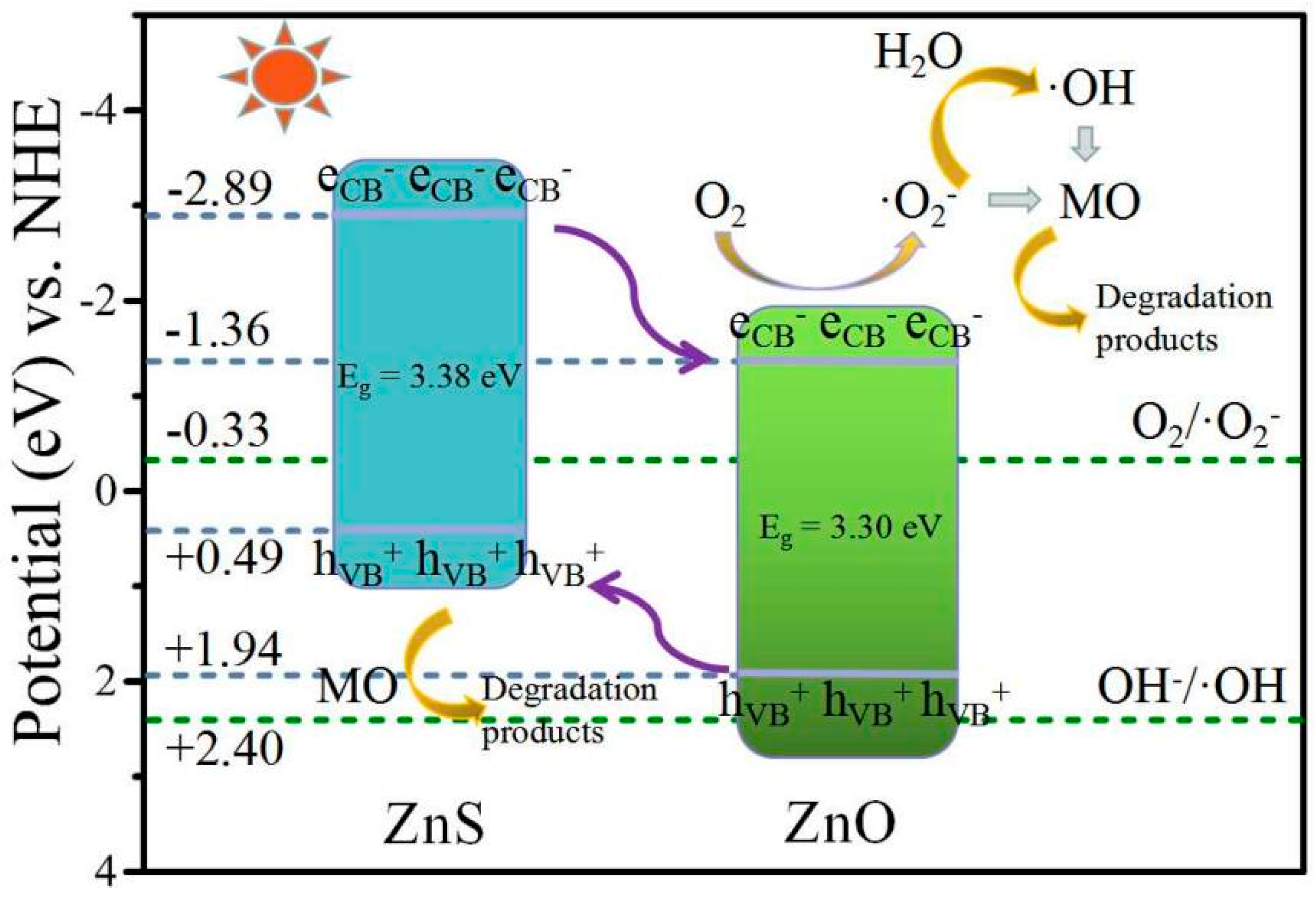
2.3.3. Photocatalytic Mechanism
3. Materials and Methods
3.1. Synthesis
3.2. Characterization
3.3. Photocatalytic Test
3.4. Theoretical Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lu, Y.; Zhang, H.; Fan, D.Q.; Chen, Z.P.; Yang, X.F. Coupling solar-driven photothermal effect into photocatalysis for sustainable water treatment. J. Hazard. Mater. 2022, 423, 127128. [Google Scholar] [CrossRef]
- Nguyen, V.Q.; Mahadadalkar, M.A.; Rabie, A.M.; Shim, J.J. Microwave-assisted synthesis of a Z-scheme heterojunction Ag/AgBr@BiOBr/Bi2O3 photocatalyst for efficient organic pollutant degradation under visible light. Environ. Sci. Nano 2022, 9, 1724–1737. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.C.; Xu, X.F. Hydrothermal synthesis and photocatalytic activity of CdO2 nanocrystals. J. Hazard. Mater. 2009, 163, 1310–1314. [Google Scholar] [CrossRef]
- Choi, J.; Kim, H.H.; Lee, K.M.; Chen, N.; Kim, M.S.; Seo, J.; Lee, D.; Cho, H.; Kim, H.; Lee, J.; et al. Bicarbonate-enhanced generation of hydroxyl radical by visible light-induced photocatalysis of H2O2 over WO3: Alteration of electron transfer mechanism. Chem. Eng. J. 2022, 432, 134401. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Wu, J.; Han, Y.D.; Huang, H.; Liu, Y.; Kang, Z.H. Photo-charge regulation of metal-free photocatalyst by carbon dots for efficient and stable hydrogen peroxide production. J. Mater. Chem. A 2021, 9, 25453–25462. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.C.; Zhang, G.S.; Yang, Z.J.; Dionysiou, D.D.; Zhu, A.P. Exceptional synergistic enhancement of the photocatalytic activity of SnS2 by coupling with polyaniline and N-doped reduced graphene oxide. Appl. Catal. B Environ. 2018, 236, 53–63. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Wang, X.; Zhao, Y.N.; Sun, H.Y.; Tan, H.Q.; Qiu, T.Y.; Zhao, Z.; Zhao, X.; Cheng, S.H.; Li, Y.G. Polyoxometalates-doped TiO2/Ag hybrid heterojunction: Removal of multiple pollutants and mechanism investigation. Environ. Sci. Nano 2021, 8, 3855–3864. [Google Scholar] [CrossRef]
- Li, K.; Ye, Y.; Zhang, W.C.; Hu, Y.Z.; Yang, Y.; Zhou, Y.; Liu, C. Modulation of the optical properties of ZnS QD-embedded glass through aluminum and manganese doping. J. Mater. Chem. C 2021, 9, 11261–11271. [Google Scholar] [CrossRef]
- Zhan, W.Q.; Yuan, Y.; Yang, B.Q.; Jia, F.F.; Song, S.X. Construction of MoS2 nano-heterojunction via ZnS doping for enhancing in-situ photocatalytic reduction of gold thiosulfate complex. Chem. Eng. J. 2020, 394, 124866. [Google Scholar] [CrossRef]
- Rao, V.N.; Ravi, P.; Sathish, M.; Reddy, N.L.; Lee, K.; Sakar, M.; Prathap, P.; Kumari, M.M.; Reddy, K.R.; Nadagouda, M.N.; et al. Monodispersed core/shell nanospheres of ZnS/NiO with enhanced H2 generation and quantum efficiency at versatile photocatalytic conditions. J. Hazard. Mater. 2021, 413, 125359. [Google Scholar]
- Shukla, R.S.; Zala, V.B.; Gupta, S.K.; Gajjar, P.N. ZnS/CdX (X = S, Se, Te) core/shell nanowires: An attempt at tuning the electronic bandgaps and SQ Efficiencies. J. Mater. Chem. C 2021, 9, 6605–6617. [Google Scholar] [CrossRef]
- Gao, T.T.; Lu, C.; Lyu, L. H2O2 inducing dissolved oxygen activation and electron donation of pollutants over Fe-ZnS quantum dots through surface electron-poor/rich microregion construction for water treatment. J. Hazard. Mater. 2021, 420, 126579. [Google Scholar] [CrossRef]
- Amani-Ghadim, A.R.; Khodam, F.; Dorraji, M.S.S. ZnS quantum dot intercalated layered double hydroxide semiconductors for solar water splitting and organic pollutant degradation. J. Mater. Chem. A 2019, 7, 11408–11422. [Google Scholar] [CrossRef]
- Chen, S.H.; Xiao, X.Y.; Li, P.H.; Li, Y.X.; Yang, M.; Guo, Z.; Huang, X.J. A direct Z-scheme ZnS/Co9S8 heterojunction based photoelectrochemical sensor for the highly sensitive and selective detection of chlorpyrifos. Environ. Sci. Nano 2020, 7, 753–763. [Google Scholar] [CrossRef]
- Serrà, A.; Philippe, L. Simple and scalable fabrication of hairy ZnO@ZnS core@shell Cu cables for continuous sunlight-driven photocatalytic water remediation. Chem. Eng. J. 2020, 401, 126164. [Google Scholar] [CrossRef]
- Fatima, H.; Azhar, M.R.; Zhong, Y.J.; Arafat, Y.; Khiadani, M.; Shao, Z.P. Rational design of ZnO-zeolite imidazole hybrid nanoparticles with reduced charge recombination for enhanced photocatalysis. J. Colloid Interf. Sci. 2022, 614, 538–546. [Google Scholar] [CrossRef]
- Rong, P.; Jiang, Y.F.; Wang, Q.; Gu, M.; Jiang, X.L.; Yu, Q. Photocatalytic degradation of methylene blue (MB) with Cu1-ZnO single atom catalysts on graphenecoated flexible substrates. J. Mater. Chem. A 2022, 10, 6231–6241. [Google Scholar] [CrossRef]
- Chinnathambi, A. Synthesis and characterization of spinel FeV2O4 coupled ZnO nanoplates for boosted white light photocatalysis and antibacterial applications. J. Alloys Compd. 2021, 890, 161742. [Google Scholar] [CrossRef]
- Guo, X.Y.; Liu, S.; Wang, W.J.; Li, C.Y.; Yang, Y.; Tian, Q.H.; Liu, Y. Plasmon-induced ultrafast charge transfer in single-particulate Cu1.94S–ZnS nanoheterostructures. Nanoscale Adv. 2021, 3, 3481–3490. [Google Scholar] [CrossRef]
- Wang, X.W.; Cao, Z.Q.; Zhang, Y.; Xu, H.P.; Cao, S.S.; Zhang, R.B. All-solid-state Z-scheme Pt/ZnS-ZnO heterostructure sheets for photocatalytic simultaneous evolution of H2 and O2. Chem. Eng. J. 2020, 385, 123782. [Google Scholar] [CrossRef]
- Giroux, M.S.; Zahra, Z.; Salawu, O.A.; Burgess, R.M.; Ho, K.T.; Adeleye, A.S. Assessing the environmental effects related to quantum dot structure, function, synthesis and exposure. Environ. Sci. Nano 2022, 9, 867–910. [Google Scholar] [CrossRef]
- Jing, M.J.; Chen, Z.G.; Li, Z.; Li, F.Y.; Chen, M.J.; Zhou, M.J.; He, B.H.; Chen, L.; Hou, Z.H.; Chen, X.B. Facile Synthesis of ZnS/N,S Co-doped Carbon Composite from Zinc Metal Complex for High-Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 704–712. [Google Scholar] [CrossRef]
- Li, H.B.; Sun, T.Q.; Zhang, L.X.; Cao, Y.A. Matching and adjusting energy band structures of Pd-modifified sulphides (ZnS, In2S3 and CuS) and improving the photocatalytic activity of CO2 photoreduction. Nanoscale 2020, 12, 18180–18192. [Google Scholar] [CrossRef]
- Gao, F.; Yuan, J.; Huang, X.Y.; Lei, R.; Jiang, C.K.; Zhuang, J.D.; Liu, P. Directional transfer of photo-generated charges mediated by cascaded dual defects in ternary photocatalyst ZnS/ZnO-In2O3 with enhanced photocatalytic performance. Chem. Eng. J. 2021, 416, 129159. [Google Scholar] [CrossRef]
- Jin, X.K.; Chen, F.J.; Jia, D.Z.; Cao, Y.L.; Duan, H.M.; Long, M.Q.; Yang, L.Y. Influences of synthetic conditions on the photocatalytic performance of ZnS/graphene composites. J. Alloys Compd. 2019, 780, 299–305. [Google Scholar] [CrossRef]
- Liu, B.L.; Li, Y.Z.; Wang, K.; Cao, Y.L. The solid-state in situ construction of Cu2O/CuO heterostructures with adjustable phase compositions to promote CO oxidation activity. CrystEngComm 2020, 22, 7808–7815. [Google Scholar] [CrossRef]
- Wang, R.Y.; Jia, D.Z.; Cao, Y.L. Facile synthesis and enhanced electrocatalytic activities of organic–inorganic hybrid ionic liquid polyoxometalate nanomaterials by solid-state chemical reaction. Electrochim. Acta 2012, 72, 101–107. [Google Scholar] [CrossRef]
- Chen, F.J.; Cao, Y.L.; Jia, D.Z. A room-temperature solid-state route for the synthesis of graphene oxide-metal sulfide composites with excellent photocatalytic activity. CrystEngComm 2013, 15, 4747–4754. [Google Scholar] [CrossRef]
- Chen, F.J.; Jin, X.K.; Cao, Y.L.; Jia, D.Z.; Liu, A.J.; Wu, R.; Long, M.Q. Effects of the synthesis conditions on the photocatalytic activities of sulfide-graphene oxide composites. Dyes Pigments 2019, 163, 177–188. [Google Scholar] [CrossRef]
- Li, Y.Z.; Cao, Y.L.; Jia, D.Z. A general strategy for synthesis of metal nanoparticles by a solid-state redox route under ambient conditions. J. Mater. Chem. A 2014, 2, 3761–3765. [Google Scholar] [CrossRef]
- Du, X.J.; Hu, J.D.; Xie, J.; Hao, A.Z.; Lu, Z.J.; Cao, Y.L. Simultaneously tailor band structure and accelerate charge separation by constructing novel In(OH)3-TiO2 heterojunction for enhanced photocatalytic water reduction. Appl. Surf. Sci. 2022, 593, 153305. [Google Scholar] [CrossRef]
- Chen, F.J.; Jin, X.K.; Jia, D.Z.; Cao, Y.L.; Duan, H.M.; Long, M.Q. Efficient treament of organic pollutants over CdS/graphene composites photocatalysts. Appl. Surf. Sci. 2020, 504, 144422. [Google Scholar] [CrossRef]
- Liu, B.L.; Cao, Y.L.; Zhang, H.Y.; Wang, S.Q.; Geng, Q.; Li, Y.Z. Constructing ultrafine Cu nanoparticles encapsulated by N-doped carbon nanosheets with fast kinetics for high-performance lithium/sodium storage. Chem. Eng. J. 2022, 446, 136918. [Google Scholar] [CrossRef]
- Zhou, M.; Ying, Y.; Huang, H.; Tan, Y.M.; Deng, W.F.; Xie, Q.J. Photoelectrochemical immunoassay of interleukin-6 based on covalent reaction-triggered photocurrent polarity switching of ZnO@fullerenol. Chem. Commun. 2021, 57, 10903–10906. [Google Scholar] [CrossRef]
- Zhang, K.; Zhuo, Z.H.; Fan, G.R.; Wang, Z.D.; Chen, S.X.; Xu, L.L.; Wen, Y.P.; Wang, P. Nano-ZnS decorated hierarchically porous carbon electrocatalyst with multiple enzyme-like activities as a nanozyme sensing platform for simultaneous detection of dopamine, uric acid, guanine, and adenine. Nanoscale 2021, 13, 20078–20090. [Google Scholar] [CrossRef]
- Dadigala, R.; Bandi, R.; Gangapuram, B.R.; Guttena, V. Construction of in situ self-assembled FeWO4/g-C3N4 nanosheet heterostructured Z-scheme photocatalysts for enhanced photocatalytic degradation of rhodamine B and tetracycline. Nanoscale Adv. 2019, 1, 322–333. [Google Scholar] [CrossRef]
- Su, T.M.; Hood, Z.D.; Naguib, M.; Bai, L.; Luo, S.; Rouleau, C.M.; Ivanov, I.N.; Ji, H.B.; Qin, Z.Z.; Wu, Z.L. 2D/2D heterojunction of Ti3C2/g-C3N4 nanosheets for enhanced photocatalytic hydrogen evolution. Nanoscale 2019, 11, 8138–8149. [Google Scholar] [CrossRef]
- Li, Z.H.; Pan, X.Y.; Yi, Z.G. Photocatalytic oxidation of methane over CuO decorated ZnO nanocatalysts. J. Mater. Chem. A 2019, 7, 469–475. [Google Scholar] [CrossRef]
- Xiong, J.H.; Li, Y.L.; Lu, S.J.; Guo, W.; Zou, J.H.; Fang, Z.B. Controllable sulphur vacancies confined in nanoporous ZnS nanoplates for visible-light photocatalytic hydrogen evolution. Chem. Commun. 2021, 57, 8186–8189. [Google Scholar] [CrossRef]
- Wu, Q.H.; Abdeta, A.B.; Kuo, D.H.; Zhang, H.Y.; Lu, Q.X.; Zhang, J.B.; Zelekew, O.A.; Mosisa, M.T.; Lin, J.G.; Chen, X.Y. A molybdenum sulfo-oxide/cobalt oxysulfifide Z scheme heterojunction catalyst for efficient photocatalytic hydrogen production and pollutant reduction. J. Mater. Chem. A 2022, 10, 5328–5349. [Google Scholar] [CrossRef]
- Ma, X.Y.; Gao, Y.F.; Yang, B.; Lou, X.J.; Huang, J.B.; Ma, L.J.; Jing, D.W. Enhanced charge separation in La2NiO4 nanoplates by coupled piezocatalysis and photocatalysis for efficient H2 evolution. Nanoscale 2022, 14, 7083–7095. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.Y.; Li, J.Y.; Li, W.; Liu, X.Y.; Dang, Y.Y.; Ma, T.H.; Wang, C.Y. Simulated-sunlight-driven Cr(VI) reduction on a type-II heterostructured Sb2S3/CdS photocatalyst. Environ. Sci. Nano 2022, 9, 1738–1747. [Google Scholar] [CrossRef]
- Mai, H.X.; Chen, D.H.; Tachibana, Y.; Suzuki, H.; Ab, R.; Caruso, R.A. Developing sustainable, high-performance perovskites in photocatalysis: Design strategies and applications. Chem. Soc. Rev. 2021, 50, 13692–13729. [Google Scholar] [CrossRef]
- Gao, X.X.; Wang, J.; Yu, J.L.; Xu, H.B. Novel ZnO–ZnS nanowire arrays with heterostructures and enhanced photocatalytic properties. CrystEngComm 2015, 17, 6328–6337. [Google Scholar] [CrossRef]
- Bano, K.; Mittal, S.K.; Singh, P.P.; Kaushal, S. Sunlight driven photocatalytic degradation of organic pollutants using a MnV2O6/BiVO4 heterojunction: Mechanistic perception and degradation pathways. Nanoscale Adv. 2021, 3, 6446–6458. [Google Scholar] [CrossRef]
- Zhou, Q.L.; Li, L.; Xin, Z.C.; Yu, Y.; Wang, L.X.; Zhang, W.Z. Visible light response and heterostructure of composite CdS@ZnS–ZnO to enhance its photocatalytic activity. J. Alloys Compd. 2020, 813, 152190. [Google Scholar] [CrossRef]
- Deng, F.; Zhao, L.N.; Luo, X.B.; Luo, S.L.; Dionysiou, D.D. Highly efficient visible-light photocatalytic performance of Ag/AgIn5S8 for degradation of tetracycline hydrochloride and treatment of real pharmaceutical industry wastewater. Chem. Eng. J. 2018, 333, 423–433. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Wang, L.; Yang, Y.; Jiao, L.; Wu, Z.H.; Gao, X.; Cheng, S.; Lin, M.Z. A novel nano-sized red phosphorus decorated borocarbonitride heterojunction with enhanced photocatalytic performance for tetracycline degradation. Environ. Sci. Nano 2022, 9, 1869–1878. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.C.; Wang, Y.Y.; Zhu, A.P.; Zhang, Y. Efficient photocatalytic reduction of aqueous Cr (VI) by Zr4+ doped and polyaniline coupled SnS2 nanoflakes. Sep. Purif. Technol. 2022, 283, 120161. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Review B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154119. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2010, 32, 1456–1465. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Chen, J.; Chen, F.; Duan, H.; Wang, Z.; Li, J. Solid-State Synthesis of ZnO/ZnS Photocatalyst with Efficient Organic Pollutant Degradation Performance. Catalysts 2022, 12, 981. https://doi.org/10.3390/catal12090981
Jin X, Chen J, Chen F, Duan H, Wang Z, Li J. Solid-State Synthesis of ZnO/ZnS Photocatalyst with Efficient Organic Pollutant Degradation Performance. Catalysts. 2022; 12(9):981. https://doi.org/10.3390/catal12090981
Chicago/Turabian StyleJin, Xuekun, Jianjun Chen, Fengjuan Chen, Haiming Duan, Ziyu Wang, and Junhua Li. 2022. "Solid-State Synthesis of ZnO/ZnS Photocatalyst with Efficient Organic Pollutant Degradation Performance" Catalysts 12, no. 9: 981. https://doi.org/10.3390/catal12090981



