Aluminosilicates Catalysts Synthesis from Low-Grade Indonesian Kaolin for the Acetalization Reaction
Abstract
:1. Introduction
2. Results and Discussion
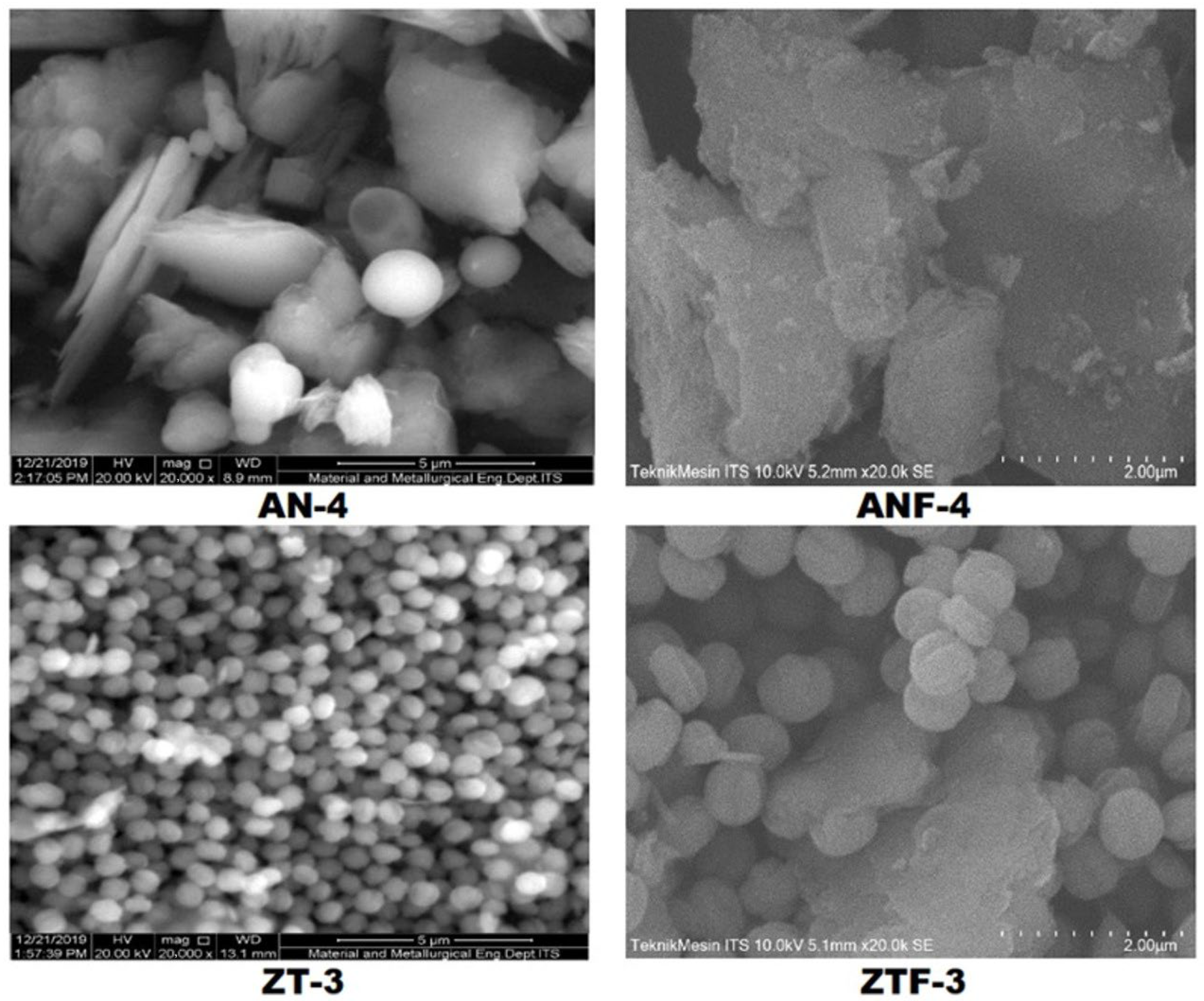
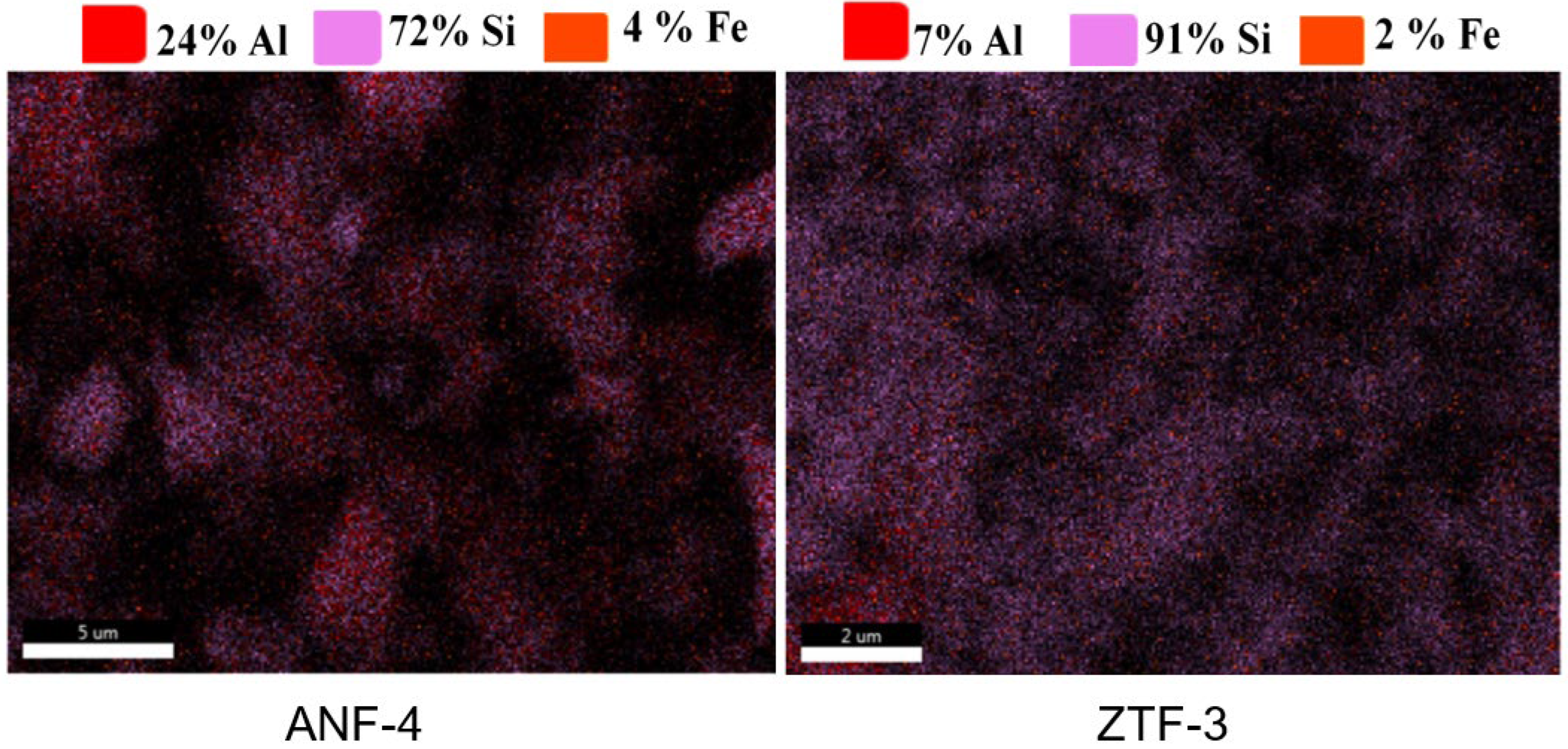
2.1. Characterisation of Kaolin and Catalysts
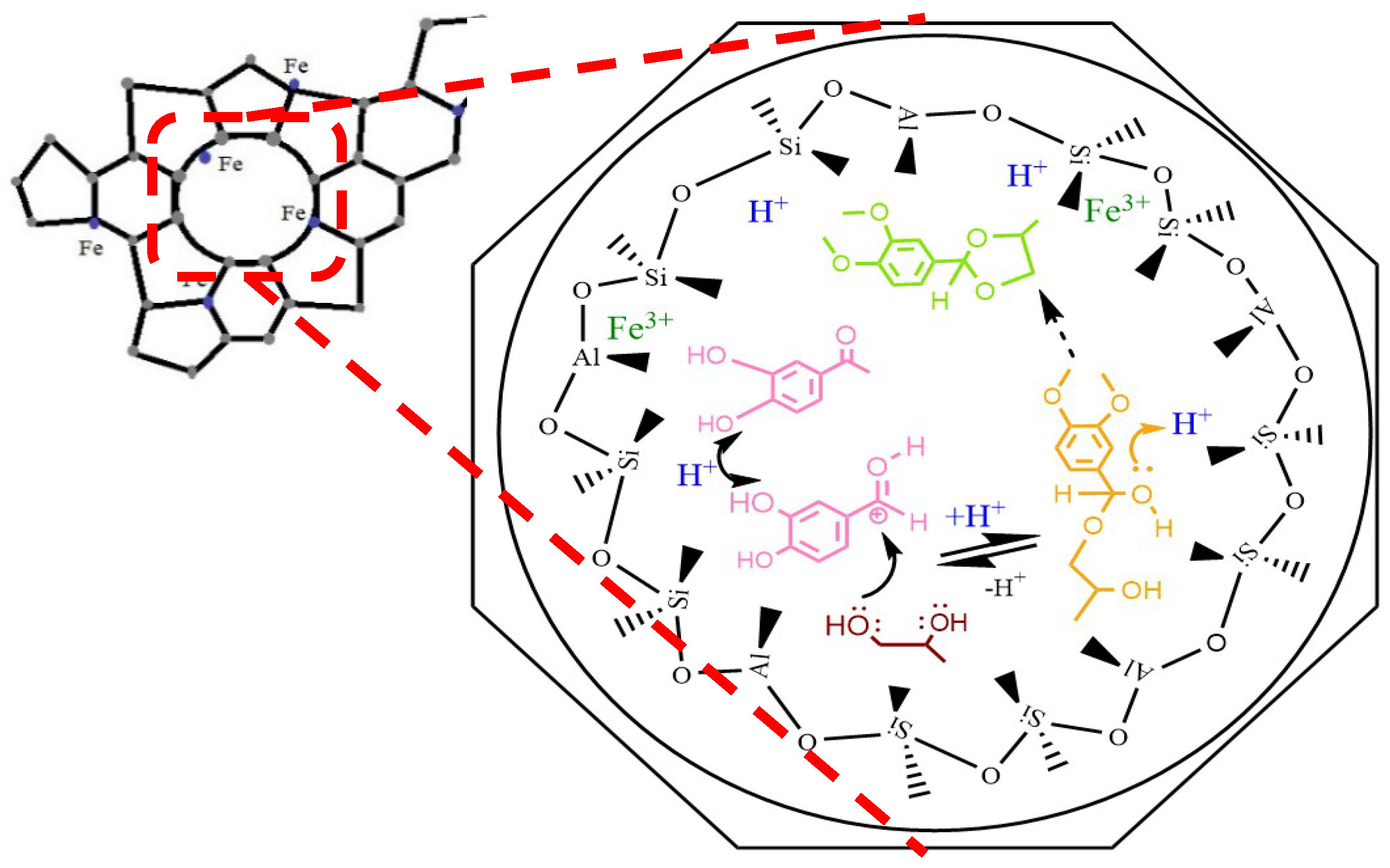
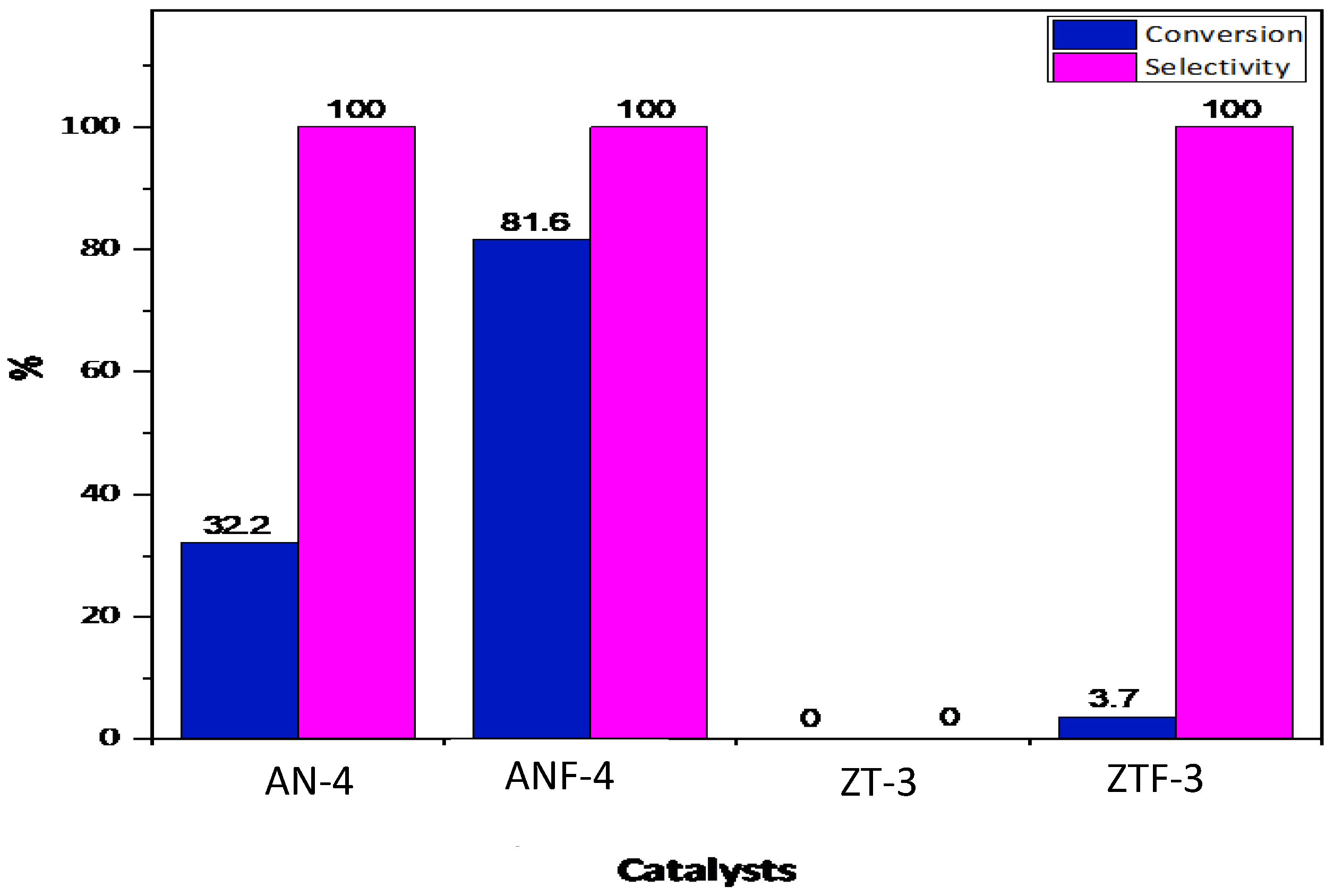
2.2. Catalytic Activity Test
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Catalysts
3.3. Catalyst Characterization
3.4. Catalytic Activity Test in Acetalization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grand View Research. Perfume Market Size, Share & Trends Analysis Report By Product (Mass, Premium), By End User (Men, Women), By Distribution Channel (Offline, Online), By Region, And Segment Forecasts, 2019–2025; Grand View Research: San Fransisco, CA, USA, 2019. [Google Scholar]
- Müller, P.M.; Lamparsky, D. Perfumes: Art, Science and Technology; Springer-Science+Business Media, B.V: Zürich, Switzerland, 1994; ISBN 9789401057011. [Google Scholar]
- Hartati; Firda, P.B.D.; Bahruji, H.; Bakar, M.B. Review on Heterogeneous Catalysts for the Synthesis of Perfumery Chemicals via Isomerization, Acetalization and Hydrogenation. Flavour Fragr. J. 2021, 36, 509–525. [Google Scholar] [CrossRef]
- Wei, G.; Yuping, L.; Zhiwei, M.; Deli, S.; Yu, C. Synthesis of Flavor 3-Methylthiopropionaldehyde Diethyl Acetal. Adv. Mater. Res. 2011, 335–336, 1352–1357. [Google Scholar] [CrossRef]
- Solomons, G.; Fryhle, C.; Snyder, S. Organic Chemistry, 11th ed.; Wiley: Hoboken, NJ, USA, 2014; ISBN 9788490225370. [Google Scholar]
- Thomas, B.; Prathapan, S.; Sugunan, S. Effect of Pore Size on the Catalytic Activities of K-10 Clay and H-Zeolites for the Acetalizatiion of Ketones with Methanol. Appl. Catal. A Gen. 2004, 277, 247–252. [Google Scholar] [CrossRef]
- Gonçalves, M.; Rodrigues, R.; Galhardo, T.S.; Carvalho, W.A. Highly Selective Acetalization of Glycerol with Acetone to Solketal over Acidic Carbon-Based Catalysts from Biodiesel Waste. Fuel 2016, 181, 46–54. [Google Scholar] [CrossRef]
- Zheng, Z.; Han, B.; Wu, F.; Shi, T.; Liu, J.; Zhang, Y.; Hao, J. One-Step for the Preparation of α-Haloacetal of Ketones with N-Bromosuccinimide/N-Chlorosuccinimide (NBS/NCS) and Ethylene Glycol. Tetrahedron 2016, 72, 7738–7743. [Google Scholar] [CrossRef]
- Chen, L.; Nohair, B.; Zhao, D.; Kaliaguine, S. Glycerol Acetalization with Formaldehyde Using Heteropolyacid Salts Supported on Mesostructured Silica. Appl. Catal. A Gen. 2018, 549, 207–215. [Google Scholar] [CrossRef]
- Weitkamp, J.; Puppe, L. Catalysis and Zeolites; Springer: Berlin/Heidelberg, Germany, 1999; ISBN 9783642083471. [Google Scholar]
- Hartati, H.; Santoso, M.; Triwahyono, S.; Prasetyoko, D. Activities of Heterogeneous Acid-Base Catalysts for Fragrances Synthesis: A Review. Bull. Chem. React. Eng. Catal. 2013, 8, 14–33. [Google Scholar] [CrossRef] [Green Version]
- Glaser, J.A. Green Chemistry with Nanocatalysts. Clean Technol. Environ. Policy 2012, 14, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Romero-Sáez, M.; Divakar, D.; Aranzabal, A.; González-Velasco, J.R.; González-Marcos, J.A. Catalytic Oxidation of Trichloroethylene over Fe-ZSM-5: Influence of the Preparation Method on the Iron Species and the Catalytic Behavior. Appl. Catal. B Environ. 2016, 180, 210–218. [Google Scholar] [CrossRef]
- Mariana Balu, A.; Pineda, A.; Yoshida, K.; Manuel Campelo, J.; Gai, P.L.; Luque, R.; Angel Romero, A. Fe/Al Synergy in Fe2O3 Nanoparticles Supported on Porous Aluminosilicate Materials: Excelling Activities in Oxidation Reactions. Chem. Commun. 2010, 46, 7825–7827. [Google Scholar] [CrossRef]
- Qoniah, I.; Prasetyoko, D.; Bahruji, H.; Triwahyono, S.; Jalil, A.A.; Suprapto; Hartati; Purbaningtias, T.E. Direct Synthesis of Mesoporous Aluminosilicates from Indonesian Kaolin Clay without Calcination. Appl. Clay Sci. 2015, 118, 290–294. [Google Scholar] [CrossRef]
- Gonçalves, M.L.; Dimitrov, L.D.; Jordão, M.H.; Wallau, M.; Urquieta-González, E.A. Synthesis of Mesoporous ZSM-5 by Crystallisation of Aged Gels in the Presence of Cetyltrimethylammonium Cations. Catal. Today 2008, 133–135, 69–79. [Google Scholar] [CrossRef]
- International Zeolite Association. Collection of Simulated XRD Powder Patterns for Zeolites, 4th ed.; Treacy, M.M., Higgins, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; ISBN 0144-2449. [Google Scholar]
- Qoniah, I.; Septiyana, B.; Rustam, R.; Prasetyoko, D.; Bahruji, H.; Hartati, H.; Zein, Y.M.; Ediati, R.; Nur, H. Direct Synthesis of ZSM-5 from Kaolin and the Influence of Organic Template. Malays. J. Fundam. Appl. Sci. 2017, 13, 137–142. [Google Scholar] [CrossRef]
- Flaningen, E.M.; Khatami, H.; Szymanski, H.A. Infrared Structural Studies of Zeolites Frameworks. Adv. Chem. Ser. 1971, 101, 201–229. [Google Scholar]
- Deguchi, M.; Iyoki, K.; Anand, C.; Yanaba, Y.; Yoshikawa, T.; Okubo, T.; Wakihara, T. Temperature-Controlled, Two-Stage Synthesis of ZSM-5 Zeolite Nanoparticles with Al Atoms Tetrahedrally Coordinated in the Framework. Microporous Mesoporous Mater. 2018, 270, 200–203. [Google Scholar] [CrossRef]
- Sundaram, T.T.; Prasanna, N.; Jayamurugan, P. Facile Synthesis of Aluminosilicate. Orient. J. Chem. 2016, 32, 1711–1715. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.D.; Noh, S.H.; Park, J.W.; Kim, W.J. Organic-Free Synthesis of ZSM-5 with Narrow Crystal Size Distribution Using Two-Step Temperature Process. Microporous Mesoporous Mater. 2006, 92, 181–188. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The Hydrothermal Synthesis of Zeolites: Precursors, Intermediates and Reaction Mechanism. Microporous Mesoporous Mater. 2005, 82, 1–78. [Google Scholar] [CrossRef]
- Hartati; Prasetyoko, D.; Santoso, M.; Bahruji, H.; Triwahyono, S. Highly Active Aluminosilicates with a Hierarchical Porous Structure for Acetalization of 3,4-Dimethoxybenzaldehyde. J. Teknol. 2014, 69, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Gorzin, F.; Towfighi Darian, J.; Yaripour, F.; Mousavi, S.M. Preparation of Hierarchical HZSM-5 Zeolites with Combined Desilication with NaAlO2/Tetrapropylammonium Hydroxide and Acid Modification for Converting Methanol to Propylene. RSC Adv. 2018, 8, 41131–41142. [Google Scholar] [CrossRef] [Green Version]
- Plana-Pallejà, J.; Abelló, S.; Berrueco, C.; Montané, D. Effect of Zeolite Acidity and Mesoporosity on the Activity of Fischer-Tropsch Fe/ZSM-5 Bifunctional Catalysts. Appl. Catal. A Gen. 2016, 515, 126–135. [Google Scholar] [CrossRef]
- Sreenivasulu, P.; Viswanadham, N.; Saxena, S.K. Facile Synthesis of Mesoporous Aluminosilicate Nanoparticles for the Selective Production of N-Benzylidenaniline in a Solvent-Free Reaction of Aniline with Benzyl Alcohol. J. Mater. Chem. A 2014, 2, 7354–7359. [Google Scholar] [CrossRef]
- Hartati, H.; Widati, A.A.; Dewi, T.K.; Prasetyoko, D. Direct Synthesis of Highly Crystalline ZSM-5 from Indonesian Kaolin. Bull. Chem. React. Eng. Catal. 2017, 12, 251–255. [Google Scholar] [CrossRef] [Green Version]
- Soltanali, S.; Halladj, R.; Rashidi, A.; Hajjar, Z.; Shafeghat, A. The Effect of Copper Loading Method on the Performance of Cu/HZSM-5 Nanocatalysts in Methanol to Gasoline Conversion. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 44, 1–7. [Google Scholar] [CrossRef]
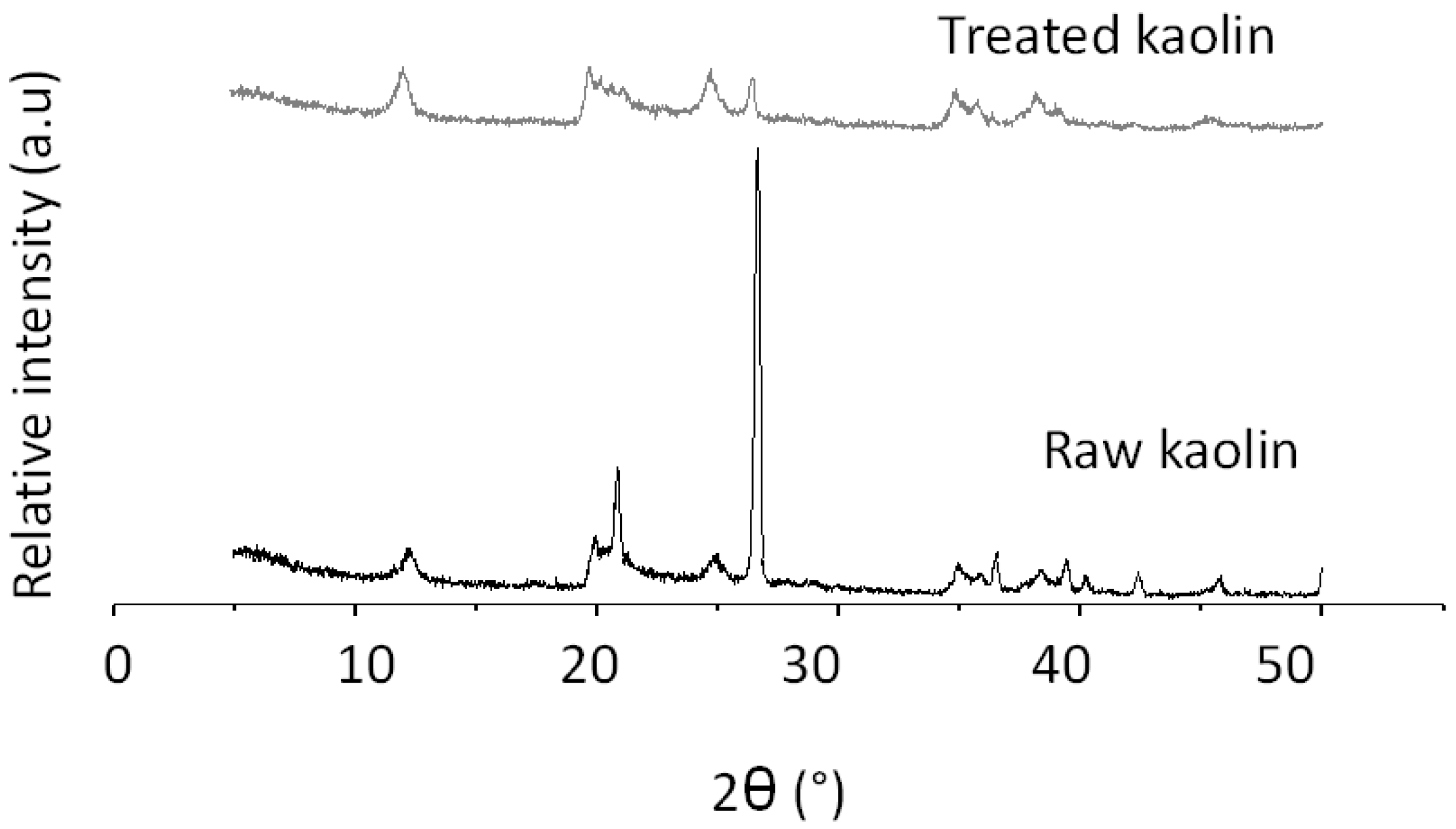
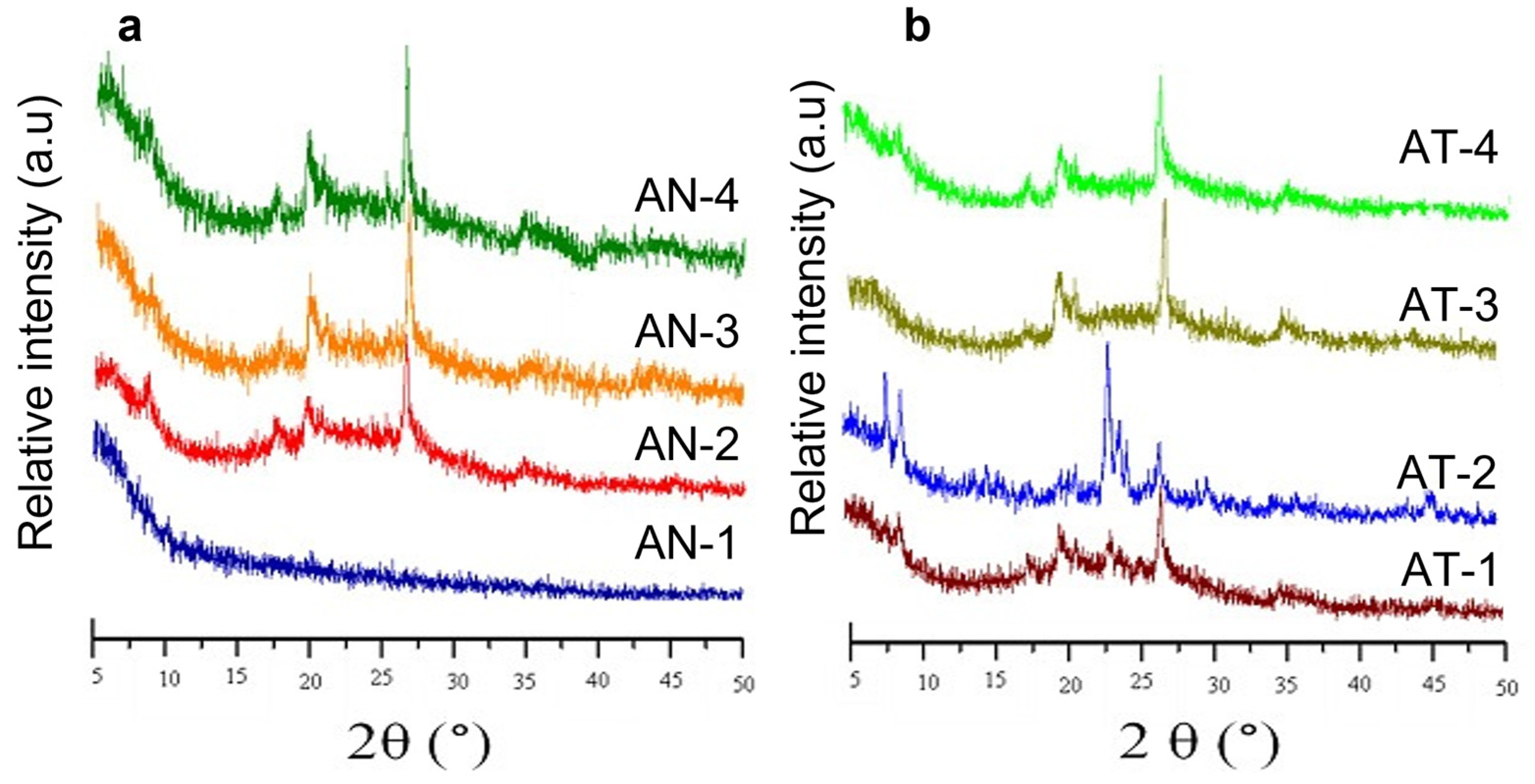
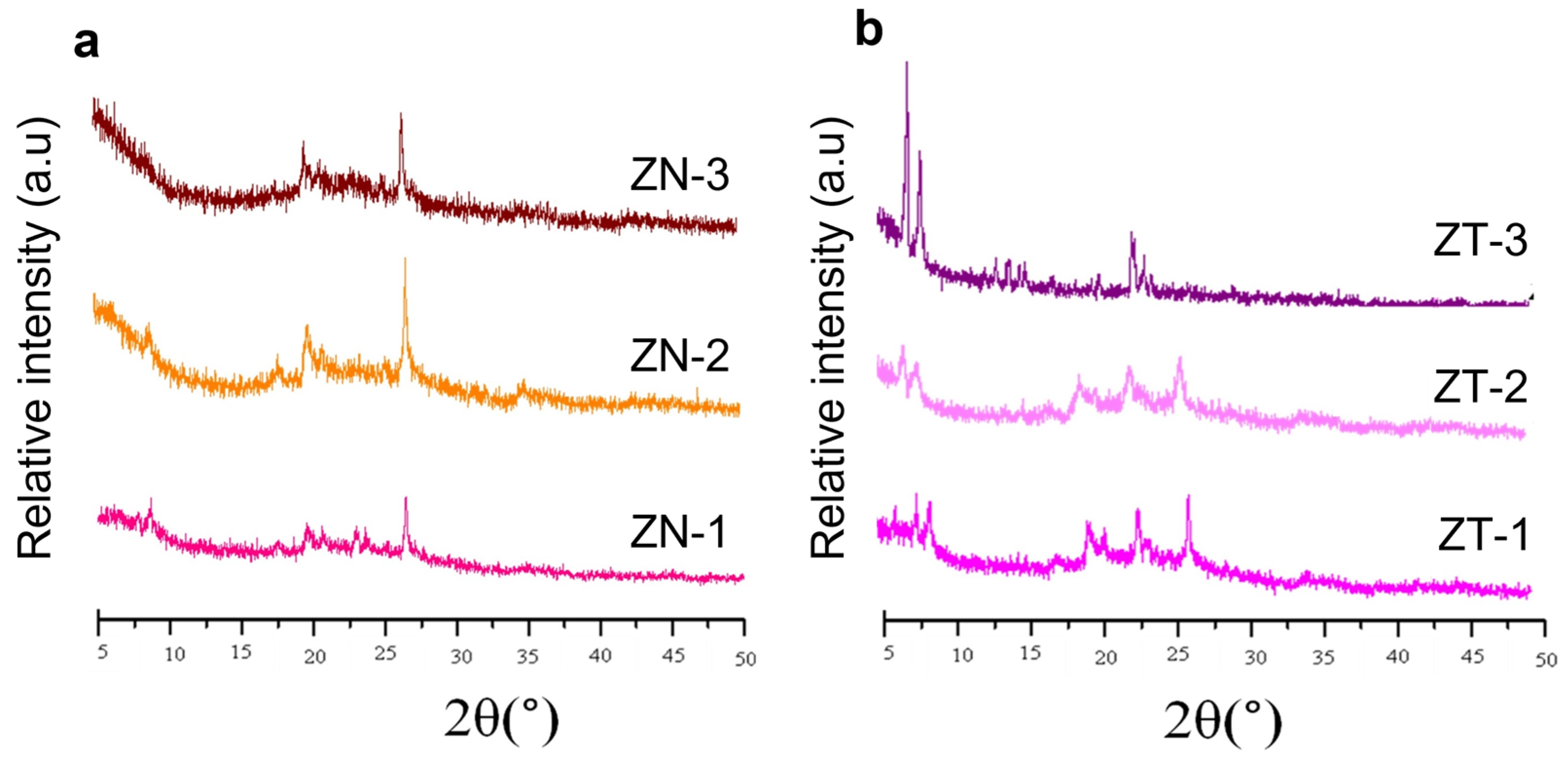

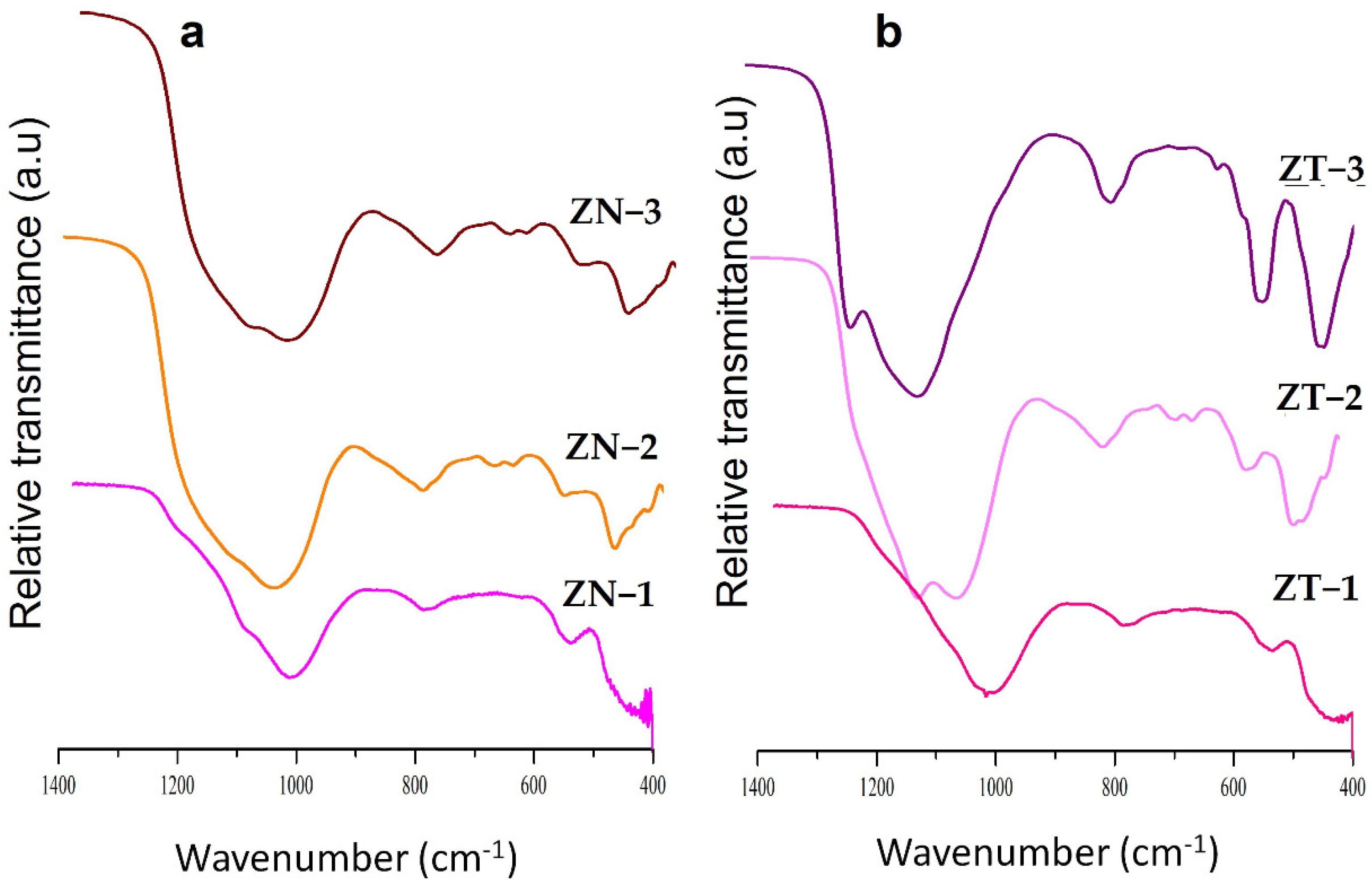


| Compound | Composition (%b/b) | |
|---|---|---|
| Raw Kaolin | Treated Kaolin | |
| Al2O3 | 22.0 | 32.5 |
| SiO2 | 69.4 | 58.1 |
| K2O | 0.86 | 2.53 |
| CaO | 2.34 | 0.58 |
| TiO2 | 1.28 | 1.21 |
| V2O5 | 0.02 | 0.03 |
| Cr2O3 | 0.13 | 0.03 |
| MnO | 3.1 | 0.15 |
| Fe2O3 | 0.08 | 4.39 |
| CuO | 0.51 | 0.06 |
| ZnO | 0.06 | 0.06 |
| BaO | 0.15 | 0.08 |
| Sample | Micropore Surface Area (m2/g) | Mesopore Surface Area (m2/g) | Average Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|---|
| ANF-4 | 154.5 | 81.8 | 3.5890 | 0.1405 |
| AN-4 | 21.6 | 43.5 | 3.5680 | 0.0889 |
| ZTF-3 | 37.1 | 66.9 | 3.5820 | 0.0589 |
| ZT-3 | 26.9 | 32.2 | 3.5790 | 0.0395 |
| Sample Code | Base | Fe3+ Addition | Hydrothermal | ||
|---|---|---|---|---|---|
| NaOH | TPAOH | Time (h) | Temp (°C) | ||
| AN-1 | √ | 12 | 60 | ||
| 24 | 80 | ||||
| AT-1 | √ | 12 | 60 | ||
| 24 | 80 | ||||
| AN-2 | √ | 24 | 60 | ||
| 48 | 80 | ||||
| AT-2 | √ | 24 | 60 | ||
| 48 | 80 | ||||
| AN-3 | √ | 24 | 80 | ||
| AT-3 | √ | 24 | 80 | ||
| AN-4 | √ | 36 | 80 | ||
| AT-4 | √ | 36 | 80 | ||
| ANF-4 | √ | √ | 36 | 80 | |
| Sample Code | Base | Fe3+ Addition | Hydrothermal | ||
|---|---|---|---|---|---|
| NaOH | TPAOH | Time (h) | Temp (°C) | ||
| ZN-1 | √ | 48 | 80 | ||
| ZN-2 | √ | 72 | 80 | ||
| ZN-3 | √ | 96 | 80 | ||
| ZT-1 | √ | 48 | 80 | ||
| ZT-2 | √ | 72 | 80 | ||
| ZT-3 | √ | 96 | 80 | ||
| ZTF-3 | √ | √ | 96 | 80 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartati, H.; A’yuni, Q.; Saputri, N.H.; Mardho, D.Z.; Firda, P.B.D.; Hartono, H.; Bahruji, H.; Nugraha, R.E.; Sholeha, N.A.; Prasetyoko, D. Aluminosilicates Catalysts Synthesis from Low-Grade Indonesian Kaolin for the Acetalization Reaction. Catalysts 2023, 13, 122. https://doi.org/10.3390/catal13010122
Hartati H, A’yuni Q, Saputri NH, Mardho DZ, Firda PBD, Hartono H, Bahruji H, Nugraha RE, Sholeha NA, Prasetyoko D. Aluminosilicates Catalysts Synthesis from Low-Grade Indonesian Kaolin for the Acetalization Reaction. Catalysts. 2023; 13(1):122. https://doi.org/10.3390/catal13010122
Chicago/Turabian StyleHartati, Hartati, Qurrota A’yuni, Nastiti Heru Saputri, Dea Zaqiatul Mardho, Putri Bintang Dea Firda, Hartono Hartono, Hasliza Bahruji, Reva Edra Nugraha, Novia Amalia Sholeha, and Didik Prasetyoko. 2023. "Aluminosilicates Catalysts Synthesis from Low-Grade Indonesian Kaolin for the Acetalization Reaction" Catalysts 13, no. 1: 122. https://doi.org/10.3390/catal13010122






