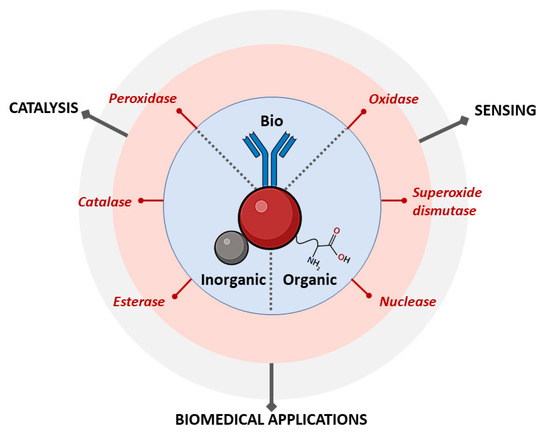
Gold Nanozymes: Smart Hybrids with Outstanding Applications
Abstract
:1. Introduction
- The chemical equilibrium of the reaction is not affected since the catalyst is recovered unaltered after the reaction is finished.
- The role of the catalyst is to lower the activation energy required for the reactants (or substrates) to be converted into products, increasing the reaction rate.
- The reaction takes place near the catalyst’s surface.
- Each catalytic reaction presents a specific mechanism.
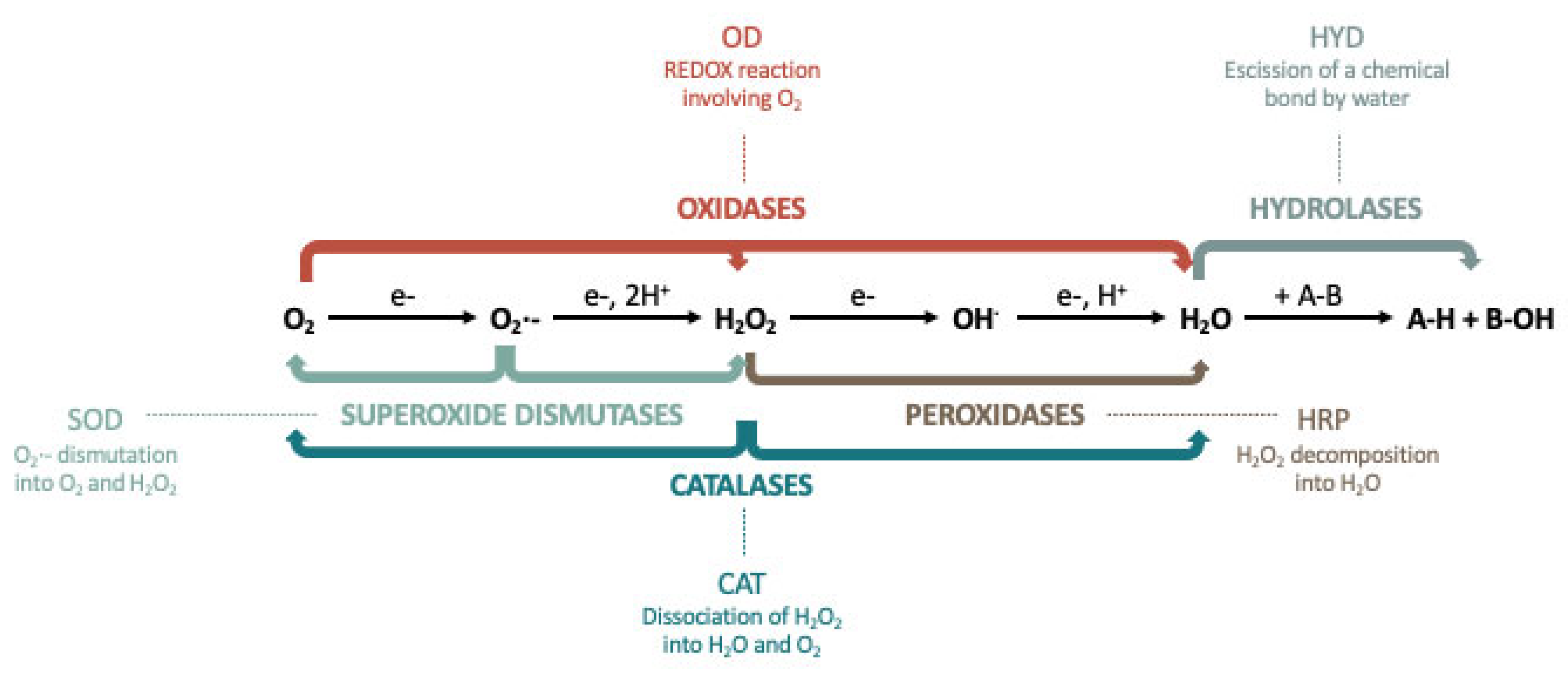
2. Types of Gold Nanozyme Activity
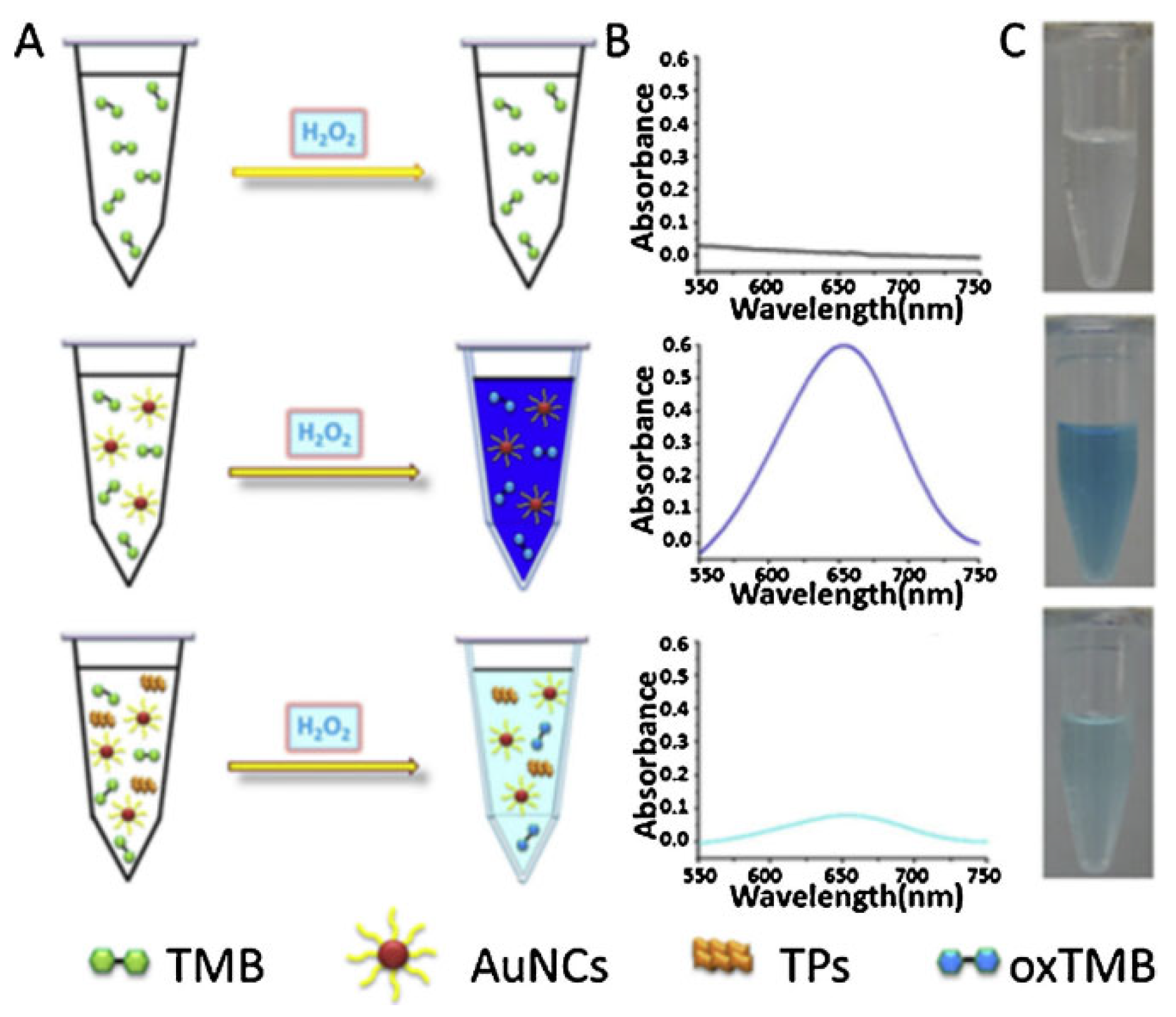
2.1. Peroxidase (HRP)
2.2. Superoxide Dismutase (SOD)
2.3. Catalase (CAT)
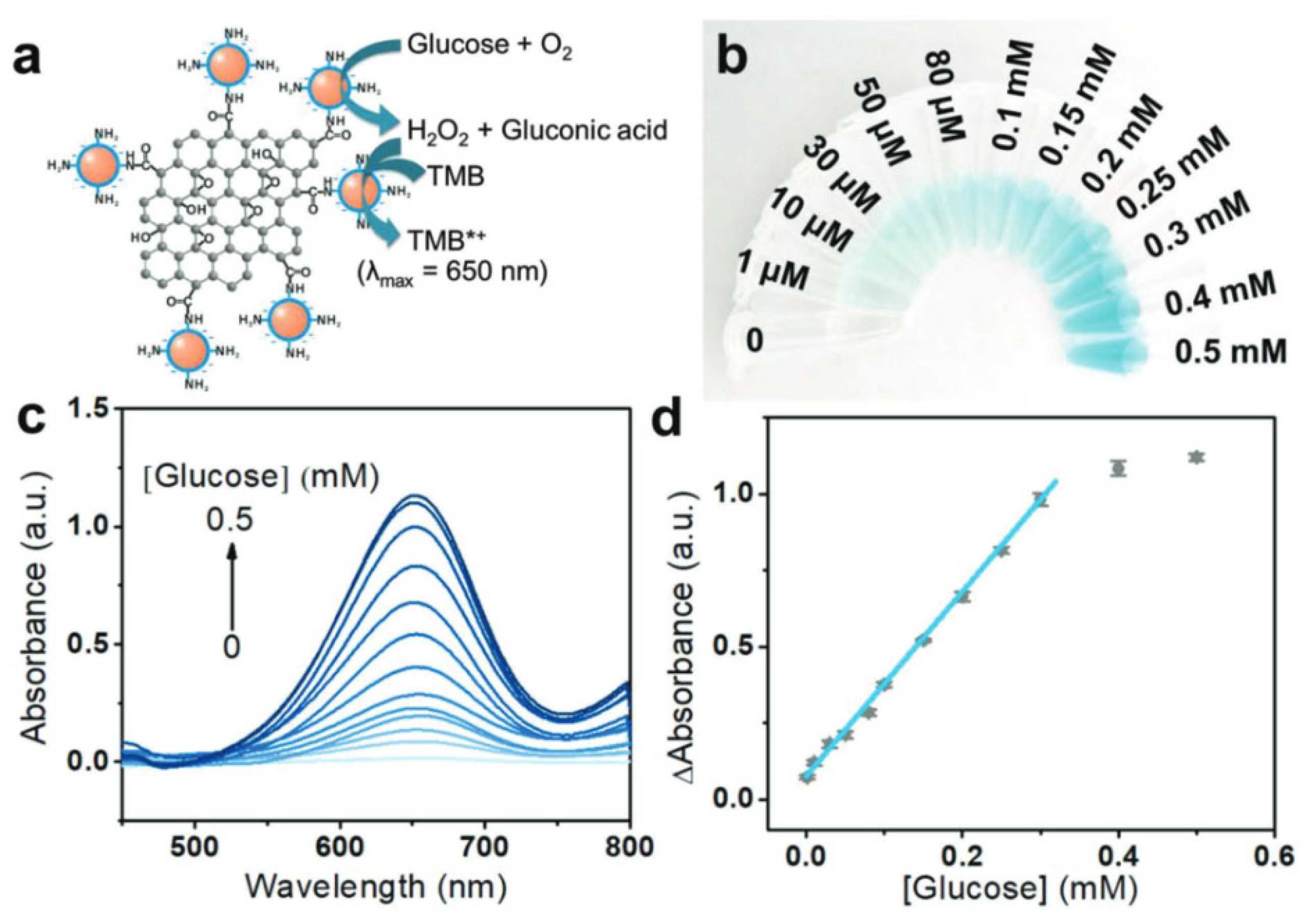
2.4. Glucose Oxidase (GOD)
2.5. Esterase
2.6. Nuclease
2.7. Combined Activity
3. Nanozymes Based on Gold Hybrids
3.1. Inorganic Hybrids
3.1.1. Carbon-Based Supports
3.1.2. MOF-Based Supports
3.1.3. Metal-Based Supports
3.2. Organic Hybrids
3.2.1. Amino Acids
3.2.2. Organic Polymers
3.3. Biohybrids
3.3.1. Protein
Antibodies
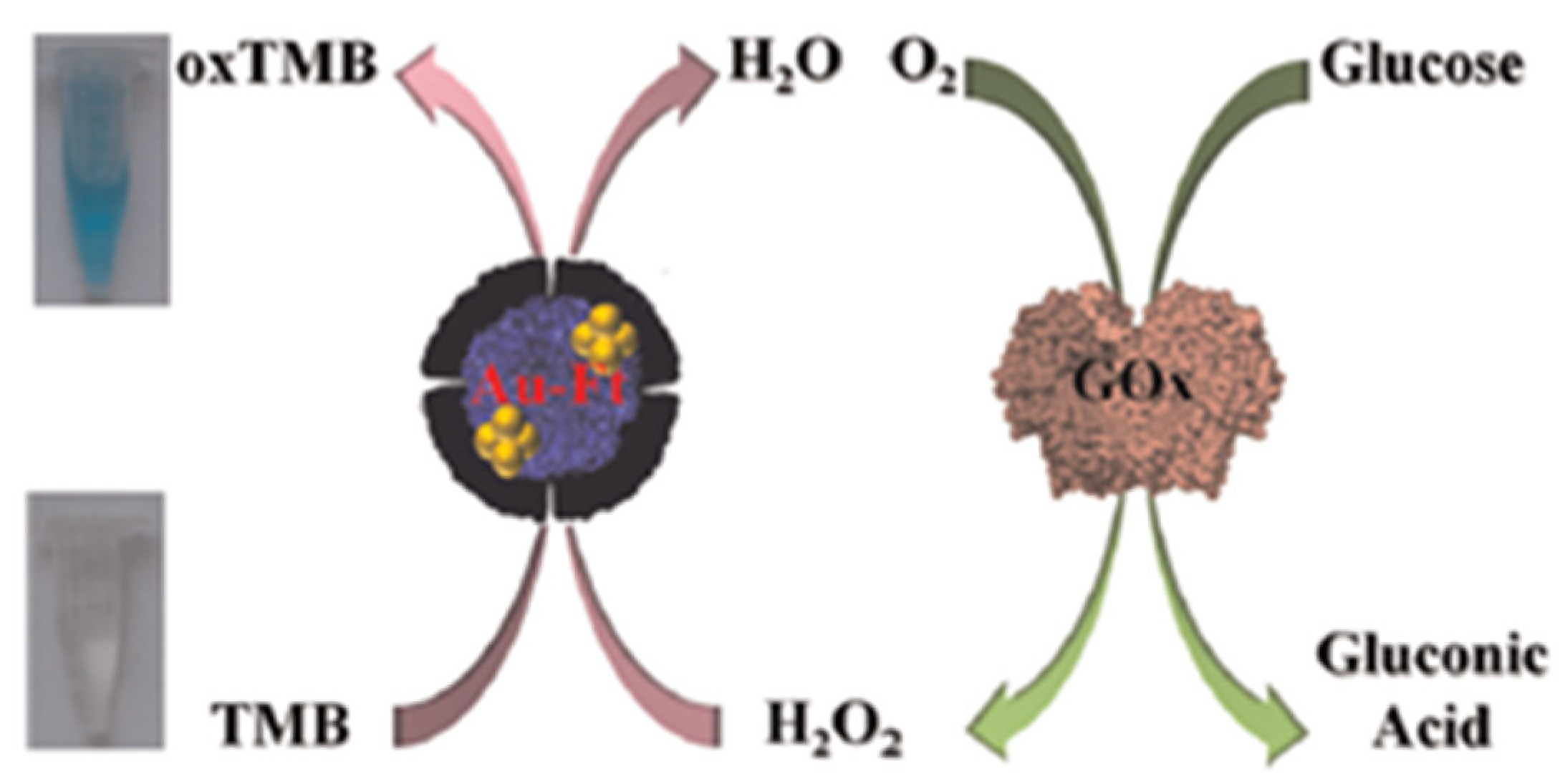
Apoferritin
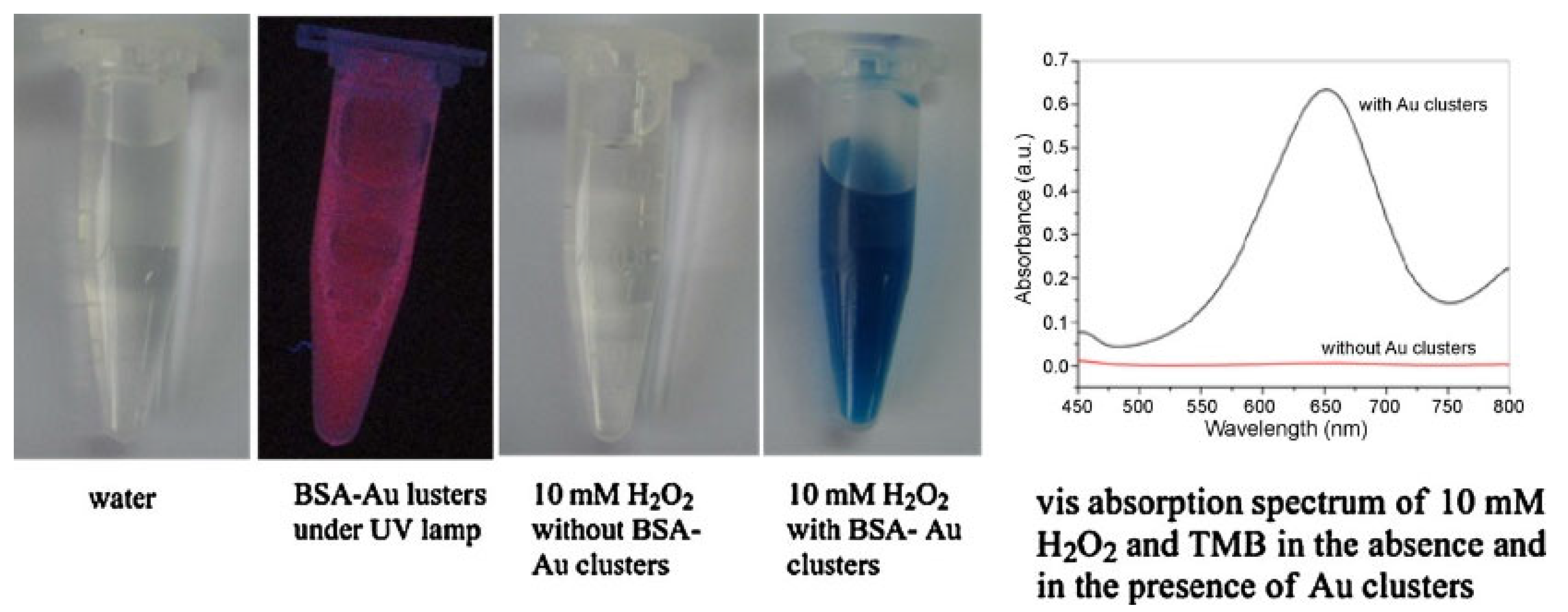
Bovine Serum Albumin
β-Casein
3.3.2. Nucleic Acids
3.3.3. Polysaccharides
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robert, A.; Meunier, B. How to Define a Nanozyme. ACS Nano 2022, 16, 6956–6959. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M. The Central Role of Enzymes as Biological Catalysts. In The Central Role of Enzymes as Biological Catalysts; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Twigg, M.V. Catalyst Handbook; Routledge: Boca Raton, UK, 1996; pp. 17–18. [Google Scholar]
- Fasim, A.; More, V.S.; More, S.S. Large-Scale Production of Enzymes for Biotechnology Uses. Curr. Opin. Biotechnol. 2021, 69, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Tikhonov, B.B.; Sulman, E.M.; Stadol’nikova, P.Y.; Sulman, A.M.; Golikova, E.P.; Sidorov, A.I.; Matveeva, V.G. Immobilized Enzymes from the Class of Oxidoreductases in Technological Processes: A Review. Biocatalysis 2019, 11, 251–263. [Google Scholar] [CrossRef]
- Abhari, K.; Hosseini, H. Enzymes in Meat, Fish, and Poultry Products Processing and Preservation-I. In Novel Food Grade Enzymes: Applications in Food Processing and Preservation Industries; Dutt Tripathi, A., Darani, K.K., Srivastava, S.K., Eds.; Springer Nature: Singapore, 2022; pp. 183–191. ISBN 978-981-19128-8-7. [Google Scholar]
- Nakai, S.; Modler, H.W. Food Proteins: Properties and Characterization; Wiley-VCH: Hoboken, NJ, USA, 1996; p. 262. [Google Scholar]
- Royer, G.P. Synthetic Enzyme Analogs (Synzymes). In Enzymes and Immobilized Cells in Biotechnology; Benjamin/Cummings: London, UK, 1985; p. 297. [Google Scholar]
- Huang, L.; Sun, D.-W.; Wei, Q. Development of Nanozymes for Food Quality and Safety Detection: Principles and Recent Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1496–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manea, F.; Bodar Houillon, F.; Pasquato, L.; Scrimin, P. Nanozymes: Gold-Nanoparticle-Based Transphosphorylation. Angew. Chem. 2004, 43, 6165–6169. [Google Scholar] [CrossRef]
- Wei, H.; Gao, L.; Fan, K.; Liu, J.; He, J.; Qu, X.; Dong, S.; Wang, E.; Yan, X. Nanozymes: A Clear Definition with Fuzzy Edges. Nano Today 2021, 40, 101269. [Google Scholar] [CrossRef]
- Yang, W.; Yang, X.; Zhu, L.; Chu, H.; Li, X.; Xu, W. Nanozymes: Activity Origin, Catalytic Mechanism, and Biological Application. Coord. Chem. Rev. 2021, 448, 214170. [Google Scholar] [CrossRef]
- Ren, X.; Chen, D.; Wang, Y.; Li, H.; Zhang, Y.; Chen, H.; Li, X.; Huo, M. Nanozymes-Recent Development and Biomedical Applications. J. Nanobiotechnol. 2022, 20, 92. [Google Scholar] [CrossRef]
- Das, B.; Franco, J.L.; Logan, N.; Balasubramanian, P.; Kim, M.I.; Cao, C. Nanozymes in Point-of-Care Diagnosis: An Emerging Futuristic Approach for Biosensing. Nano-Micro Lett. 2021, 13, 193. [Google Scholar] [CrossRef]
- Wong, E.L.; Vuong, K.Q.; Chow, E. Nanozymes for Environmental Pollutant Monitoring and Remediation. Sensors 2021, 21, 408. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, J.; Qu, X. Nano-Gold as Artificial Enzymes: Hidden Talents. Adv. Mater. 2014, 26, 4200–4217. [Google Scholar] [CrossRef] [PubMed]
- Lou-Franco, J.; Das, B.; Elliott, C.; Cao, C. Gold Nanozymes: From Concept to Biomedical Applications. Nano-Micro Lett. 2021, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Mandani, S.; Sharma, B.; Dey, D.; Sarma, T.K. Carbon Nanodots as Ligand Exchange Probes in Au@C-Dot Nanobeacons for Fluorescent Turn-on Detection of Biothiols. Nanoscale 2015, 7, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Gong, N.; Ma, X.; Ye, X.; Zhou, Q.; Chen, X.; Tan, X.; Yao, S.; Huo, S.; Zhang, T.; Chen, S.; et al. Carbon-Dot-Supported Atomically Dispersed Gold as a Mitochondrial Oxidative Stress Amplifier for Cancer Treatment. Nat. Nanotechnol. 2019, 14, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, C.; Wang, F.; Liao, M.; Jiang, G. Bimetallic Nanoparticles Decorated Hollow Nanoporous Carbon Framework as Nanozyme Biosensor for Highly Sensitive Electrochemical Sensing of Uric Acid. Biosens. Bioelectron. 2020, 150, 111869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Pan, S.; Zhang, Y.; Chang, J.; Cheng, J.; Huang, Z.; Li, T.; Zhang, C.; de la Fuentea, J.M.; Zhang, Q.; et al. Carbon-Gold Hybrid Nanoprobes for Real-Time Imaging, Photothermal/Photodynamic and Nanozyme Oxidative Therapy. Theranostics 2019, 9, 3443–3458. [Google Scholar] [CrossRef]
- Qi, G.; Zhang, Y.; Wang, J.; Wang, D.; Wang, B.; Li, H.; Jin, Y. Smart Plasmonic Nanozyme Enhances Combined Chemo-Photothermal Cancer Therapy and Reveals Tryptophan Metabolic Apoptotic Pathway. Anal. Chem. 2019, 91, 12203–12211. [Google Scholar] [CrossRef]
- Wu, N.; Wang, Y.-T.; Wang, X.-Y.; Guo, F.-N.; Wen, H.; Yang, T.; Wang, J.-H. Enhanced Peroxidase-like Activity of AuNPs Loaded Graphitic Carbon Nitride Nanosheets for Colorimetric Biosensing. Anal. Chim. Acta 2019, 1091, 69–75. [Google Scholar] [CrossRef]
- Gan, H.; Han, W.; Fu, Z.; Wang, L. The Chain-like Au/Carbon Dots Nanocomposites with Peroxidase-like Activity and Their Application for Glucose Detection. Colloids Surf. B Biointerfaces 2021, 199, 111553. [Google Scholar] [CrossRef]
- Li, Q.; Yang, D.; Tammina, S.K.; Yang, Y. Construction of AuNPs/Cu,I-CD-Based Colorimetric Sensor: Catalytic Oxidation of TBHQ and the Catalytic Inhibition of HCHO. Food Chem. 2022, 373, 131438. [Google Scholar] [CrossRef]
- Shang, Q.; Dong, H.; Liu, S.; Jiang, F.; Li, Y.; Wang, S.; Liu, Q.; Li, Y.; Tang, F. Sandwich-Type Electrochemical Immunosensor Based on Nitrogen-Doped Porous Carbon and Nanoporous Trimetallic Nanozyme (PdAgCu) for Determination of Prostate Specific Antigen. Microchim. Acta 2022, 189, 359. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Wang, H.; Zhang, Y.; Yan, B.; Shen, G.; Yu, R. A Direct Electrochemical Biosensing Platform Constructed by Incorporating Carbon Nanotubes and Gold Nanoparticles onto Redox Poly(Thionine) Film. Anal. Sci. 2007, 23, 235–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
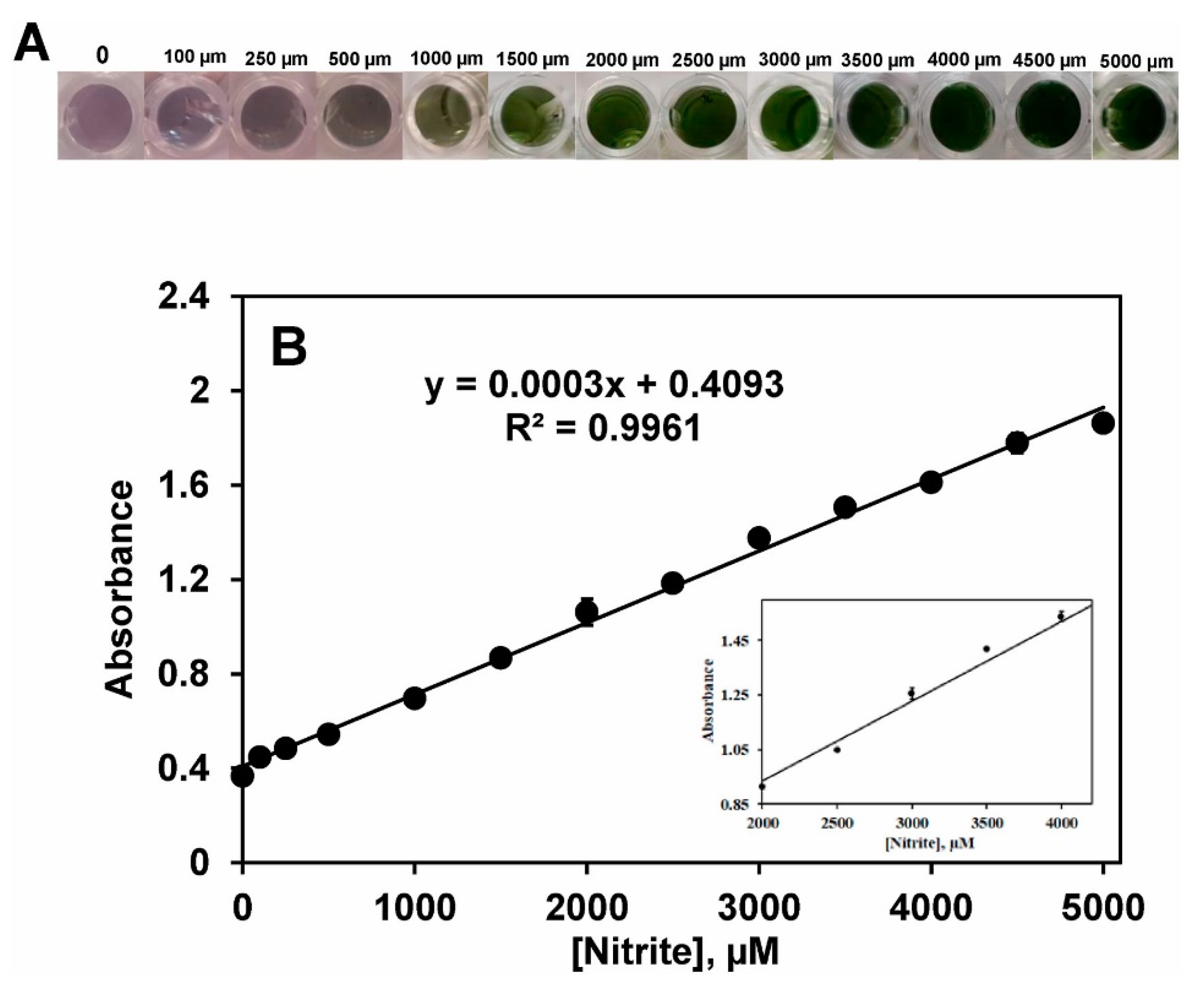
- Adegoke, O.; Zolotovskaya, S.; Abdolvand, A.; Daeid, N.N. Rapid and Highly Selective Colorimetric Detection of Nitrite Based on the Catalytic-Enhanced Reaction of Mimetic Au Nanoparticle-CeO2 Nanoparticle-Graphene Oxide Hybrid Nanozyme. Talanta 2021, 224, 121875. [Google Scholar] [CrossRef] [PubMed]
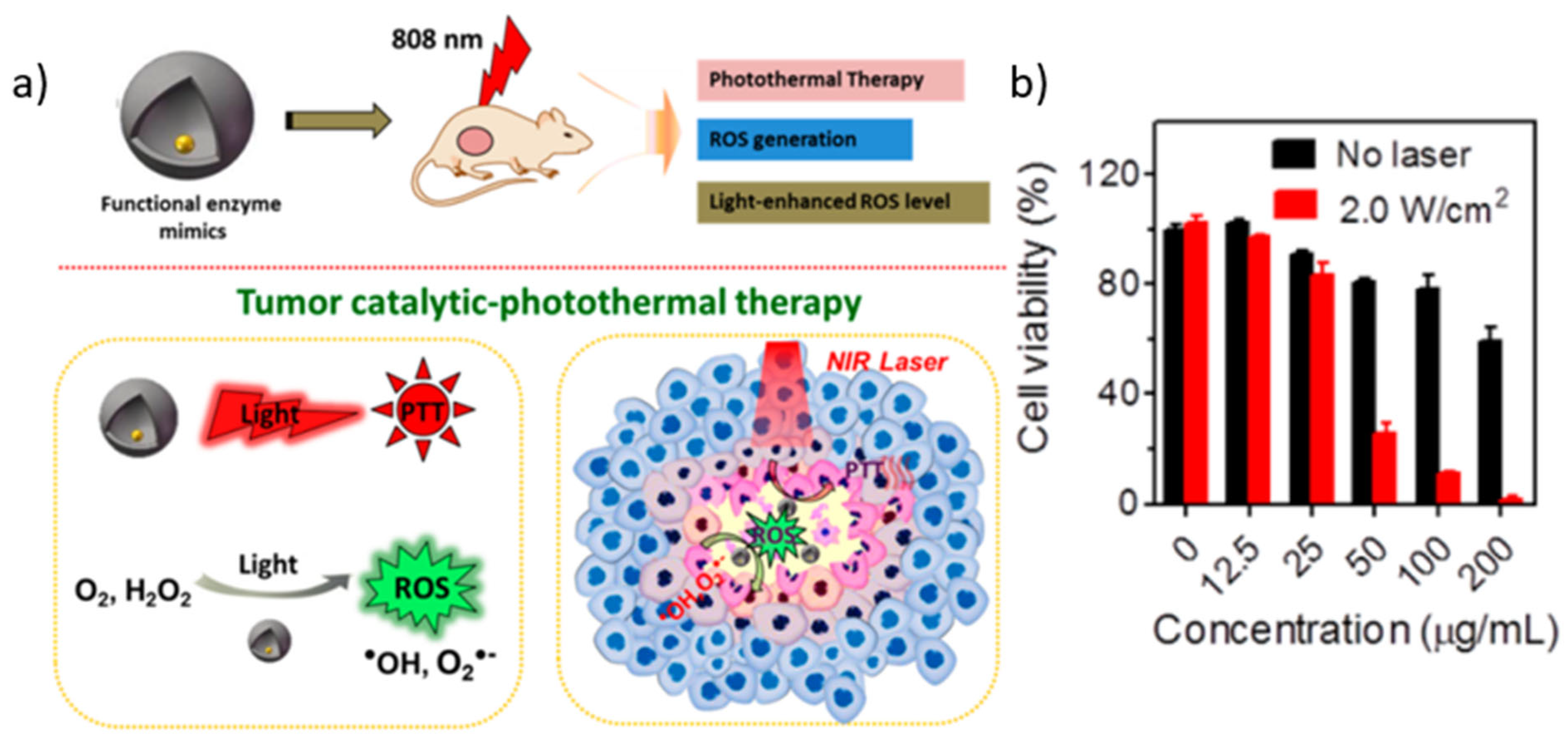
- Fan, L.; Xu, X.; Zhu, C.; Han, J.; Gao, L.; Xi, J.; Guo, R. Tumor Catalytic–Photothermal Therapy with Yolk–Shell Gold@Carbon Nanozymes. ACS Appl. Mater. Interfaces 2018, 10, 4502–4511. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H.; Zhao, X.; Wu, J.; Muhammad, F.; Lin, S.; He, J.; Zhou, L.; Zhang, C.; Deng, Y.; et al. Surface-Enhanced Raman Scattering Active Gold Nanoparticles with Enzyme-Mimicking Activities for Measuring Glucose and Lactate in Living Tissues. ACS Nano 2017, 11, 5558–5566. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, J.; Pan, Q.; Zhang, X.; Shao, W.; Zhang, X.; Quan, C.; Li, J. An Au@NH2-MIL-125(Ti)-Based Multifunctional Platform for Colorimetric Detections of Biomolecules and Hg2+. J. Mater. Chem. B 2019, 8, 114–124. [Google Scholar] [CrossRef]
- Hu, W.-C.; Younis, M.R.; Zhou, Y.; Wang, C.; Xia, X.-H. In Situ Fabrication of Ultrasmall Gold Nanoparticles/2D MOFs Hybrid as Nanozyme for Antibacterial Therapy. Small 2020, 16, 2000553. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, T.; Wang, D.; Zhang, N.; Yang, H.; Jing, X.; Niu, R.; Yang, Z.; Xie, Y.; Meng, L. Gold Nanorods/Metal–Organic Framework Hybrids: Photo-Enhanced Peroxidase-like Activity and SERS Performance for Organic Dyestuff Degradation and Detection. Anal. Chem. 2022, 94, 4484–4494. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Shen, B.; Li, C.; Chen, J.; Wang, Y.; Tian, S.; Li, X.; Luo, N.; Liu, R.; et al. Electrochemiluminescent (ECL) Biosensor for Burkholderia Pseudomallei Based on Cobalt-Doped MOF Decorated with Gold Nanoparticles and N-(4-Aminobutyl)-N-(Ethylisoluminol). Microchim. Acta 2022, 189, 355. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Liu, S.; Zhang, Q.; Liu, X.; Wong, D.K.Y. Gold Nanoparticle Encapsulated-Tubular TiO2 Nanocluster as a Scaffold for Development of Thiolated Enzyme Biosensors. Anal. Chem. 2013, 85, 4350–4356. [Google Scholar] [CrossRef]
- Navya, P.N.; Madhyastha, H.; Madhyastha, R.; Nakajima, Y.; Maruyama, M.; Srinivas, S.P.; Jain, D.; Amin, M.H.; Bhargava, S.K.; Daima, H.K. Single Step Formation of Biocompatible Bimetallic Alloy Nanoparticles of Gold and Silver Using Isonicotinylhydrazide. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Xu, M.; Lu, M.; Chen, G.; Tang, D. Urchin-like (Gold Core)@(Platinum Shell) Nanohybrids: A Highly Efficient Peroxidase-Mimetic System for in Situ Amplified Colorimetric Immunoassay. Biosens. Bioelectron. 2015, 70, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Yang, K.; Tao, J.; Liu, Y.; Zhang, Q.; Habibi, S.; Nie, Z.; Xia, X. An Enzyme-Free Signal Amplification Technique for Ultrasensitive Colorimetric Assay of Disease Biomarkers. ACS Nano 2017, 11, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, M.; Shekari-Far, A.; Hakimian, F.; Haghiralsadat, B.F.; Fatemi, S.K.; Dashtestani, F. Core-Shell Au@Co-Fe Hybrid Nanoparticles as Peroxidase Mimetic Nanozyme for Antibacterial Application. Process Biochem. 2020, 95, 131–138. [Google Scholar] [CrossRef]
- Peng, X.; Wan, G.; Wu, L.; Zeng, M.; Lin, S.; Wang, G. Peroxidase-like Activity of Au@TiO2 Yolk-Shell Nanostructure and Its Application for Colorimetric Detection of H2O2 and Glucose. Sens. Actuators B Chem. 2018, 257, 166–177. [Google Scholar] [CrossRef]
- Boriachek, K.; Masud, M.K.; Palma, C.; Phan, H.-P.; Yamauchi, Y.; Hossain, M.S.A.; Nguyen, N.-T.; Salomon, C.; Shiddiky, M.J.A. Avoiding Pre-Isolation Step in Exosome Analysis: Direct Isolation and Sensitive Detection of Exosomes Using Gold-Loaded Nanoporous Ferric Oxide Nanozymes. Anal. Chem. 2019, 91, 3827–3834. [Google Scholar] [CrossRef] [Green Version]
- Masud, M.K.; Yadav, S.; Islam, M.N.; Nguyen, N.-T.; Salomon, C.; Kline, R.; Alamri, H.R.; Alothman, Z.A.; Yamauchi, Y.; Hossain, M.S.A.; et al. Gold-Loaded Nanoporous Ferric Oxide Nanocubes with Peroxidase-Mimicking Activity for Electrocatalytic and Colorimetric Detection of Autoantibody. Anal. Chem. 2017, 89, 11005–11013. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Hu, X.; Hou, S.; Wen, T.; Liu, W.; Zhu, X.; Yin, J.-J.; Wu, X. Au@Pt Core/Shell Nanorods with Peroxidase- and Ascorbate Oxidase-like Activities for Improved Detection of Glucose. Sens. Actuators B Chem. 2012, 166–167, 708–714. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, D.; Zhen, Y.; Guo, R. Amino Acid-Mediated ‘Turn-off/Turn-on’ Nanozyme Activity of Gold Nanoclusters for Sensitive and Selective Detection of Copper Ions and Histidine. Biosens. Bioelectron. 2017, 92, 140–146. [Google Scholar] [CrossRef]
- Liu, L.; Du, J.; Liu, W.; Guo, Y.; Wu, G.; Qi, W.; Lu, X. Enhanced His@AuNCs Oxidase-like Activity by Reduced Graphene Oxide and Its Application for Colorimetric and Electrochemical Detection of Nitrite. Anal. Bioanal. Chem. 2019, 411, 2189–2200. [Google Scholar] [CrossRef]
- Song, Y.; Qiao, J.; Liu, W.; Qi, L. Colorimetric Detection of Serum Doxycycline with D-Histidine-Functionalized Gold Nanoclusters as Nanozymes. Analyst 2020, 145, 3564–3568. [Google Scholar] [CrossRef] [PubMed]
- Pezhhan, H.; Akhond, M.; Shamsipur, M. Histidine Capped-Gold Nanoclusters Mediated Fluorescence Detection of Glucose and Hydrogen Peroxide Based on Glucose Oxidase-Mimicking Property of Gold Nanoparticles via an Inner Filter Effect Mechanism. J. Lumin. 2020, 228, 117604. [Google Scholar] [CrossRef]
- Ansar, S.M.; Kitchens, C.L. Impact of Gold Nanoparticle Stabilizing Ligands on the Colloidal Catalytic Reduction of 4-Nitrophenol. ACS Catal. 2016, 6, 5553–5560. [Google Scholar] [CrossRef]
- Karthiga, D.; Choudhury, S.; Chandrasekaran, N.; Mukherjee, A. Effect of Surface Charge on Peroxidase Mimetic Activity of Gold Nanorods (GNRs). Mater. Chem. Phys. 2019, 227, 242–249. [Google Scholar] [CrossRef]
- Park, G.; Seo, D.; Chung, I.S.; Song, H. Poly(Ethylene Glycol)- and Carboxylate-Functionalized Gold Nanoparticles Using Polymer Linkages: Single-Step Synthesis, High Stability, and Plasmonic Detection of Proteins. Langmuir 2013, 29, 13518–13526. [Google Scholar] [CrossRef]
- Cheng, C.; Qiao, J.; Zhang, H.; Zhao, Z.; Qi, L. Polymer-Capped Gold Nanoparticles as Nanozymes with Improved Catalytic Activity for the Monitoring of Serum Ciprofloxacin. Analyst 2022, 147, 1509–1514. [Google Scholar] [CrossRef]
- Giorgi-Coll, S.; Blunt-Foley, H.; Hutchinson, P.J.; Carpenter, K.L.H. Heparin-Gold Nanoparticles for Enhanced Microdialysis Sampling. Anal. Bioanal. Chem. 2017, 409, 5031–5042. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yang, J.; Wang, L.; Ran, B.; Jia, Y.; Zhang, L.; Yang, G.; Shao, H.; Jiang, X. Pharmaceutical Intermediate-Modified Gold Nanoparticles: Against Multidrug-Resistant Bacteria and Wound-Healing Application via an Electrospun Scaffold. ACS Nano 2017, 11, 5737–5745. [Google Scholar] [CrossRef]
- Chong, Y.; Huang, J.; Xu, X.; Yu, C.; Ning, X.; Fan, S.; Zhang, Z. Hyaluronic Acid-Modified Au-Ag Alloy Nanoparticles for Radiation/Nanozyme/Ag+ Multimodal Synergistically Enhanced Cancer Therapy. Bioconjug. Chem. 2020, 31, 1756–1765. [Google Scholar] [CrossRef]
- Gomaa, M.M. Early Diagnosis of Experimental Trichinella Spiralis Infection by Nano-Based Enzyme-Linked Immunosorbent Assay (Nano-Based ELISA). Exp. Parasitol. 2020, 212, 107867. [Google Scholar] [CrossRef]
- Oh, S.; Kim, J.; Tan Tran, V.; Lee, D.K.; Rahin Ahmed, S.; Hong, J.C.; Lee, J.; Park, E.Y.; Lee, J. Magnetic Nanozyme-Linked Immunosorbent Assay for Ultrasensitive Influenza A Virus Detection. ACS Appl. Mater. Interfaces 2018, 10, 12534–12543. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Fan, K.; Zhang, D.; Tan, S.; Liang, M.; Liu, Y.; Zhang, J.; Zhang, P.; Liu, W.; Qiu, X.; et al. Nanozyme-Strip for Rapid Local Diagnosis of Ebola. Biosens. Bioelectron. 2015, 74, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.R.; Corredor, J.C.; Nagy, É.; Neethirajan, S. Amplified Visual Immunosensor Integrated with Nanozyme for Ultrasensitive Detection of Avian Influenza Virus. Nanotheranostics 2017, 1, 338–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Sun, C.; Guo, Y.; Nie, G.; Xu, L. Peroxidase-like Activity of Apoferritin Paired Gold Clusters for Glucose Detection. Biosens. Bioelectron. 2015, 64, 165–170. [Google Scholar] [CrossRef]
- Dashtestani, F.; Ghourchian, H.; Najafi, A. Silver-Gold-Apoferritin Nanozyme for Suppressing Oxidative Stress during Cryopreservation. Mater. Sci. Eng. C 2019, 94, 831–840. [Google Scholar] [CrossRef]
- Wang, S.; Liu, P.; Qin, Y.; Chen, Z.; Shen, J. Rapid Synthesis of Protein Conjugated Gold Nanoclusters and Their Application in Tea Polyphenol Sensing. Sens. Actuators B Chem. 2016, 223, 178–185. [Google Scholar] [CrossRef]
- Wang, X.-X.; Wu, Q.; Shan, Z.; Huang, Q.-M. BSA-Stabilized Au Clusters as Peroxidase Mimetics for Use in Xanthine Detection. Biosens. Bioelectron. 2011, 26, 3614–3619. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, X.; Han, L.; Li, F. “Non-Naked” Gold with Glucose Oxidase-like Activity: A Nanozyme for Tandem Catalysis. Small 2018, 14, 1803256. [Google Scholar] [CrossRef]
- McVey, C.; Logan, N.; Thanh, N.T.K.; Elliott, C.; Cao, C. Unusual Switchable Peroxidase-Mimicking Nanozyme for the Determination of Proteolytic Biomarker. Nano Res. 2019, 12, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Tang, M.-Q.; Chen, J.; Zhu, Y.-H.; Lei, C.-B.; He, H.-W.; Xu, X.-H. A Sandwich ELISA-like Detection of C-Reactive Protein in Blood by Citicoline-Bovine Serum Albumin Conjugate and Aptamer-Functionalized Gold Nanoparticles Nanozyme. Talanta 2020, 217, 121070. [Google Scholar] [CrossRef]
- Tripathi, P.; Kumar, A.; Sachan, M.; Gupta, S.; Nara, S. Aptamer-Gold Nanozyme Based Competitive Lateral Flow Assay for Rapid Detection of CA125 in Human Serum. Biosens. Bioelectron. 2020, 165, 112368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, W.; Cheng, Y. Aptamer Labeled Nanozyme-Based ELISA for Ampicillin Residue Detection in Milk. Chem. Pap. 2022, 76, 3077–3085. [Google Scholar] [CrossRef]
- Weerathunge, P.; Ramanathan, R.; Torok, V.A.; Hodgson, K.; Xu, Y.; Goodacre, R.; Behera, B.K.; Bansal, V. Ultrasensitive Colorimetric Detection of Murine Norovirus Using NanoZyme Aptasensor. Anal. Chem. 2019, 91, 3270–3276. [Google Scholar] [CrossRef] [PubMed]
- Weerathunge, P.; Ramanathan, R.; Shukla, R.; Sharma, T.K.; Bansal, V. Aptamer-Controlled Reversible Inhibition of Gold Nanozyme Activity for Pesticide Sensing. Anal. Chem. 2014, 86, 11937–11941. [Google Scholar] [CrossRef]
- Sharma, T.K.; Ramanathan, R.; Weerathunge, P.; Mohammadtaheri, M.; Daima, H.K.; Shukla, R.; Bansal, V. Aptamer-Mediated ‘Turn-off/Turn-on’ Nanozyme Activity of Gold Nanoparticles for Kanamycin Detection. Chem. Commun. 2014, 50, 15856–15859. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Zhao, R.; Feng, S.; Xie, Y. Colorimetric Zearalenone Assay Based on the Use of an Aptamer and of Gold Nanoparticles with Peroxidase-like Activity. Mikrochim. Acta 2018, 185, 535. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Y.; Tao, H.; Chen, H.; Yang, W.; Qiu, S. Colorimetric Detection of Streptomycin in Milk Based on Peroxidase-Mimicking Catalytic Activity of Gold Nanoparticles. RSC Adv. 2017, 7, 38471–38478. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.-X.; Wang, B.-B.; Zhao, X.; Chen, L.-J.; Yan, X.-P. A PH-Responsive Persistent Luminescence Nanozyme for Selective Imaging and Killing of Helicobacter Pylori and Common Resistant Bacteria. ACS Appl. Mater. Interfaces 2021, 13, 60955–60965. [Google Scholar] [CrossRef]
- Lee, G.; Kim, C.; Kim, D.; Hong, C.; Kim, T.; Lee, M.; Lee, K. Multibranched Au–Ag–Pt Nanoparticle as a Nanozyme for the Colorimetric Assay of Hydrogen Peroxide and Glucose. ACS Omega 2022, 7, 40973–40982. [Google Scholar] [CrossRef]
- Jiang, C.; Li, Z.; Wu, Y.; Guo, W.; Wang, J.; Jiang, Q. Colorimetric Detection of Hg2+ Based on Enhancement of Peroxidase-like Activity of Chitosan-Gold Nanoparticles. Bull. Korean Chem. Soc. 2018, 39, 625–630. [Google Scholar] [CrossRef]
- Jiang, C.; Zhu, J.; Li, Z.; Luo, J.; Wang, J.; Sun, Y. Chitosan–Gold Nanoparticles as Peroxidase Mimic and Their Application in Glucose Detection in Serum. RSC Adv. 2017, 7, 44463–44469. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, G.; Wang, L.; Shen, A.; Hu, J. Simultaneous Enzymatic and SERS Properties of Bifunctional Chitosan-Modified Popcorn-like Au-Ag Nanoparticles for High Sensitive Detection of Melamine in Milk Powder. Talanta 2015, 140, 204–211. [Google Scholar] [CrossRef] [PubMed]
- de Souza Vandenberghe, L.P.; Karp, S.G.; Pagnoncelli, M.G.B.; von Linsingen Tavares, M.; Junior, N.L.; Diestra, K.V.; Viesser, J.A.; Soccol, C.R. Chapter 2—Classification of Enzymes and Catalytic Properties. In Biomass, Biofuels, Biochemicals. Advances in Enzyme Catalysis and Technologies; Singh, S.P., Pandey, A., Singhania, R.R., Larroche, C., Li, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 11–30. ISBN 978-0-12-819820-9. [Google Scholar]
- Enzyme Kinetics and Mechanism; Garland Science: New York, NY, USA, 2007; ISBN 978-0-203-83357-5.
- Heine, A.; DeSantis, G.; Luz, J.G.; Mitchell, M.; Wong, C.-H.; Wilson, I.A. Observation of Covalent Intermediates in an Enzyme Mechanism at Atomic Resolution. Science 2001, 294, 369–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfenden, R.; Frick, L. Mechanisms of Enzyme Action and Inhibition: Transition State Analogues for Acid-Base Catalysis. J. Protein Chem. 1986, 5, 147–155. [Google Scholar] [CrossRef]
- Warshel, A.; Sharma, P.K.; Kato, M.; Xiang, Y.; Liu, H.; Olsson, M.H.M. Electrostatic Basis for Enzyme Catalysis. Chem. Rev. 2006, 106, 3210–3235. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, G.; Mendel, R.R.; Ribbe, M.W. Molybdenum Cofactors, Enzymes and Pathways. Nature 2009, 460, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Fan, Y.; Zhang, W.; Gu, N.; Zhang, Y. Catalytic Mechanisms of Nanozymes and Their Applications in Biomedicine. Bioconjug. Chem. 2019, 30, 1273–1296. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, S.; Guo, X.; Dong, Y.; Yao, C.; Liu, Y.; Song, Y.; Tan, X.; Gao, L.; Yang, D. Chiral Carbon Dots Mimicking Topoisomerase I To Mediate the Topological Rearrangement of Supercoiled DNA Enantioselectively. Angew. Chem. Int. Ed. 2020, 59, 11087–11092. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Z.; Chen, Z.; Ren, J.; Qu, X. Mesoporous Silica-Encapsulated Gold Nanoparticles as Artificial Enzymes for Self-Activated Cascade Catalysis. Biomaterials 2013, 34, 2600–2610. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, X.; Carmona, U.; Yang, F.; Gao, X.; Knez, M.; Zhang, L.; Qin, Y. Control of Stepwise Hg2+ Reduction on Gold to Selectively Tune Its Peroxidase and Catalase-like Activities and the Mechanism. Adv. Mater. Interfaces 2021, 8, 2100086. [Google Scholar] [CrossRef]
- Bindoli, A.; Rigobello, M.P. Peroxidase Biochemistry and Redox Signaling. In Encyclopedia of Biological Chemistry, 2nd ed.; Lennarz, W.J., Lane, M.D., Eds.; Academic Press: Waltham, MA, USA, 2013; pp. 407–412. ISBN 978-0-12-378631-9. [Google Scholar]
- Jv, Y.; Li, B.; Cao, R. Positively-Charged Gold Nanoparticles as Peroxidiase Mimic and Their Application in Hydrogen Peroxide and Glucose Detection. Chem. Commun. 2010, 46, 8017–8019. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Dhiman, A.; Kapil, A.; Bansal, V.; Sharma, T.K. Aptamer-Mediated Colorimetric and Electrochemical Detection of Pseudomonas Aeruginosa Utilizing Peroxidase-Mimic Activity of Gold NanoZyme. Anal. Bioanal. Chem. 2019, 411, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Assady, M.; Farahnak, A.; Golestani, A.; Esharghian, M. Superoxide Dismutase (SOD) Enzyme Activity Assay in Fasciola spp. Parasites and Liver Tissue Extract. Iran. J. Parasitol. 2011, 6, 17–22. [Google Scholar] [PubMed]
- Zhao, L.; Ren, X.; Zhang, J.; Zhang, W.; Chen, X.; Meng, X. Dendritic Silica with Carbon Dots and Gold Nanoclusters for Dual Nanozymes. New J. Chem. 2020, 44, 1988–1992. [Google Scholar] [CrossRef]
- Shangari, N.; O’Brien, P.J. Catalase Activity Assays. In Current Protocols in Toxicology; Wiley: Hoboken, NJ, USA, 2006; Chapter 7, Unit 7.7.1-15. [Google Scholar]
- Zhang, H.; Yang, K.-L. In Situ Formation and Immobilization of Gold Nanoparticles on Polydimethylsiloxane (PDMS) Exhibiting Catalase-Mimetic Activity. Chem. Commun. 2020, 56, 6416–6419. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-P.; Wu, T.-H.; Liu, C.-Y.; Chen, K.-C.; Chen, Y.-X.; Chen, G.-S.; Lin, S.-Y. Self-Supplying O2 through the Catalase-like Activity of Gold Nanoclusters for Photodynamic Therapy against Hypoxic Cancer Cells. Small 2017, 13, 1700278. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.B. Carbohydrates. In Clinical chemistry and Molecular Diagnostics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 518–538. [Google Scholar]
- Comotti, M.; Della Pina, C.; Falletta, E.; Rossi, M. Aerobic Oxidation of Glucose with Gold Catalyst: Hydrogen Peroxide as Intermediate and Reagent. Adv. Synth. Catal. 2006, 348, 313–316. [Google Scholar] [CrossRef]
- Chen, J.; Wu, W.; Huang, L.; Ma, Q.; Dong, S. Self-Indicative Gold Nanozyme for H2O2 and Glucose Sensing. Chem.-Eur. J. 2019, 25, 11940–11944. [Google Scholar] [CrossRef]
- Cao, L.; Wang, P.; Chen, L.; Wu, Y.; Di, J. A Photoelectrochemical Glucose Sensor Based on Gold Nanoparticles as a Mimic Enzyme of Glucose Oxidase. RSC Adv. 2019, 9, 15307–15313. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Pan, Y.; Yang, J.; Gao, Y.; Huang, T.; Luan, X.; Wang, Y.; Song, Y. Gold Nanoparticles Doped Metal-Organic Frameworks as near-Infrared Light-Enhanced Cascade Nanozyme against Hypoxic Tumors. Nano Res. 2020, 13, 653–660. [Google Scholar] [CrossRef]
- Gopalan, N.; Nampoothiri, K.M. Chapter 14—Biotechnological Production of Enzymes Using Agro-Industrial Wastes: Economic Considerations, Commercialization Potential, and Future Prospects. In Agro-Industrial Wastes as Feedstock for Enzyme Production; Dhillon, G.S., Kaur, S., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 313–330. ISBN 978-0-12-802392-1. [Google Scholar]
- Pengo, P.; Polizzi, S.; Pasquato, L.; Scrimin, P. Carboxylate−Imidazole Cooperativity in Dipeptide-Functionalized Gold Nanoparticles with Esterase-like Activity. J. Am. Chem. Soc. 2005, 127, 1616–1617. [Google Scholar] [CrossRef] [PubMed]
- Yang, W. Nucleases: Diversity of Structure, Function and Mechanism. Q. Rev. Biophys. 2011, 44, 1–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ji, H.; Liu, C.; Bing, W.; Wang, Z.; Qu, X. A Multinuclear Metal Complex Based DNase-Mimetic Artificial Enzyme: Matrix Cleavage for Combating Bacterial Biofilms. Angew. Chem. 2016, 128, 10890–10894. [Google Scholar] [CrossRef]
- Czescik, J.; Zamolo, S.; Darbre, T.; Rigo, R.; Sissi, C.; Pecina, A.; Riccardi, L.; De Vivo, M.; Mancin, F.; Scrimin, P. A Gold Nanoparticle Nanonuclease Relying on a Zn(II) Mononuclear Complex. Angew. Chem. 2020, 133, 1443–1452. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.; Wu, X.; Gao, X. Mechanism of PH-Switchable Peroxidase and Catalase-like Activities of Gold, Silver, Platinum and Palladium. Biomaterials 2015, 48, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, S.; Vallabani, N.V.S.; Shutthanandan, V.; Bowden, M.; Karakoti, A.S.; Singh, S. Gold Core/Ceria Shell-Based Redox Active Nanozyme Mimicking the Biological Multienzyme Complex Phenomenon. J. Colloid Interface Sci. 2018, 513, 831–842. [Google Scholar] [CrossRef]
- Zhou, X.; Fan, C.; Tian, Q.; Han, C.; Yin, Z.; Dong, Z.; Bi, S. Trimetallic AuPtCo Nanopolyhedrons with Peroxidase- and Catalase-like Catalytic Activity for Glow-Type Chemiluminescence Bioanalysis. Anal. Chem. 2022, 94, 847–855. [Google Scholar] [CrossRef]
- Wang, P.; Min, D.; Chen, G.; Li, M.; Tong, L.; Cao, Y. Inorganic Nanozymes: Prospects for Disease Treatments and Detection Applications. Front. Chem. 2021, 9, 773285. [Google Scholar] [CrossRef]
- He, W.; Zhou, Y.-T.; Wamer, W.G.; Hu, X.; Wu, X.; Zheng, Z.; Boudreau, M.D.; Yin, J.-J. Intrinsic Catalytic Activity of Au Nanoparticles with Respect to Hydrogen Peroxide Decomposition and Superoxide Scavenging. Biomaterials 2013, 34, 765–773. [Google Scholar] [CrossRef]
- Comotti, M.; Della Pina, C.; Matarrese, R.; Rossi, M. The Catalytic Activity of “Naked” Gold Particles. Angew. Chem. Int. Ed. 2004, 43, 5812–5815. [Google Scholar] [CrossRef]
- Khan, A.; Jawaid, M.; Inamuddin; Asiri, A.M. Nanocarbon and Its Composites, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; Available online: https://www.elsevier.com/books/nanocarbon-and-its-composites/khan/978-0-08-102509-3 (accessed on 2 November 2022).
- Li, Q.; Yu, D.; Fan, C.; Huang, Q.; Tang, Y.; Guo, R.; Huang, Y.; Wang, H.; Lin, C.; Lin, Y. Gold Nanoparticles Adsorbed on Graphene as Nanozymes for the Efficient Elimination of Dye Pollutants. ACS Appl. Nano Mater. 2022, 5, 94–100. [Google Scholar] [CrossRef]
- Niu, X.; Li, X.; Lyu, Z.; Pan, J.; Ding, S.; Ruan, X.; Zhu, W.; Du, D.; Lin, Y. Metal–Organic Framework Based Nanozymes: Promising Materials for Biochemical Analysis. Chem. Commun. 2020, 56, 11338–11353. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent Metal–Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Espallargas, G.M. Dynamic Magnetic MOFs. Chem. Soc. Rev. 2013, 42, 1525–1539. [Google Scholar] [CrossRef]
- Maurin, G.; Serre, C.; Cooper, A.; Férey, G. The New Age of MOFs and of Their Porous-Related Solids. Chem. Soc. Rev. 2017, 46, 3104–3107. [Google Scholar] [CrossRef]
- Wang, D.; Jana, D.; Zhao, Y. Metal–Organic Framework Derived Nanozymes in Biomedicine. Acc. Chem. Res. 2020, 53, 1389–1400. [Google Scholar] [CrossRef]
- Liu, H.; Syama, L.; Zhang, L.; Lee, C.; Liu, C.; Dai, Z.; Yan, Q. High-Entropy Alloys and Compounds for Electrocatalytic Energy Conversion Applications. SusMat 2021, 1, 482–505. [Google Scholar] [CrossRef]
- Tekade, R. Biomaterials and Bionanotechnology, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; Available online: https://www.elsevier.com/books/biomaterials-and-bionanotechnology/tekade/978-0-12-814427-5 (accessed on 2 November 2022).
- Wu, Z.; Huang, C.; Dong, Y.; Zhao, B.; Chen, Y. Gold Core@Platinum Shell Nanozyme-Mediated Magnetic Relaxation Switching DNA Sensor for the Detection of Listeria Monocytogenes in Chicken Samples. Food Control 2022, 137, 108916. [Google Scholar] [CrossRef]
- Amino Acids Functionalized Inorganic Metal Nanoparticles: Synthetic Nanozymes for Target Specific Binding, Sensing and Catalytic Applications. Available online: https://www.springerprofessional.de/en/amino-acids-functionalized-inorganic-metal-nanoparticles-synthet/19230674 (accessed on 13 October 2022).
- Maruyama, T.; Fujimoto, Y.; Maekawa, T. Synthesis of Gold Nanoparticles Using Various Amino Acids. J. Colloid Interface Sci. 2015, 447, 254–257. [Google Scholar] [CrossRef]
- Synthesis of Gold Nanoparticles Using Amino Acids by Light Irradiation|IntechOpen. Available online: https://www.intechopen.com/chapters/50852 (accessed on 13 October 2022).
- Ramezani, F.; Habibi, M.; Rafii-Tabar, H.; Amanlou, M. Effect of Peptide Length on the Conjugation to the Gold Nanoparticle Surface: A Molecular Dynamic Study. DARU J. Pharm. Sci. 2015, 23, 9. [Google Scholar] [CrossRef]
- Yang, X.; Shi, M.; Zhou, R.; Chen, X.; Chen, H. Blending of HAuCl4 and Histidine in Aqueous Solution: A Simple Approach to the Au10 Cluster. Nanoscale 2011, 3, 2596–2601. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Hall, C.K. Binding Preferences of Amino Acids for Gold Nanoparticles: A Molecular Simulation Study. Langmuir 2016, 32, 7888–7896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Sun, H.; Xu, H.; Matysiak, S.; Ren, J.; Qu, X. Mesoporous Encapsulated Chiral Nanogold for Use in Enantioselective Reactions. Angew. Chem. Int. Ed. 2018, 57, 16791–16795. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Li, Y.; Chen, L.; Lan, X.; Hou, C.; Liu, C.; Liu, C. An Amino Acid-Based Supramolecular Nanozyme by Coordination Self-Assembly for Cascade Catalysis and Enhanced Chemodynamic Therapy towards Biomedical Applications. Nanoscale Adv. 2021, 3, 6482–6489. [Google Scholar] [CrossRef]
- Casciaro, B.; Cappiello, F.; Cacciafesta, M.; Mangoni, M.L. Promising Approaches to Optimize the Biological Properties of the Antimicrobial Peptide Esculentin-1a(1–21)NH2: Amino Acids Substitution and Conjugation to Nanoparticles. Front. Chem. 2017, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- Daima, H.K.; Selvakannan, P.R.; Shukla, R.; Bhargava, S.K.; Bansal, V. Fine-Tuning the Antimicrobial Profile of Biocompatible Gold Nanoparticles by Sequential Surface Functionalization Using Polyoxometalates and Lysine. PLoS ONE 2013, 8, e79676. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Liu, M.; Ma, G.; Wang, Y.; Zhao, L.; Yuan, Q.; Gao, F.; Liu, R.; Zhai, J.; Chai, Z.; et al. Peptide-Conjugated Gold Nanoprobe: Intrinsic Nanozyme-Linked Immunsorbant Assay of Integrin Expression Level on Cell Membrane. ACS Nano 2015, 9, 10979–10990. [Google Scholar] [CrossRef]
- Wadhwani, P.; Heidenreich, N.; Podeyn, B.; Bürck, J.; Ulrich, A.S. Antibiotic Gold: Tethering of Antimicrobial Peptides to Gold Nanoparticles Maintains Conformational Flexibility of Peptides and Improves Trypsin Susceptibility. Biomater. Sci. 2017, 5, 817–827. [Google Scholar] [CrossRef]
- Casciaro, B.; Moros, M.; Rivera-Fernández, S.; Bellelli, A.; de la Fuente, J.M.; Mangoni, M.L. Gold-Nanoparticles Coated with the Antimicrobial Peptide Esculentin-1a(1-21)NH2 as a Reliable Strategy for Antipseudomonal Drugs. Acta Biomater. 2017, 47, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.P.P.; Lim, D.-K. Gold-Polymer Nanocomposites for Future Therapeutic and Tissue Engineering Applications. Pharmaceutics 2022, 14, 70. [Google Scholar] [CrossRef]
- Song, W.; Chi, M.; Gao, M.; Zhao, B.; Wang, C.; Lu, X. Self-Assembly Directed Synthesis of Au Nanorices Induced by Polyaniline and Their Enhanced Peroxidase-like Catalytic Properties. J. Mater. Chem. C 2017, 5, 7465–7471. [Google Scholar] [CrossRef]
- Muddineti, O.S.; Ghosh, B.; Biswas, S. Current Trends in Using Polymer Coated Gold Nanoparticles for Cancer Therapy. Int. J. Pharm. 2015, 484, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Liao, H.; Feng, L.; Wang, M.; Fu, W. Accelerating the Peroxidase-like Activity of Gold Nanoclusters at Neutral PH for Colorimetric Detection of Heparin and Heparinase Activity. Anal. Chem. 2018, 90, 6247–6252. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-C.; Yang, M.-Y.; Chang, K.-B.; Tseng, C.-H.; Yang, Y.-P.; Yang, Y.-C.; Kung, M.-L.; Lai, W.-Y.; Lin, T.-W.; Hsieh, H.-H.; et al. Fabrication of Hyaluronic Acid-Gold Nanoparticles with Chitosan to Modulate Neural Differentiation of Mesenchymal Stem Cells. J. Chin. Med. Assoc. 2021, 84, 1007–1018. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, H.; Zhang, L.; Wang, L.; Zeng, Y.; Xia, X.; Liu, F.; Chen, Y. Strategy to Fabricate Naked-Eye Readout Ultrasensitive Plasmonic Nanosensor Based on Enzyme Mimetic Gold Nanoclusters. Anal. Chem. 2016, 88, 1412–1418. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Kanagesan, S.; Pandurangan, A.; Padmanabhan, P. Chapter 4—Basics to Different Imaging Techniques, Different Nanobiomaterials for Image Enhancement. In Nanobiomaterials in Medical Imaging; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 101–129. ISBN 978-0-323-41736-5. [Google Scholar]
- Heger, Z.; Skalickova, S.; Zitka, O.; Adam, V.; Kizek, R. Apoferritin Applications in Nanomedicine. Nanomedicine 2014, 9, 2233–2245. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. Chapter 1—An Overview of Biopolymer Nanostructures for Encapsulation of Food Ingredients. In Biopolymer Nanostructures for Food Encapsulation Purposes; Jafari, S.M., Ed.; Nanoencapsulation in the Food Industry; Academic Press: Cambridge, CA, USA, 2019; pp. 1–35. ISBN 978-0-12-815663-6. [Google Scholar]
- Liu, Y.; Xiang, Y.; Ding, D.; Guo, R. Structural Effects of Amphiphilic Protein/Gold Nanoparticle Hybrid Based Nanozyme on Peroxidase-like Activity and Silver-Mediated Inhibition. RSC Adv. 2016, 6, 112435–112444. [Google Scholar] [CrossRef]
- Blanco, A.; Blanco, G. Chapter 6—Nucleic Acids. In Medical Biochemistry; Blanco, A., Blanco, G., Eds.; Academic Press: Norwich, NY, USA, 2017; pp. 121–140. ISBN 978-0-12-803550-4. [Google Scholar]
- Srivastava, S.; Abraham, P.R.; Mukhopadhyay, S. Aptamers: An Emerging Tool for Diagnosis and Therapeutics in Tuberculosis. Front. Cell. Infect. Microbiol. 2021, 11, 656421. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Revuri, V.; Huh, K.M.; Lee, Y. Chapter 14—Polysaccharide Based Nano/Microformulation: An Effective and Versatile Oral Drug Delivery System. In Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 409–433. ISBN 978-0-323-47720-8. [Google Scholar]
- Jiang, T.; James, R.; Kumbar, S.G.; Laurencin, C.T. Chapter 5—Chitosan as a Biomaterial: Structure, Properties, and Applications in Tissue Engineering and Drug Delivery. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Oxford, UK, 2014; pp. 91–113. ISBN 978-0-12-396983-5. [Google Scholar]


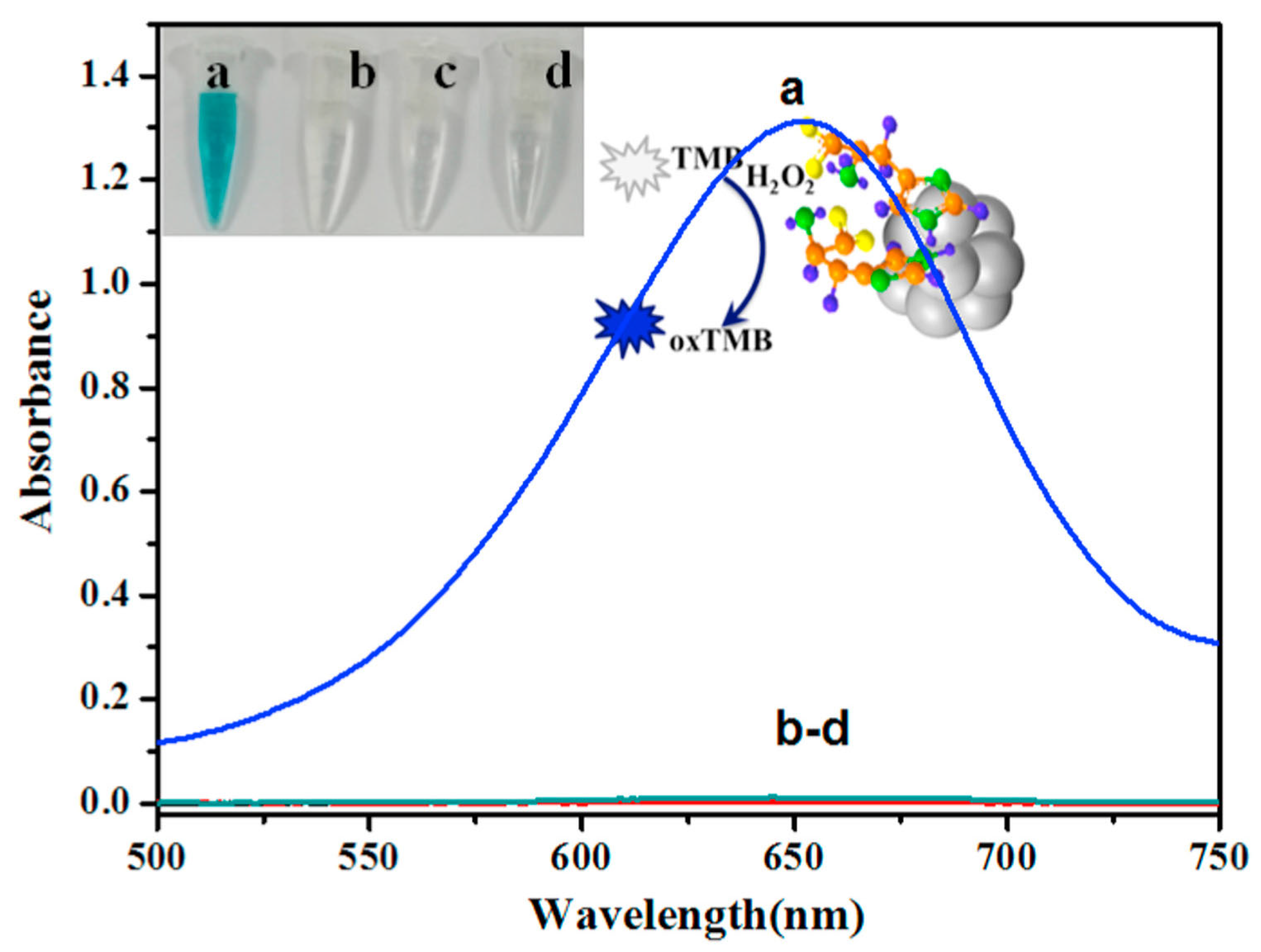
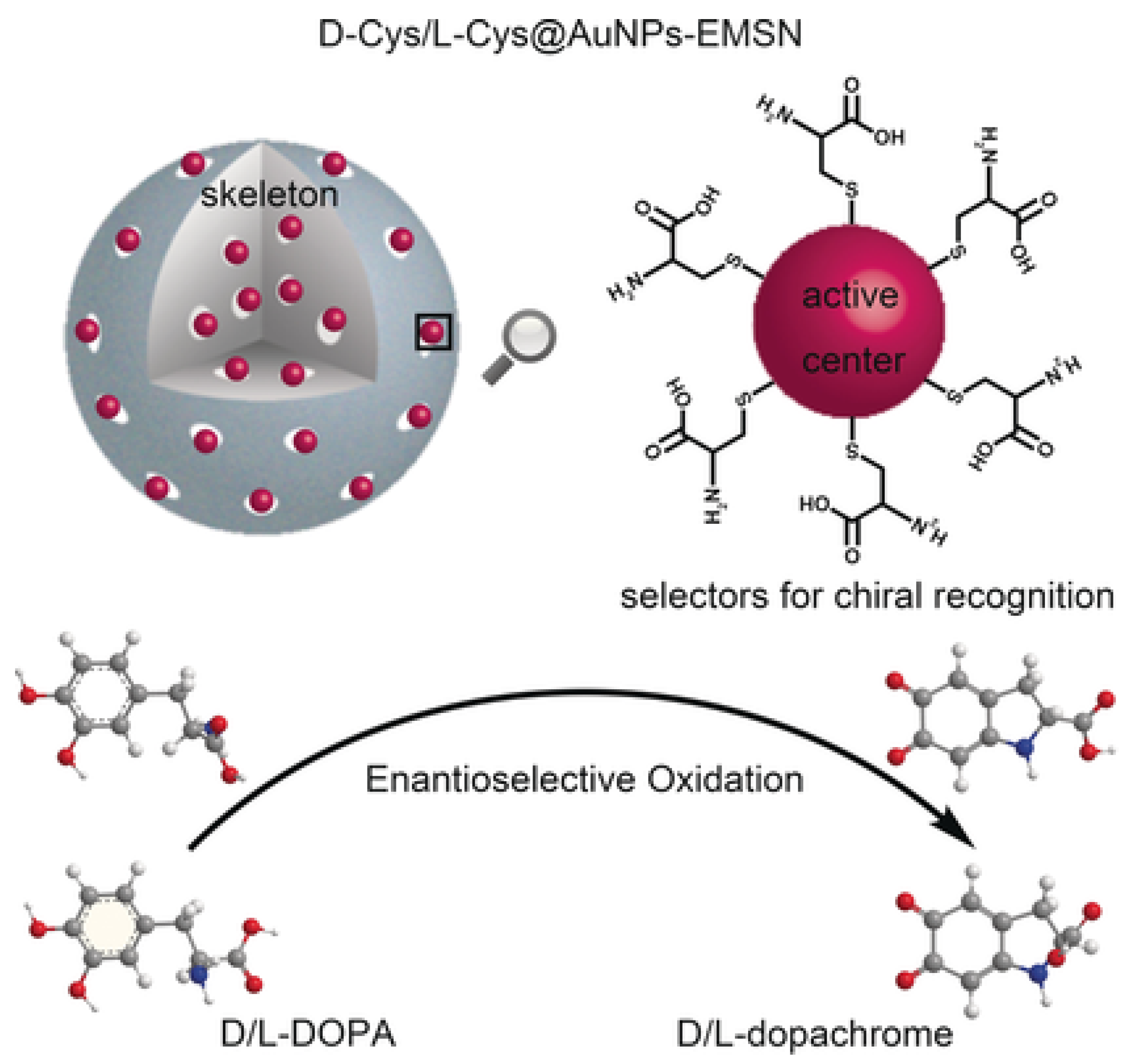


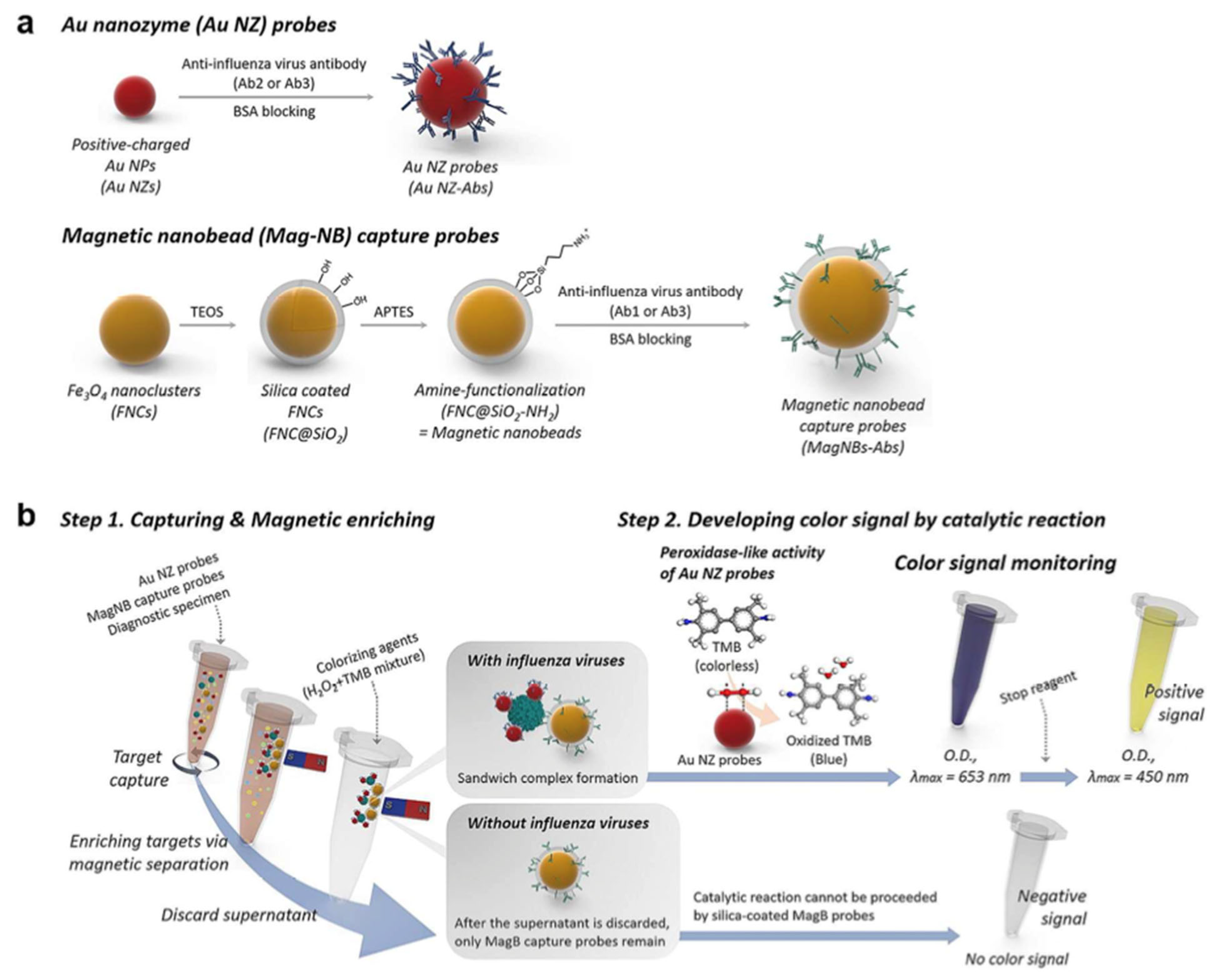
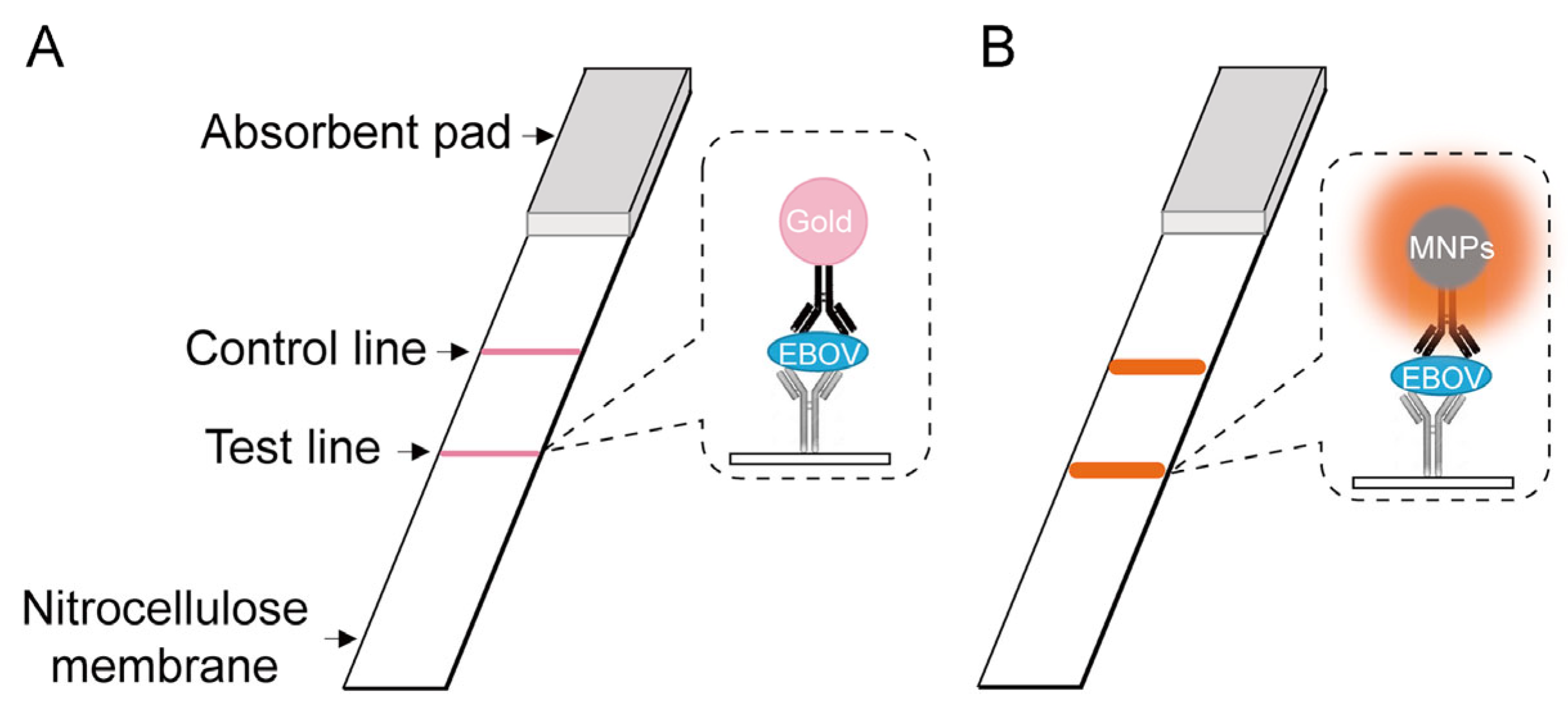





| Inorganic Biohybrids | ||||
|---|---|---|---|---|
| Carbon-Based | ||||
| Carbon-Based Material | Enzymatic Activity | Application | Ref. | |
| Carbon dots | Oxidase | Sensing | Biothiols | [18] |
| Antitumoral | Liver cancer | [19] | ||
| Nanoporous carbon | Oxidase | Sensing | Oxidase | [20] |
| Carbon nanoshell | HRP a | Antitumoral | Gastric cancer | [21] |
| - | [22] | |||
| Graphite | HRP | Sensing | H2O2 and glucose | [23] |
| Carbon dots | HRP | Sensing | Glucose | [24] |
| HRP | Sensing | Tert-butyl hydroquinone and formaldehyde | [25] | |
| HRP | Catalysis | Oxidation of tert-butyl hydroquinone | ||
| Porous carbon @PDAGCU | HRP | Sensing | PSA b | [26] |
| Carbon nanotubes | HRP | Sensing | H2O2 | [27] |
| Graphene oxide@CeO2 | HRP | Sensing | Nitrites | [28] |
| Yolk shell carbon | Oxidase + HRP | Antitumoral | Colorectal cancer | [29] |
| MOFs | ||||
| MOF | Enzymatic Activity | Application | Ref. | |
| MIL-101 | HRP | Sensing | Glucose and lactate | [30] |
| NH2-MIL-125 (Ti) | HRP | Sensing | Cysteine, H2O2 and Hg2+ | [31] |
| Al-MOF-2D | HRP | Antibacterial | Staphylococcus aureus and Escherichia coli | [32] |
| Fe-MOF | HRP | Degradation | Methylene blue | [33] |
| HRP | Sensing | Hydroxyl radical | ||
| Co-MOF | HRP | Sensing | Burkholderia pseudomallei | [34] |
| Metals | ||||
| Metal | Enzymatic Activity | Application | Ref. | |
| Tubular TiO2 | HRP | Sensing | H2O2 | [35] |
| Ag alloy | HRP | Antibacterial | Mycobacterium tuberculosis | [36] |
| Pd@Ir core–shell | HRP | Sensing | PSA | [37] |
| Pt core–shell | HRP | Sensing | Improving ELISA | [38] |
| Co-Fe core–shell | HRP | Sensing | Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Bacillus cereus | [39] |
| Yolk shell TiO2 | HRP | Sensing | H2O2 and glucose | [40] |
| Fe2O3 nanocubes | HRP | Sensing | Improving ELISA | [41] |
| HRP | Sensing | P53 autoantibodies | [42] | |
| Pt core–shell | HRP | Sensing | Glucose | [43] |
| Organic Hybrids | ||||
| Aminoacids (aa) | ||||
| aa | Enzymatic Activity | Application | Ref. | |
| Various | HRP | Sensing | Cu2+, histidine | [44] |
| Histidine | HRP | Sensing | Nitrite | [45] |
| Cysteine | HRP | Enantioselectivity | Dopamine | [45] |
| Peptide | HRP | Optical imaging | Cancer cells (HEL cells) | [30] |
| Histidine | Oxidase | Sensing | Doxycycline | [46] |
| Glucose oxidase | Sensing | Glucose | [47] | |
| Polymers | ||||
| Polymer | Enzymatic Activity | Application | Ref. | |
| PEG-SH | HRP | Sensing | H2O2 | [48,49] |
| PEG/Carboxylate | HRP | Sensing | Proteins | [50] |
| PAM-4 | HRP | Sensing | Ciprofloxacin | [51] |
| Heparin | HRP | Microdialysis | Cytokines | [52] |
| PCL/Gelatin | HRP | Antibacterial + Wound healing | MDR Bacteria | [53] |
| Hyaluronic acid | HRP | Anticancer | 4T1 breast cancer cells | [54] |
| Biohybrids | ||||
| Proteins | ||||
| Protein | Enzymatic Activity | Application | Ref. | |
| Ab c | HRP | Sensing | Trichinella spiralis | [55] |
| HRP | Sensing | Influenza A virus | [56] | |
| HRP | Sensing | Ebola | [57] | |
| HRP | Sensing | Influenza virus | [58] | |
| Apoferritin | HRP | Sensing | Glucose | [59] |
| SOD d, Catalase, HRP | ROS Scavenger | O2− | [60] | |
| BSA e | HRP | Sensing | Tea polyphenols | [61] |
| HRP | Sensing | Xanthine | [62] | |
| Glucose oxidase + HRP | Sensing | Glucose | [63] | |
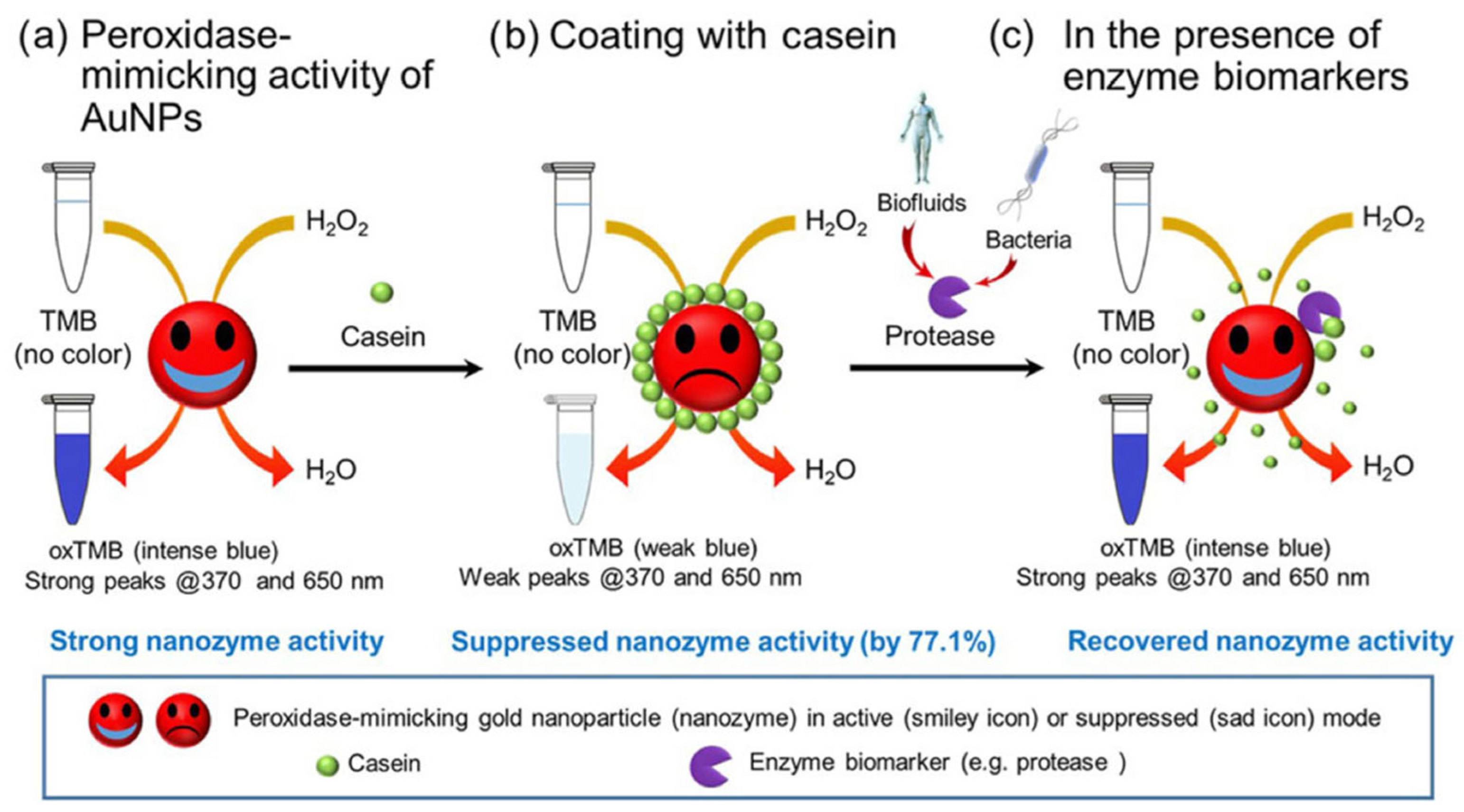
| β-Cas f | HRP | Sensing | Protease enzyme | [64] |
| Nucleic acids | ||||
| Nucleic Acid | Enzymatic Activity | Application | Ref. | |
| Apt g | HRP | Sensing | C Reactive protein | [65] |
| HRP | Sensing | CA125 Ovarian cancer biomarker | [66] | |
| HRP | Sensing | Ampicillin | [67] | |
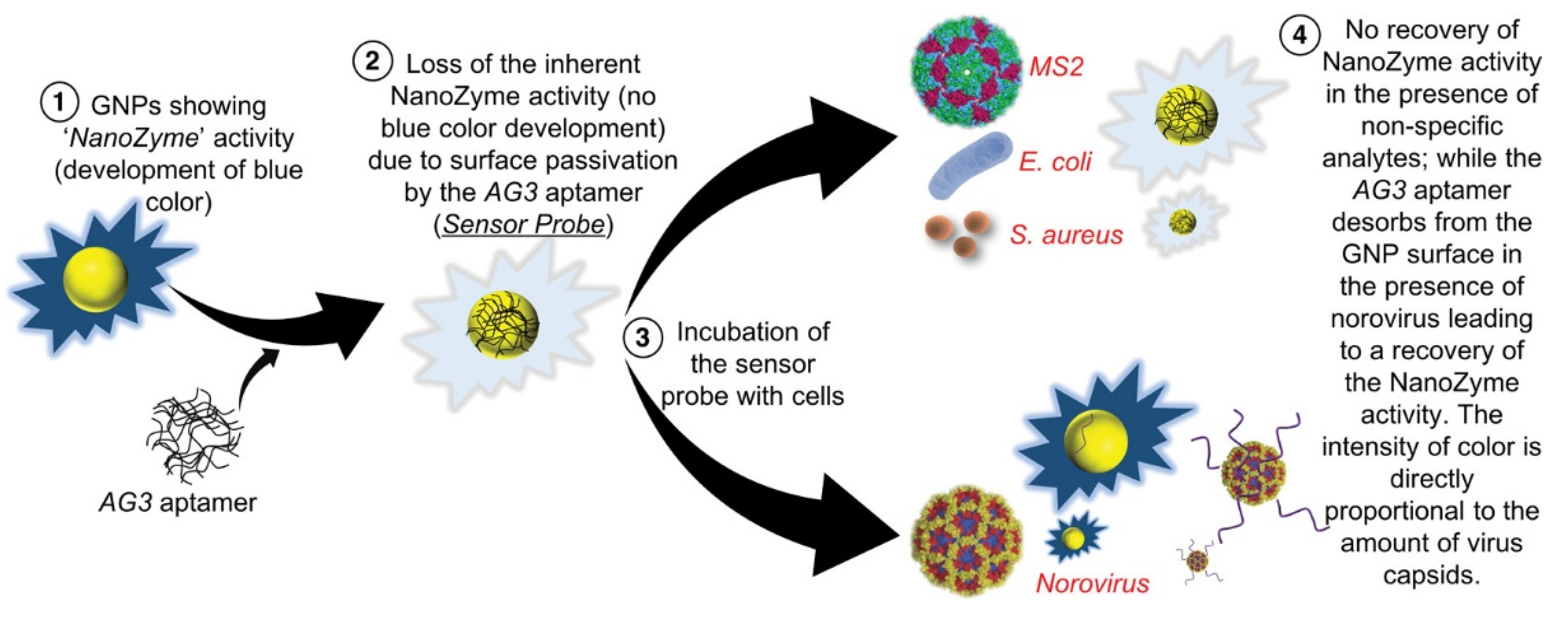
| HRP | Sensing | Norovirus | [68] | |
| HRP | Sensing | Acetamiprid pesticide | [69] | |
| HRP | Sensing | Kanamycin | [70] | |
| HRP | Sensing | Zearalenone | [71] | |
| HRP | Sensing | Streptomycin | [72] | |
| Polysaccharides | ||||
| Polysaccharide | Enzymatic Activity | Application | Ref. | |
| Chitosan | Oxidase + HRP | Antibacterial + Bacterial imaging | Helicobacter pylori | [73] |
| HRP | Sensing | H2O2 and glucose | [74] | |
| HRP | Sensing | Hg2+ | [75] | |
| HRP | Sensing | Glucose | [76] | |
| HRP | Sensing | Melamine | [77] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimenez-Falcao, S.; Méndez-Arriaga, J.M.; García-Almodóvar, V.; García-Valdivia, A.A.; Gómez-Ruiz, S. Gold Nanozymes: Smart Hybrids with Outstanding Applications. Catalysts 2023, 13, 13. https://doi.org/10.3390/catal13010013
Jimenez-Falcao S, Méndez-Arriaga JM, García-Almodóvar V, García-Valdivia AA, Gómez-Ruiz S. Gold Nanozymes: Smart Hybrids with Outstanding Applications. Catalysts. 2023; 13(1):13. https://doi.org/10.3390/catal13010013
Chicago/Turabian StyleJimenez-Falcao, Sandra, Jose M. Méndez-Arriaga, Victoria García-Almodóvar, Antonio A. García-Valdivia, and Santiago Gómez-Ruiz. 2023. "Gold Nanozymes: Smart Hybrids with Outstanding Applications" Catalysts 13, no. 1: 13. https://doi.org/10.3390/catal13010013