Oxidation Catalysis of Au Nano-Particles Immobilized on Titanium(IV)- and Alkylthiol-Functionalized SBA-15 Type Mesoporous Silicate Supports
Abstract
:1. Introduction
2. Results
2.1. Preparation and Characterization of the Catalysts
2.1.1. Preparation and Characterization of Bi-Functionalized SBA-15 Type Supports
2.1.2. Preparation and Characterization of Au-Immobilized Catalysts
2.1.3. Characterization of the Calcined Catalyst
2.2. Catalysis
2.2.1. Aerobic Alcohol Oxidation Activity
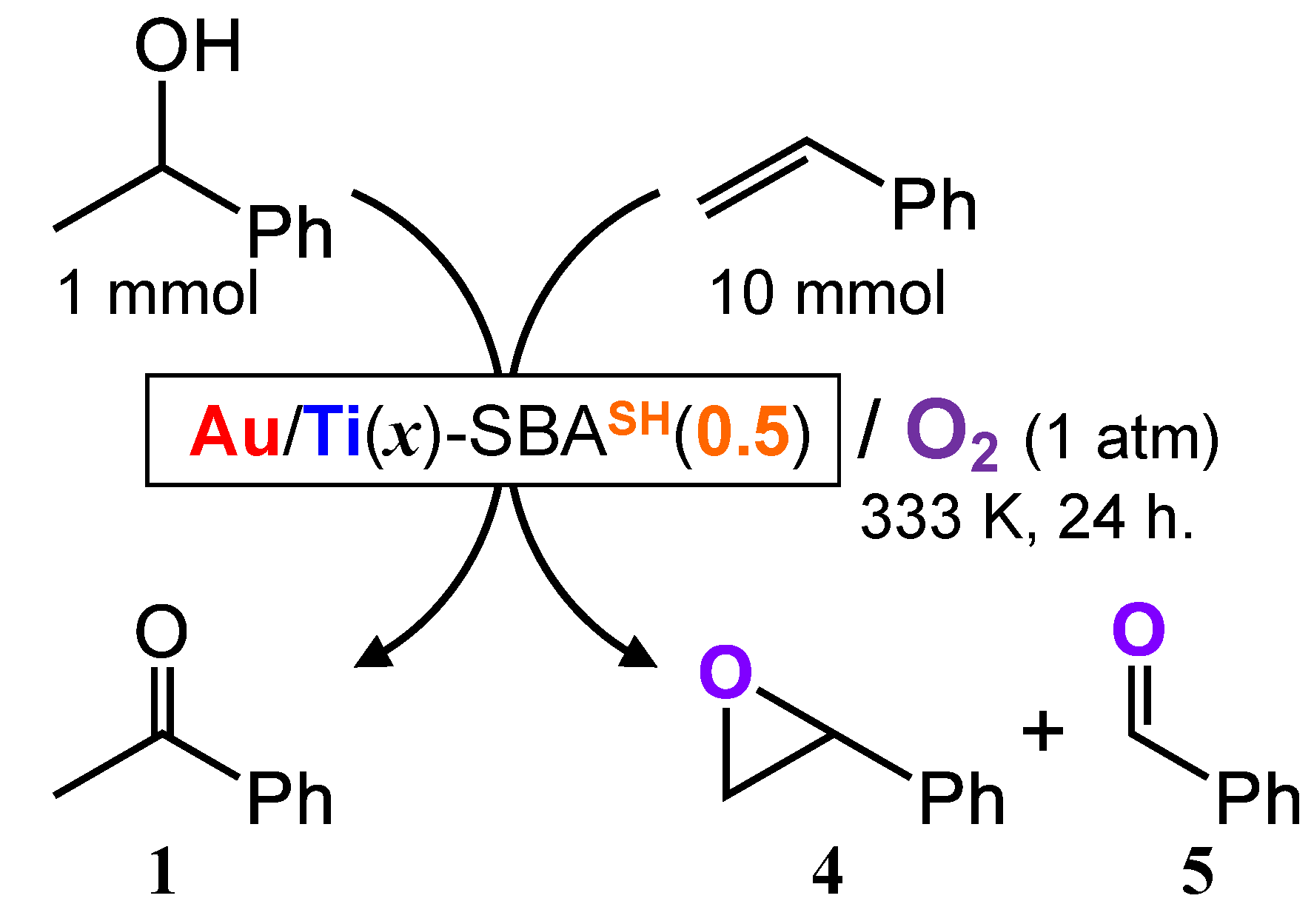
2.2.2. Co-Oxidation of Alcohol and Alkene
3. Discussion
4. Materials and Methods
4.1. General
4.2. Preparation of the Bi-Functionalized SBA-15 Type Supports Ti(x)-SBASH(y)
4.3. Preparation of the Catalysts Au/Ti(x)-SBASH(y)
4.4. Catalytic Aerobic Oxidation of 1-Phenylethanol
4.5. Catalytic Co-Oxidation of 1-Phenylethanol and Styrene
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- German, D.; Pakrieva, E.; Kolobova, E.; Carabineiro, S.A.C.; Stucchi, M.; Villa, A.; Prati, L.; Bogdanchikova, N.; Cortés Corberán, V.; Pestryakov, A. Oxidation of 5-Hydroxymethylfurfural on Supported Ag, Au, Pd and Bimetallic Pd-Au Catalysts: Effect of the Support. Catalysts 2021, 11, 115. [Google Scholar] [CrossRef]
- Cattaneo, S.; Stucchi, M.; Villa, A.; Prati, L. Gold Catalysts for the Selective Oxidation of Biomass-Derived Products. ChemCatChem 2019, 11, 309–323. [Google Scholar] [CrossRef]
- Pakrieva, E.; Kolobova, E.; German, D.; Stucchi, M.; Villa, A.; Prati, L.; Carabineiro, S.A.; Bogdanchikova, N.; Corberán, V.C.; Pestryakov, A. Glycerol Oxidation over Supported Gold Catalysts: The Combined Effect of Au Particle Size and Basicity of Support. Processes 2020, 8, 1016. [Google Scholar] [CrossRef]
- Jouve, A.; Stucchi, M.; Barlocco, I.; Evangelisti, C.; Somodic, F.; Villa, A.; Prati, L. Carbon-Supported Au Nanoparticles: Catalytic Activity Ruled Out by Carbon Support. Top. Catal. 2018, 61, 1928–1938. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C. Supported Gold Nanoparticles as Catalysts for the Oxidation of Alcohols and Alkanes. Front. Chem. 2019, 7, 709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, T.; Murayama, T.; Taketoshi, A.; Haruta, M. Importance of Size and Contact Structure of Gold Nanoparticles for the Genesis of Unique Catalytic Processes. Chem. Rev. 2020, 120, 464–525. [Google Scholar] [CrossRef] [Green Version]
- Okumura, M.; Fujitani, T.; Huang, J.; Ishida, T. A Career in Catalysis: Masatake Haruta. ACS Catal. 2015, 5, 4699–4707. [Google Scholar] [CrossRef]
- Yamazoe, S.; Koyasu, K.; Tsukuda, T. Nonscalable Oxidation Catalysis of Gold Clusters. Accounts Chem. Res. 2014, 47, 816–824. [Google Scholar] [CrossRef]
- Gutiérrez, L.-F.; Hamoudi, S.; Belkacemi, K. Synthesis of Gold Catalysts Supported on Mesoporous Silica Materials: Recent Developments. Catalysts 2011, 1, 97–154. [Google Scholar] [CrossRef]
- Aprile, C.; Abad, A.; García, H.; Corma, A. Synthesis and catalytic activity of periodic mesoporous materials incorporating gold nanoparticles. J. Mater. Chem. 2005, 15, 4408–4413. [Google Scholar] [CrossRef]
- Tsunoyama, H.; Sakurai, H.; Tsukuda, T. Size effect on the catalysis of gold clusters dispersed in water for aerobic oxidation of alcohol. Chem. Phys. Lett. 2006, 429, 528–532. [Google Scholar] [CrossRef]
- Liu, Y.; Tsunoyama, H.; Akita, T.; Xie, S.; Tsukuda, T. Aerobic Oxidation of Cyclohexane Catalyzed by Size-Controlled Au Clusters on Hydroxyapatite: Size Effect in the Sub-2 nm Regime. ACS Catal. 2011, 1, 2–6. [Google Scholar] [CrossRef]
- Miyamura, H.; Matsubara, R.; Miyazaki, Y.; Kobayashi, S. Aerobic Oxidation of Alcohols at Room Temperature and Atmospheric Conditions Catalyzed by Reusable Gold Nanoclusters Stabilized by the Benzene Rings of Polystyrene Derivatives. Angew. Chem. Int. Ed. 2007, 46, 4151–4154. [Google Scholar] [CrossRef]
- Truttmann, V.; Drexler, H.; Stöger-Pollach, M.; Kawawaki, T.; Negishi, Y.; Barrabés, N.; Rupprechter, G. CeO 2 Supported Gold Nanocluster Catalysts for CO Oxidation: Surface Evolution Influenced by the Ligand Shell. ChemCatChem 2022, 14, e2022003. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Bai, P.; Lei, Z.; Loh, K.; Zhao, X.S. Gold nanoparticles supported on functionalized mesoporous silica for selective oxidation of cyclohexane. Microporous Mesoporous Mater. 2011, 141, 222–230. [Google Scholar] [CrossRef]
- Wu, P.; Bai, P.; Yan, Z.; Zhao, G.X.S. Gold nanoparticles supported on mesoporous silica: Origin of high activity and role of Au NPs in selective oxidation of cyclohexane. Sci. Rep. 2016, 6, 18817. [Google Scholar] [CrossRef] [Green Version]
- Bravo-Suárez, J.J.; Bando, K.K.; Akita, T.; Fujitani, T.; Fuhrer, T.J.; Oyama, S.T. Propane reacts with O2 and H2 on gold supported TS-1 to form oxygenates with high selectivity. Chem. Commun. 2008, 3272–3274. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Akita, T.; Faye, J.; Fujitani, T.; Takei, T.; Haruta, M. Propene Epoxidation with Dioxygen Catalyzed by Gold Clusters. Angew. Chem. Int. Ed. 2009, 48, 7862–7866. [Google Scholar] [CrossRef] [PubMed]
- Solé-Daura, A.; Zhang, T.; Fouilloux, H.; Robert, C.; Thomas, C.M.; Chamoreau, L.-M.; Carbó, J.J.; Proust, A.; Guillemot, G.; Poblet, J.M. Catalyst Design for Alkene Epoxidation by Molecular Analogues of Heterogeneous Titanium-Silicalite Catalysts. ACS Catal. 2020, 10, 4737–4750. [Google Scholar] [CrossRef]
- Goto, Y.; Kamata, K.; Yamaguchi, K.; Uehara, K.; Hikichi, S.; Mizuno, N. Synthesis, Structural Characterization, and Catalytic Performance of Dititanium-Substituted g-Keggin Silicotungstate. Inorg. Chem. 2006, 45, 2347–2356. [Google Scholar] [CrossRef]
- Bhadra, S.; Akakura, M.; Yamamoto, H. Design of a New Bimetallic Catalyst for Asymmetric Epoxidation and Sulfoxidation. J. Am. Chem. Soc. 2015, 137, 15612–15615. [Google Scholar] [CrossRef] [PubMed]
- Talsi, E.P.; Samsonenko, D.G.; Bryliakov, K.P. Titanium Salan Catalysts for the Asymmetric Epoxidation of Alkenes: Steric and Electronic Factors Governing the Activity and Enantioselectivity. Chem. Eur. J. 2014, 20, 14329–14335. [Google Scholar] [CrossRef] [PubMed]
- Berkessel, A.; Günther, T.; Wang, Q.; Neudörfl, J.-M. Titanium Salalen Catalysts Based on cis-1,2-Diaminocyclohexane: Enan-tioselective Epoxidation of Terminal Non-Conjugated Olefins with H2O2. Angew. Chem. Int. Ed. 2013, 52, 8467–8471. [Google Scholar] [CrossRef]
- Yu, Y.; Fang, N.; Chen, Z.; Liu, D.; Liu, Y.; He, M. Catalyst Design for Alkene Epoxidation by Molecular Analogues of Het-erogeneous Titanium-Silicalite Catalysts. ACS Sustainable Chem. Eng. 2022, 10, 11641–11654. [Google Scholar] [CrossRef]
- Vinu, A.; Srinivasu, P.; Miyahara, M.; Ariga, K. Preparation and Catalytic Performances of Ultralarge-Pore TiSBA-15 Mesoporous Molecular Sieves with Very High Ti Content. J. Phys. Chem. B 2006, 110, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Y.; Yokoi, T.; Kondo, J.N.; Tatsumi, T. High-Performance Titanosilicate Catalyst Obtained through Combination of Liquid-Phase and Solid-Phase Transformation Mechanisms. ChemCatChem 2014, 6, 2719–2726. [Google Scholar] [CrossRef]
- Huybrechts, D.R.C.; De Bruycker, L.; Jacobs, P.A. Oxyfunctionalization of alkanes with hydrogen peroxide on titanium silicalite. Nature 1990, 345, 240–242. [Google Scholar] [CrossRef]
- Sasaki, M.; Sato, Y.; Tsuboi, Y.; Inagaki, S.; Kubota, Y. Ti-YNU-2: A Microporous Titanosilicate with Enhanced Catalytic Performance for Phenol Oxidation. ACS Catal. 2014, 4, 2653–2657. [Google Scholar] [CrossRef]
- Li, G.; Zhao, X.S. Characterization and Photocatalytic Properties of Titanium-Containing Mesoporous SBA-15. Ind. Eng. Chem. Res. 2006, 45, 3569–3573. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Lu, J.; Han, B.; Li, M.; Xiu, J.; Ying, P.; Li, C. Direct Synthesis and Characterization of Titanium-Substituted Mesoporous Molecular Sieve SBA-15. Chem. Mater. 2002, 14, 3413–3421. [Google Scholar] [CrossRef]
- Turner, M.; Golovko, V.B.; Vaughan, O.P.H.; Abdulkin, P.; Berenguer-Murcia, A.; Tikhov, M.S.; Johnson, B.F.G.; Lambert, R.M. Selective oxidation with dioxygen by gold nanoparticle catalysts derived from 55-atom clusters. Nature 2008, 454, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, J.; Stack, T.D.P. Controlled Loadings in a Mesoporous Material: Click-on Silica. J. Am. Chem. Soc. 2008, 130, 14360–14361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsunoyama, H.; Ichikuni, N.; Sakurai, H.; Tsukuda, T. Effect of Electronic Structures of Au Clusters Stabilized by Poly(N-vinyl-2-pyrrolidone) on Aerobic Oxidation Catalysis. J. Am. Chem. Soc. 2009, 131, 7086–7093. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Yakita, Y.; Katayama, M.; Higashimine, K.; Ebitani, K. The role of negatively charged Au states in aerobic oxidation of alcohols over hydrotalcite supported AuPd nanoclusters. Catal. Sci. Technol. 2013, 3, 351–359. [Google Scholar] [CrossRef]
- Staykov, A.; Nishimi, T.; Yoshizawa, K.; Ishihara, T. Oxygen Activation on Nanometer-Size Gold Nanoparticles. J. Phys. Chem. C 2012, 116, 15992–16000. [Google Scholar] [CrossRef]
- Nakamizu, A.; Kasai, T.; Nakazawa, J.; Hikichi, S. Immobilization of a Boron Center-Functionalized Scorpionate Ligand on Mesoporous Silica Supports for Heterogeneous Tp-Based Catalysts. ACS Omega 2017, 2, 1025–1030. [Google Scholar] [CrossRef]
- Matsubara, C.; Iwamoto, T.; Nishikawa, Y.; Takamura, K.; Yano, S.; Yoshikawa, S. Coloured species formed from the titanium(IV)-4-(2′-pyridylazo)resorcinol reagent in the spectrophotometric determination of trace amounts of hydrogen peroxide. J. Chem. Soc. Dalton Trans. 1985, 81–84. [Google Scholar] [CrossRef]

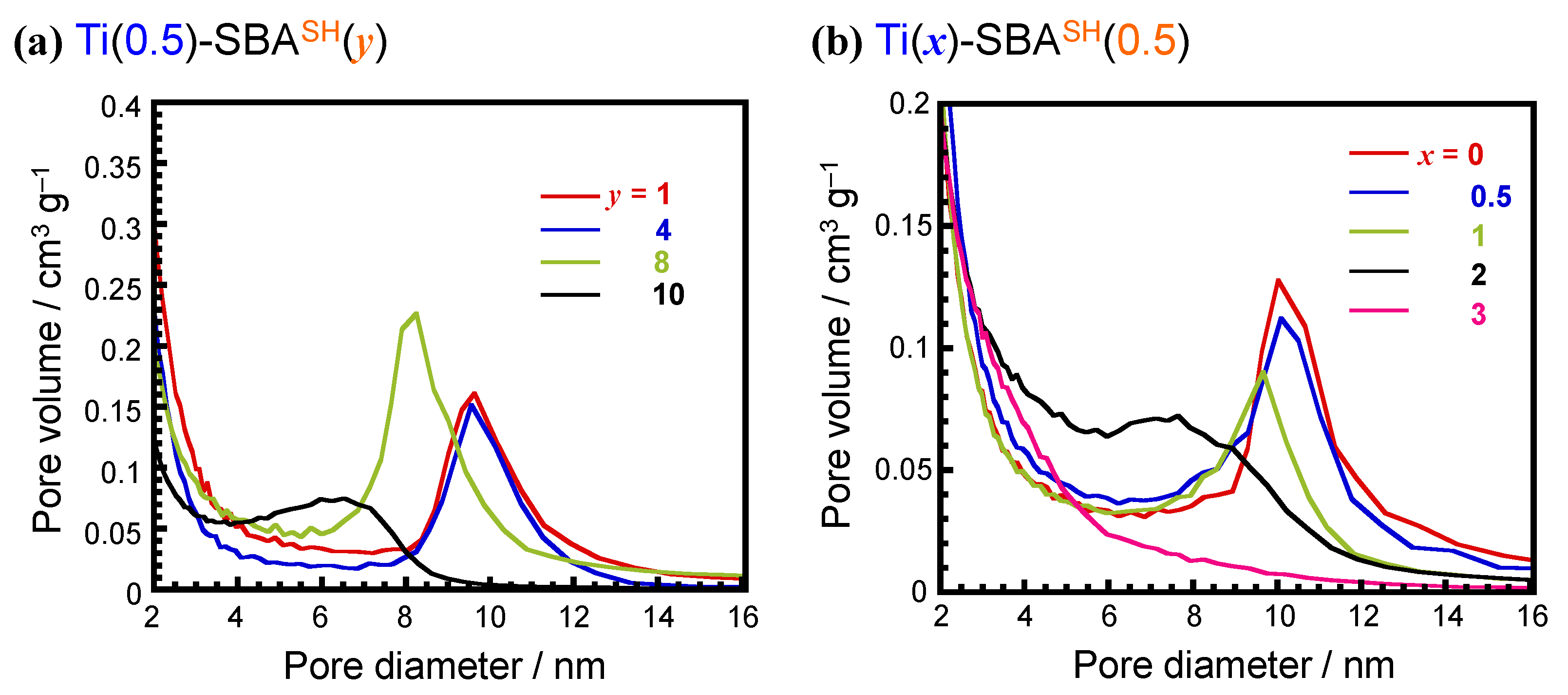
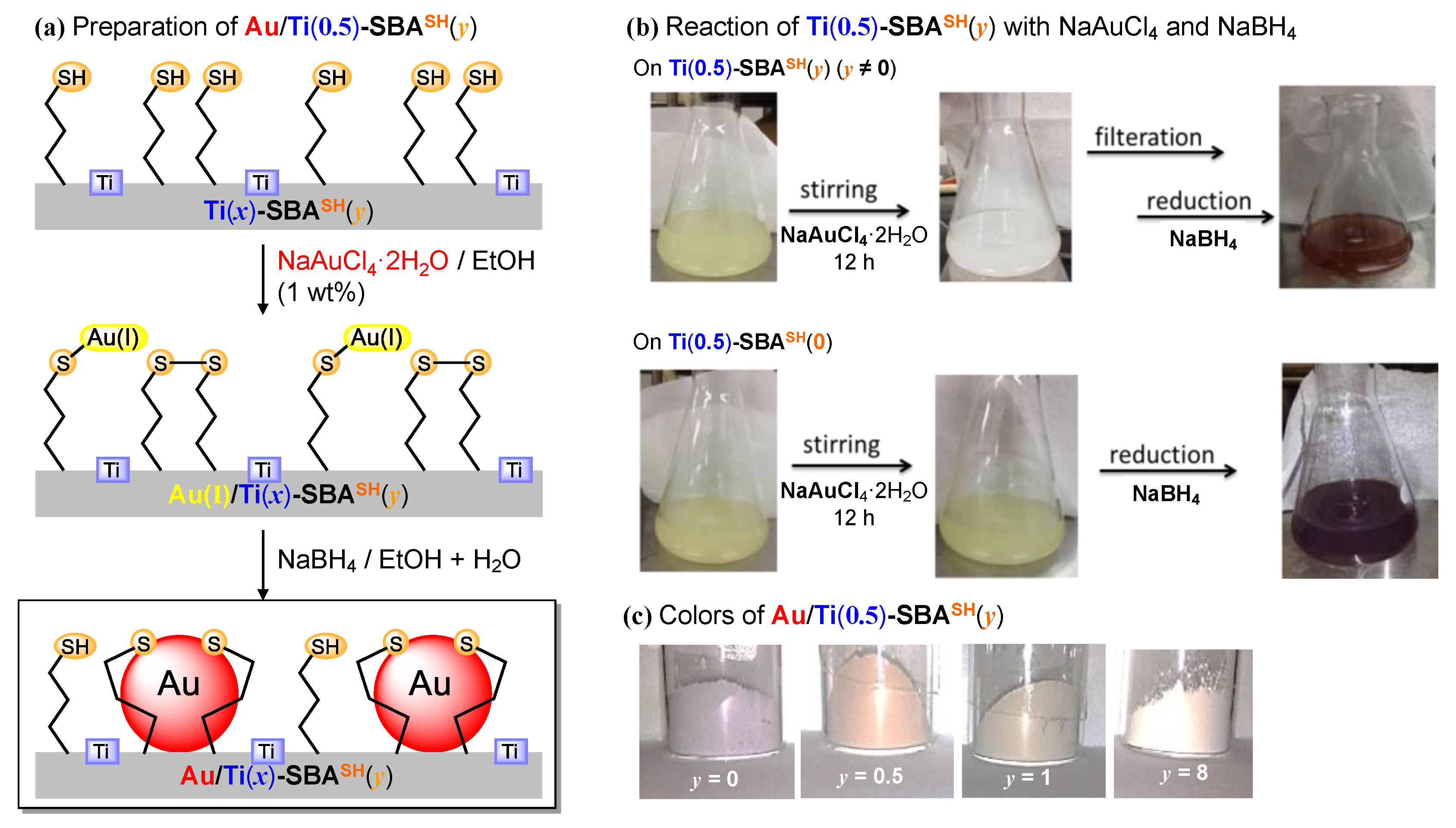

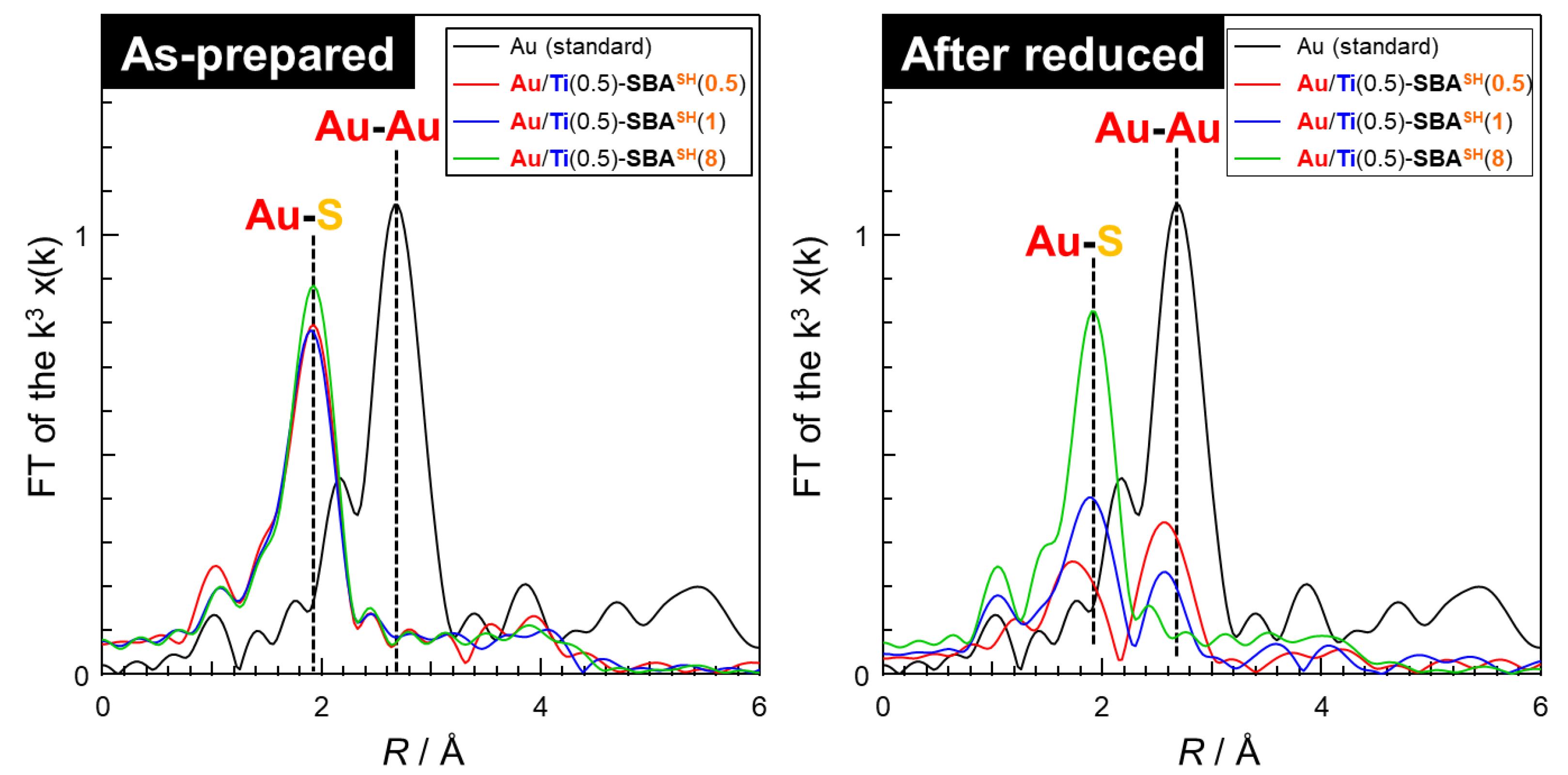
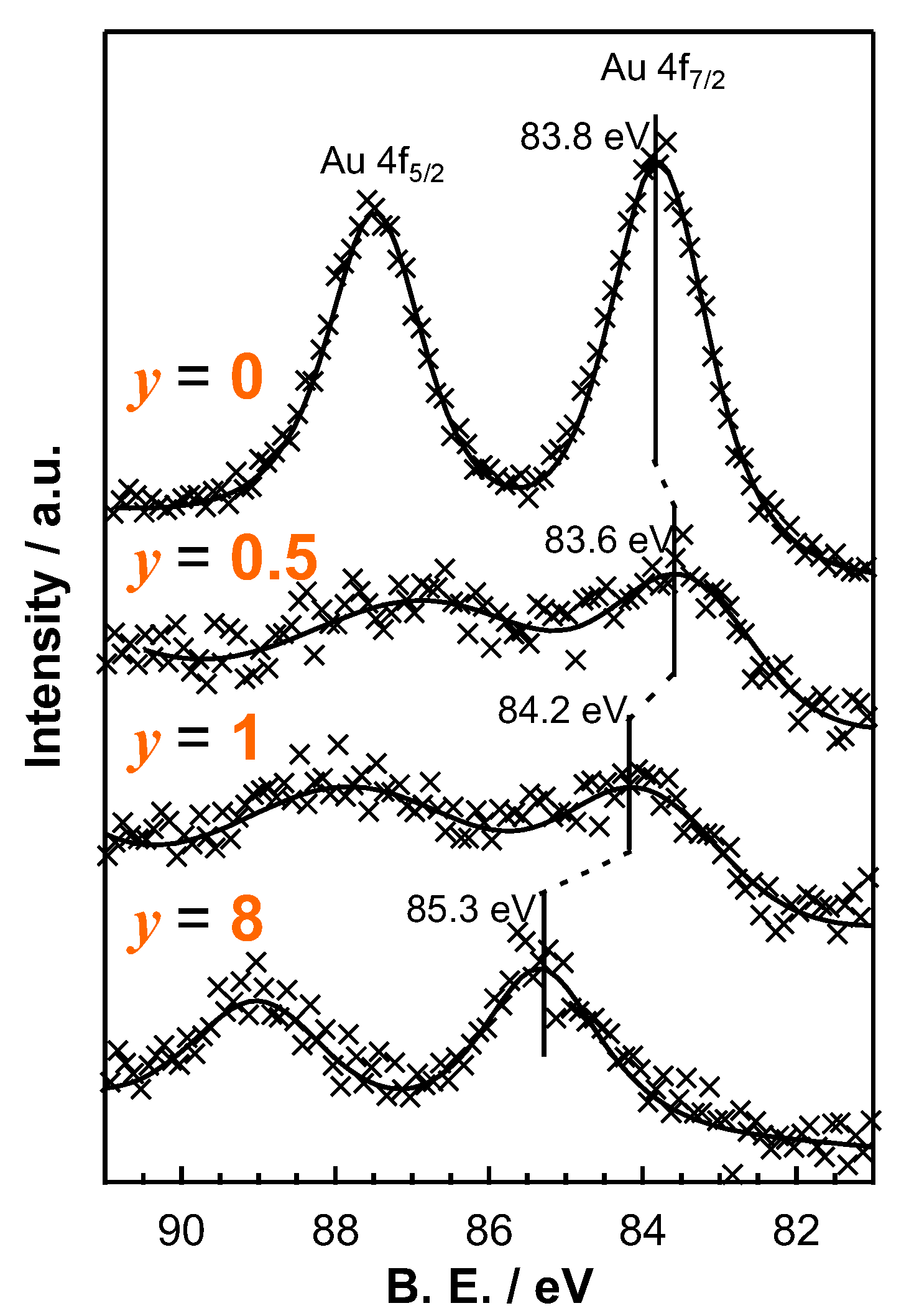
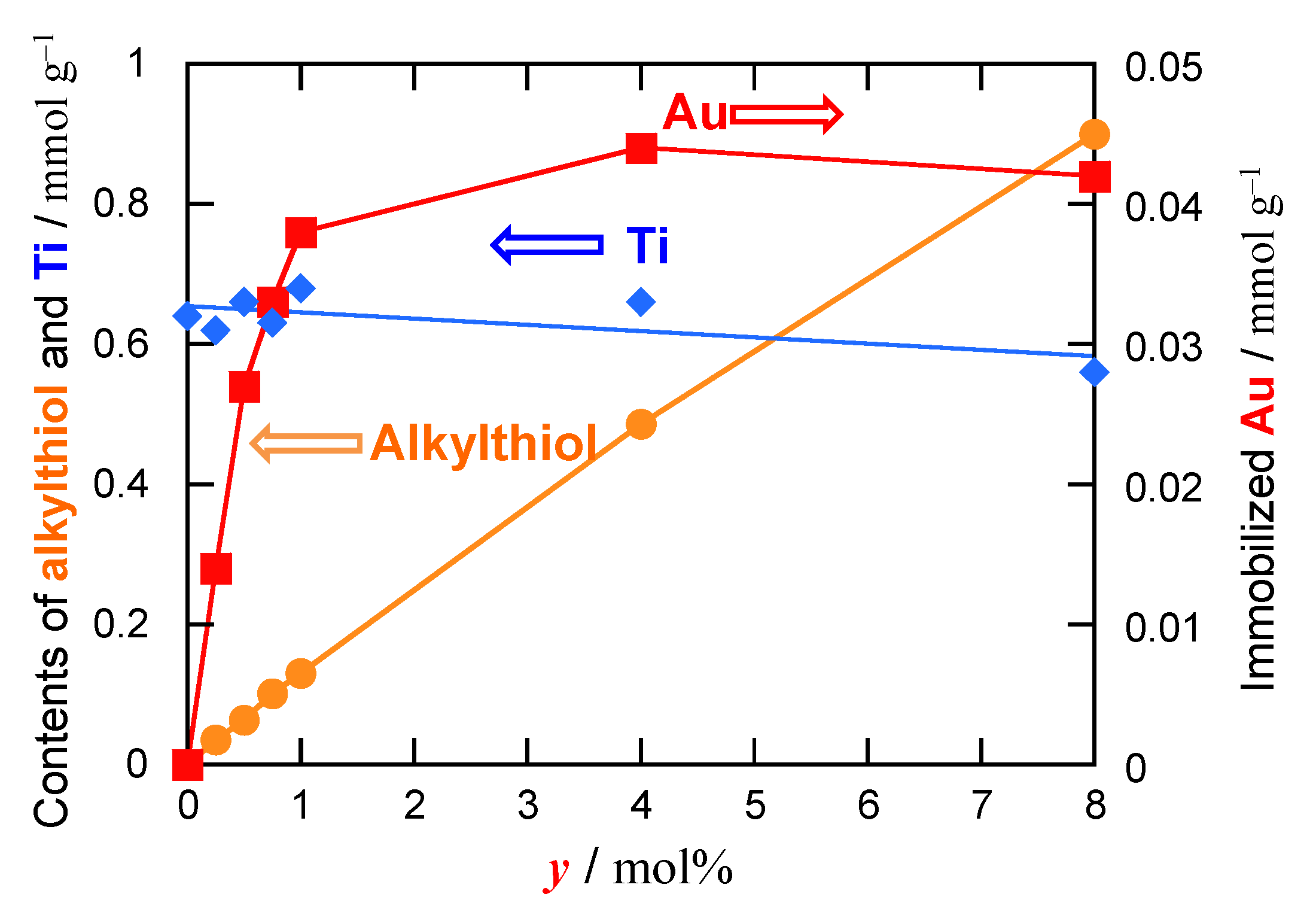
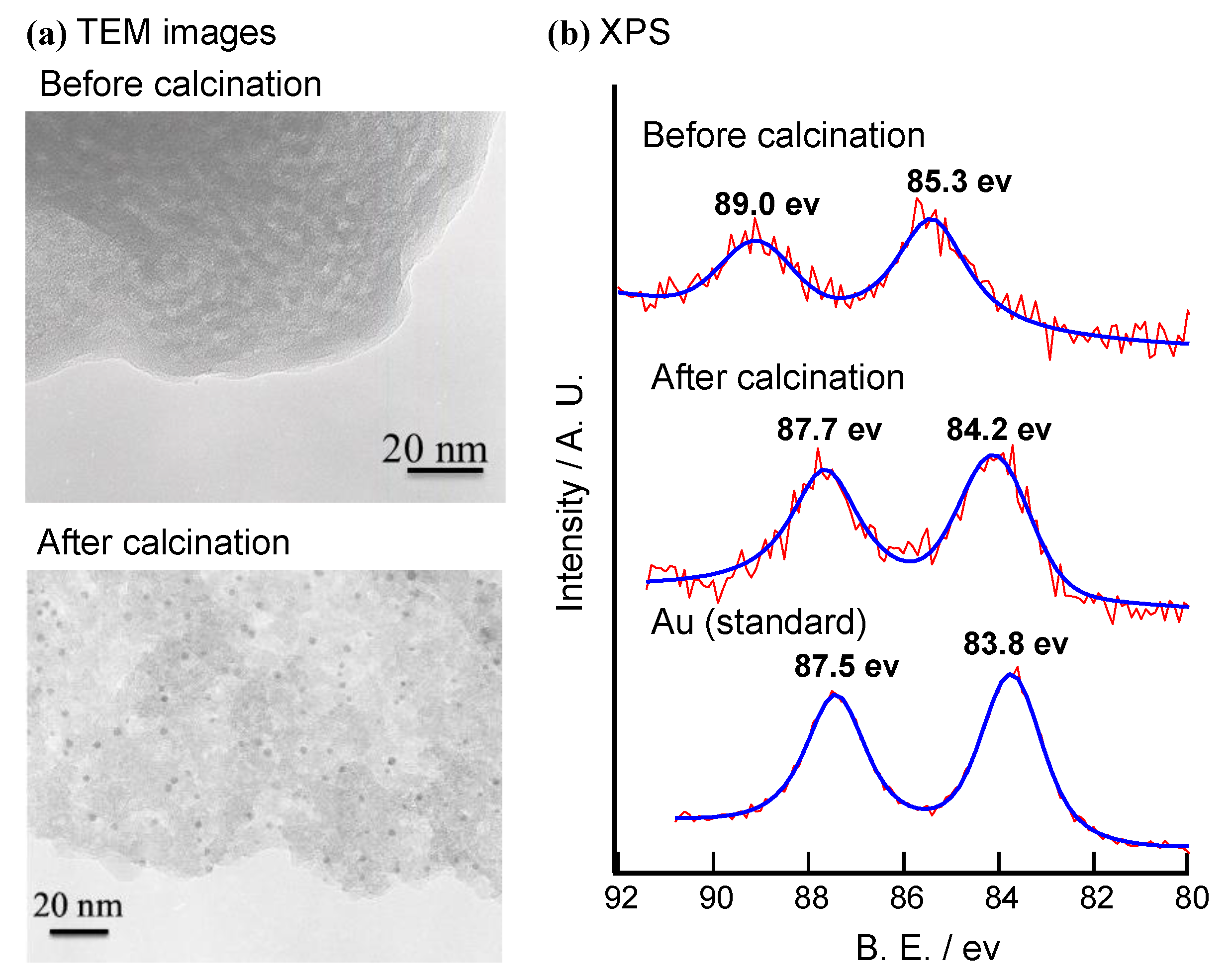


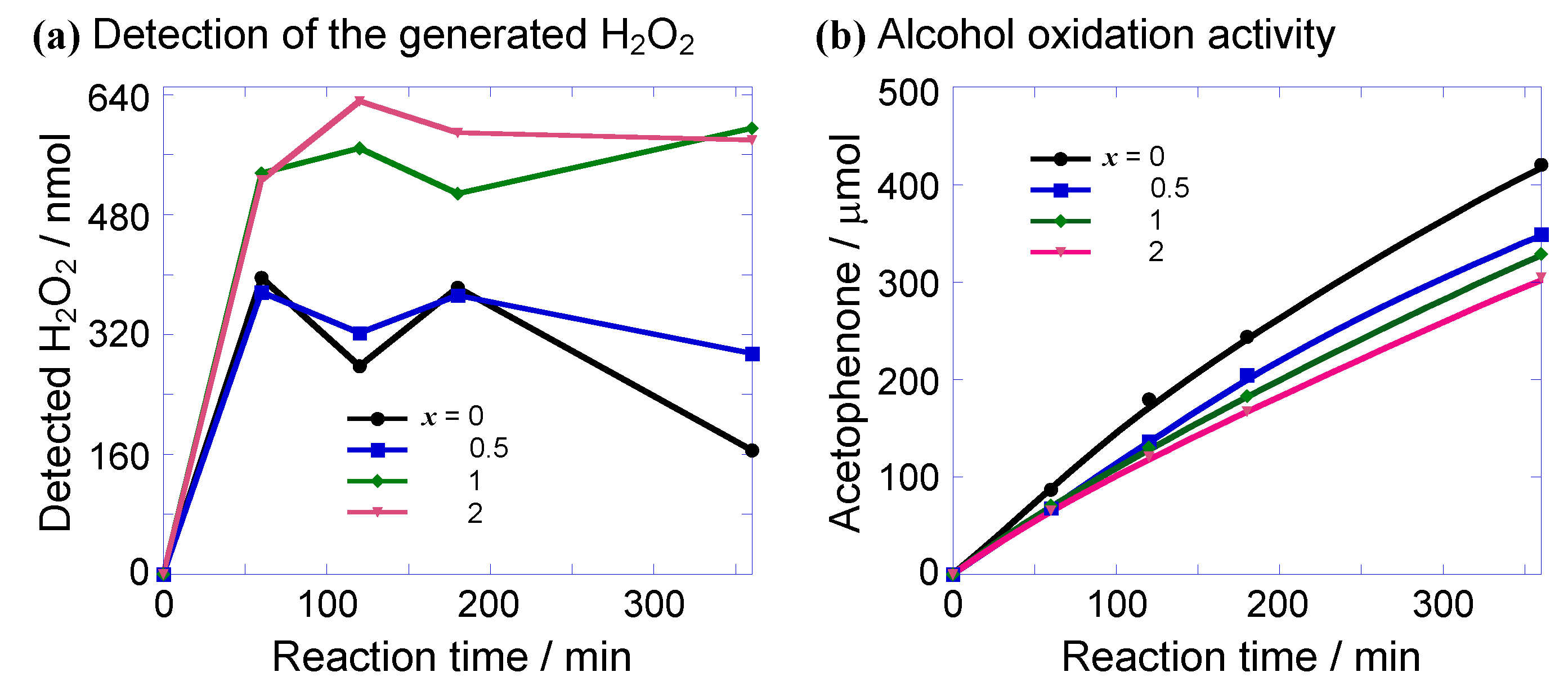
| Applied Ratio/mol% | Contents/mmol g–1 | Surface Area/m2 g–1 | Average Pore Diameter/nm | Total Pore Volume /cm3 g−1 | ||
|---|---|---|---|---|---|---|
| x | y | Ti | Alkylthiol | |||
| 0.5 | 0 | 0.064 | — | 847.3 | 8.57 | 0.82 |
| 0.25 | 0.062 | 0.035 | 1013.1 | 10.09 | 0.95 | |
| 0.5 * | 0.066 * | 0.064 * | 971.4 * | 9.58 * | 1.04 * | |
| 0.75 | 0.063 | 0.101 | 1058.3 | 8.96 | 0.97 | |
| 1 | 0.068 | 0.121 | 948.1 | 9.54 | 1.15 | |
| 4 | 0.066 | 0.486 | 710.2 | 9.46 | 0.71 | |
| 8 | 0.056 | 0.900 | 726.3 | 8.14 | 0.99 | |
| 10 | 0.058 | 0.761 | 415.8 | 6.41 | 0.43 | |
| 0 | 0.5 | — | 0.062 | 897.5 | 10.05 | 0.91 |
| 0.5 * | 0.066 * | 0.064 * | 971.4 * | 9.58 * | 1.04 * | |
| 1 | 0.152 | 0.062 | 795.2 | 9.64 | 0.79 | |
| 2 | 0.309 | 0.063 | 819.2 | 7.48 | 0.85 | |
| 3 | Not measured | 732.0 | Not calculated | 0.56 | ||
| Catalyst | Alkylthiol/mmol g–1 | Immobilized Au/mmol g–1 |
|---|---|---|
| Au/Ti(0.5)-SBASH(0) | — | <0.001 |
| Au/Ti(0.5)-SBASH(0) 1 | — | 0.049 |
| Au/Ti(0.5)-SBASH(0.25) | 0.035 | 0.014 |
| Au/Ti(0.5)-SBASH(0.5) | 0.064 | 0.027 |
| Au/Ti(0.5)-SBASH(0.75) | 0.101 | 0.033 |
| Au/Ti(0.5)-SBASH(1) | 0.121 | 0.038 |
| Au/Ti(0.5)-SBASH(4) | 0.486 | 0.044 |
| Au/Ti(0.5)-SBASH(8) | 0.900 | 0.042 |
| Au/Ti(0)-SBASH(0.5) | 0.062 | 0.028 |
| Au/Ti(1)-SBASH(0.5) | 0.062 | 0.030 |
| Au/Ti(2)-SBASH(0.5) | 0.063 | 0.029 |
| Catalyst | Calcination Condition | Amount of Products/μmol | TON of Au for 1 | ||
|---|---|---|---|---|---|
| Temp./K | Time/h | 1 | 2 + 3 | ||
| Au/Ti(0.5)-SBASH(0.5) | — | — | 349.1 | 0 | 258.6 |
| 573 | 2 | 99.7 | trace | 73.9 | |
| Au/Ti(0.5)-SBASH(1) | — | — | 53.6 | 0 | 29.8 |
| 573 | 2 | 135.1 | trace | 75.1 | |
| 673 | 2 | 114.7 | trace | 63.7 | |
| Au/Ti(0.5)-SBASH(8) | — | — | 0.2 | 0 | 0.1< |
| 573 | 2 | 0.8 | 66.9 | 0.4 | |
| 673 | 2 | 4.7 | 166.1 | 2.3 | |
| 673 | 4 | 8.3 | 110.3 | 4.0 | |
| 673 | 8 | 26.7 | trace | 12.8 | |
| 673 | 12 | 0 | 0 | 0 | |
| 673 * | 2 * | 160.6 | 0 | 77.2 | |
| Catalyst | Amount of Products/μmol | TON of Au for 1 | TON for 4 + 5 (Styrene Oxidation) | |||
|---|---|---|---|---|---|---|
| 1 | 4 | 5 | Based on Ti | Based on Au | ||
| Au/Ti(0)-SBASH(0.5) | 51.2 | 1.2 | 17.3 | 36.6 | — | 13.2 |
| Au/Ti(0.5)-SBASH(0.5) | 106.1 | 0.1 | 30.0 | 78.6 | 9.1 | 22.3 |
| Au/Ti(1)-SBASH(0.5) | 58.3 | 2.5 | 55.4 | 38.9 | 7.6 | 38.6 |
| Au/Ti(2)-SBASH(0.5) | 96.9 | 1.3 | 83.2 | 66.8 | 5.5 | 58.3 |
| Au/Ti(1)-SBASH(0.5) 1 | 0 | 4.8 | 9.1 | — | 1.8 | 9.3 |
| Ti(1)-SBASH(0.5) + Au/Ti(0)-SBASH(0.5) 2 | 39.2 | 0.4 | 30.2 | 28.0 | 4.0 | 21.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haketa, T.; Nozawa, T.; Nakazawa, J.; Okamura, M.; Hikichi, S. Oxidation Catalysis of Au Nano-Particles Immobilized on Titanium(IV)- and Alkylthiol-Functionalized SBA-15 Type Mesoporous Silicate Supports. Catalysts 2023, 13, 35. https://doi.org/10.3390/catal13010035
Haketa T, Nozawa T, Nakazawa J, Okamura M, Hikichi S. Oxidation Catalysis of Au Nano-Particles Immobilized on Titanium(IV)- and Alkylthiol-Functionalized SBA-15 Type Mesoporous Silicate Supports. Catalysts. 2023; 13(1):35. https://doi.org/10.3390/catal13010035
Chicago/Turabian StyleHaketa, Tomoki, Toshiaki Nozawa, Jun Nakazawa, Masaya Okamura, and Shiro Hikichi. 2023. "Oxidation Catalysis of Au Nano-Particles Immobilized on Titanium(IV)- and Alkylthiol-Functionalized SBA-15 Type Mesoporous Silicate Supports" Catalysts 13, no. 1: 35. https://doi.org/10.3390/catal13010035









