Synthesis of Silicotungstic Acid/Ni-Zr-O Composite Nanoparticle by Using Bimetallic Ni-Zr MOF for Fatty Acid Esterification
Abstract
:1. Introduction
2. Results and Discussion
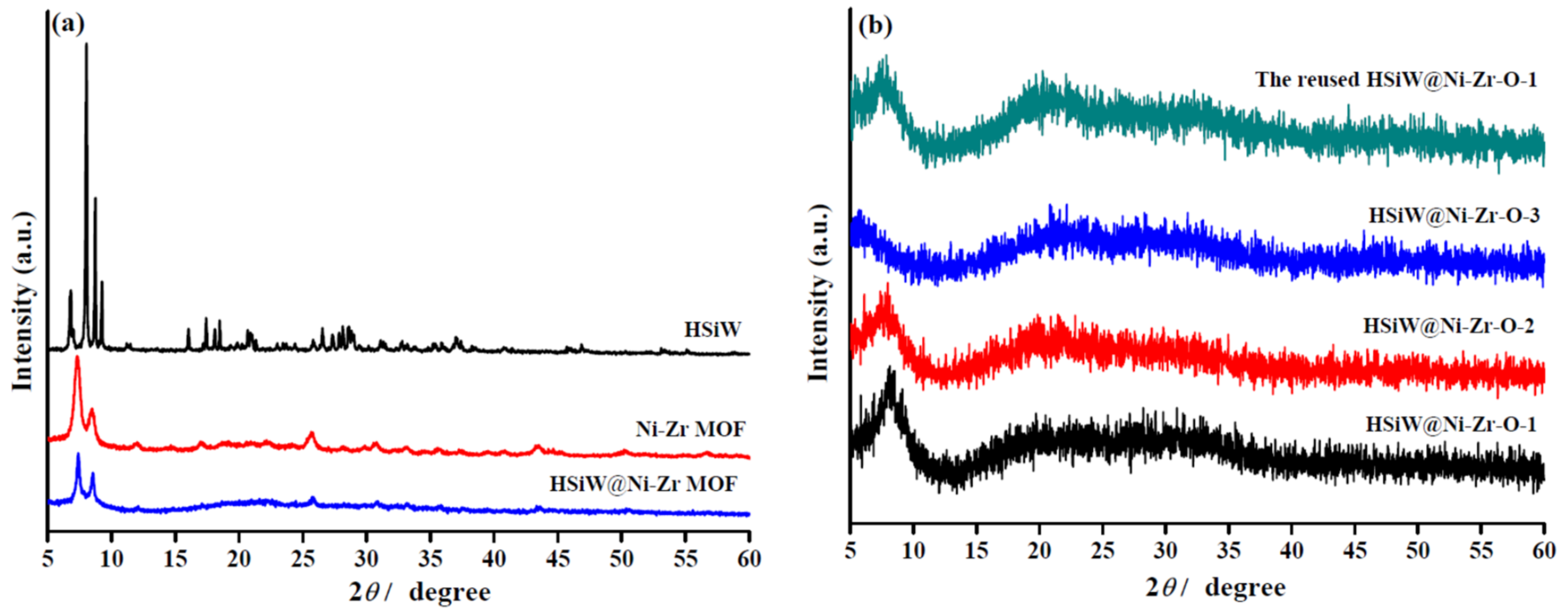
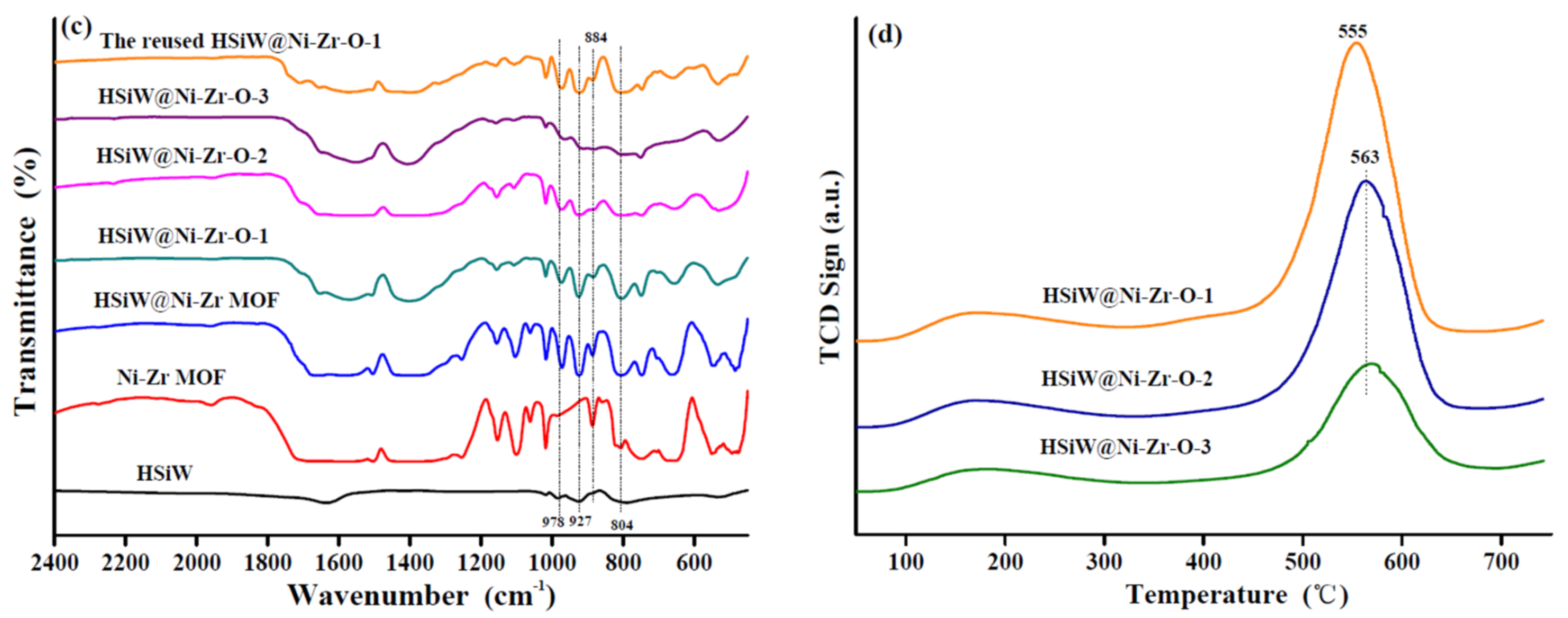
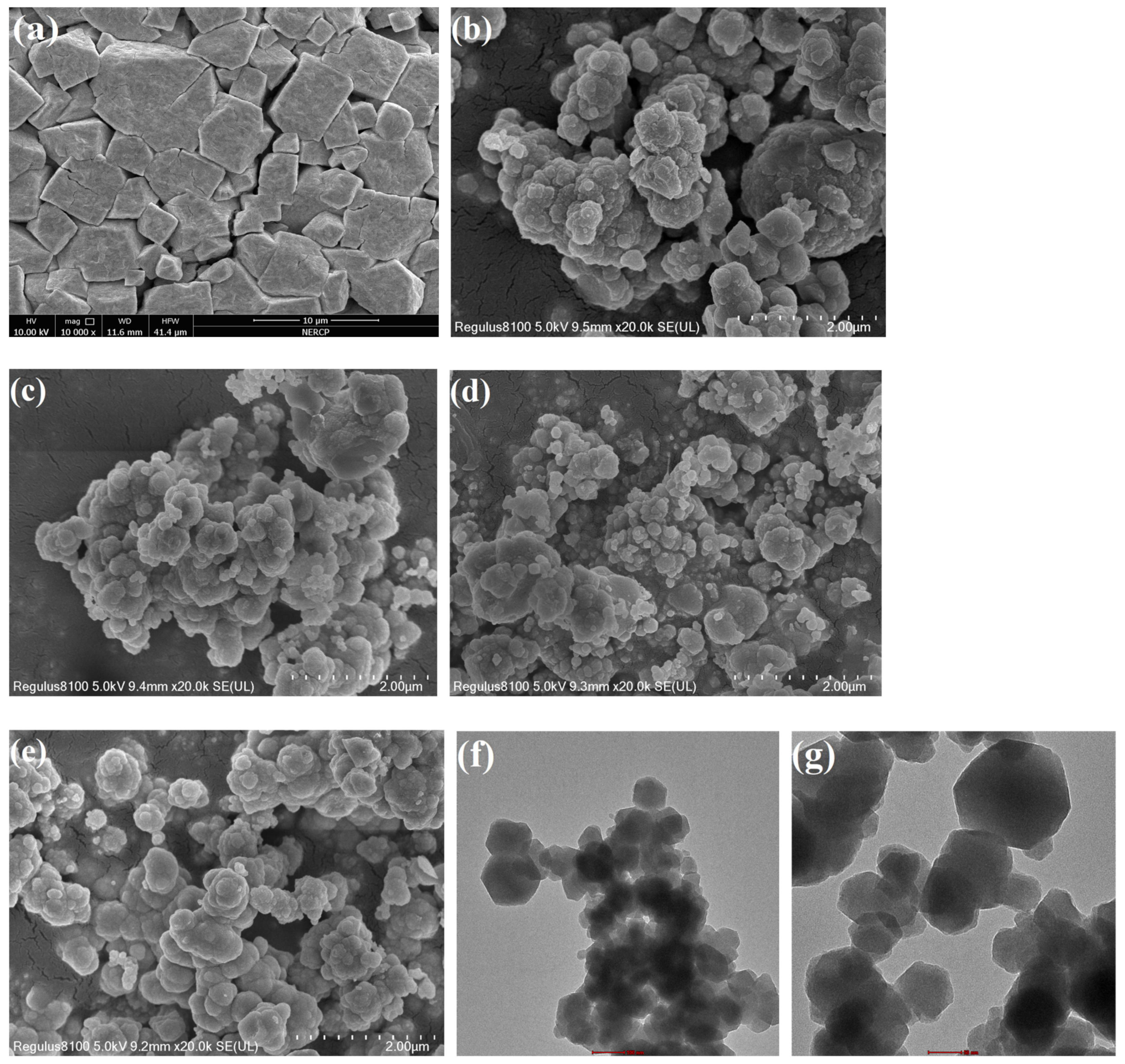
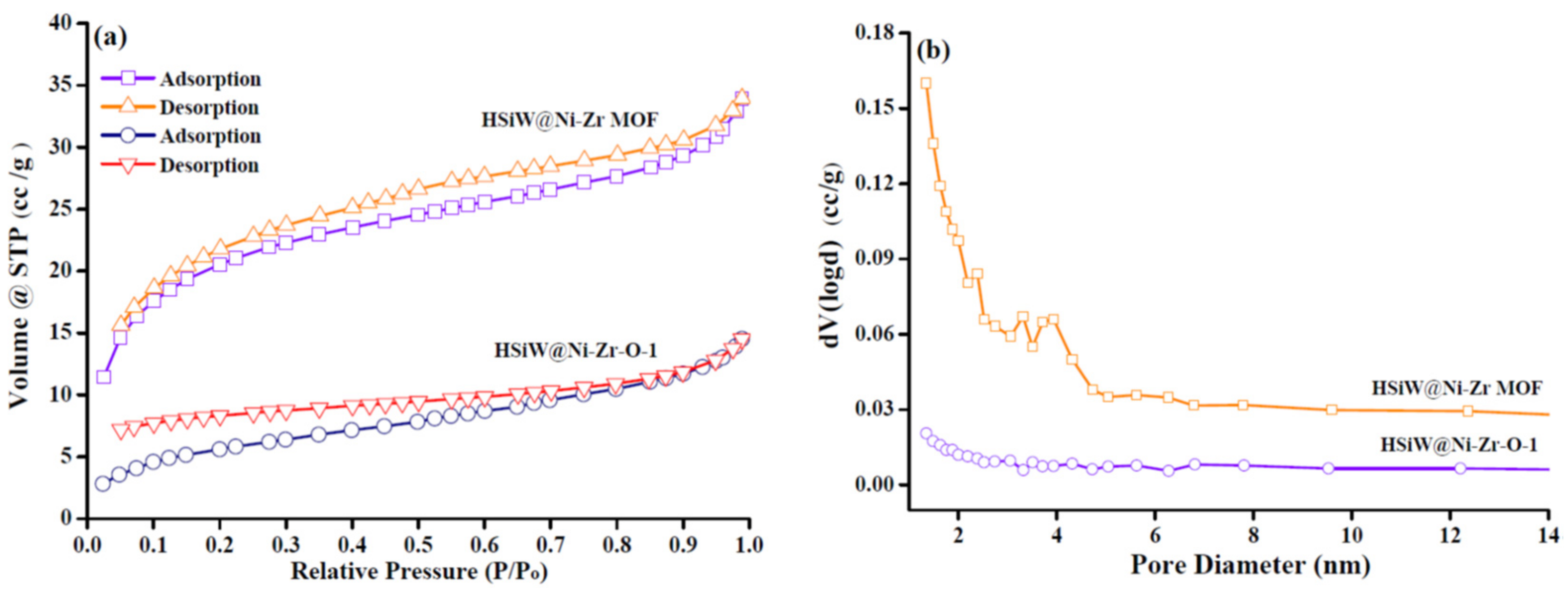
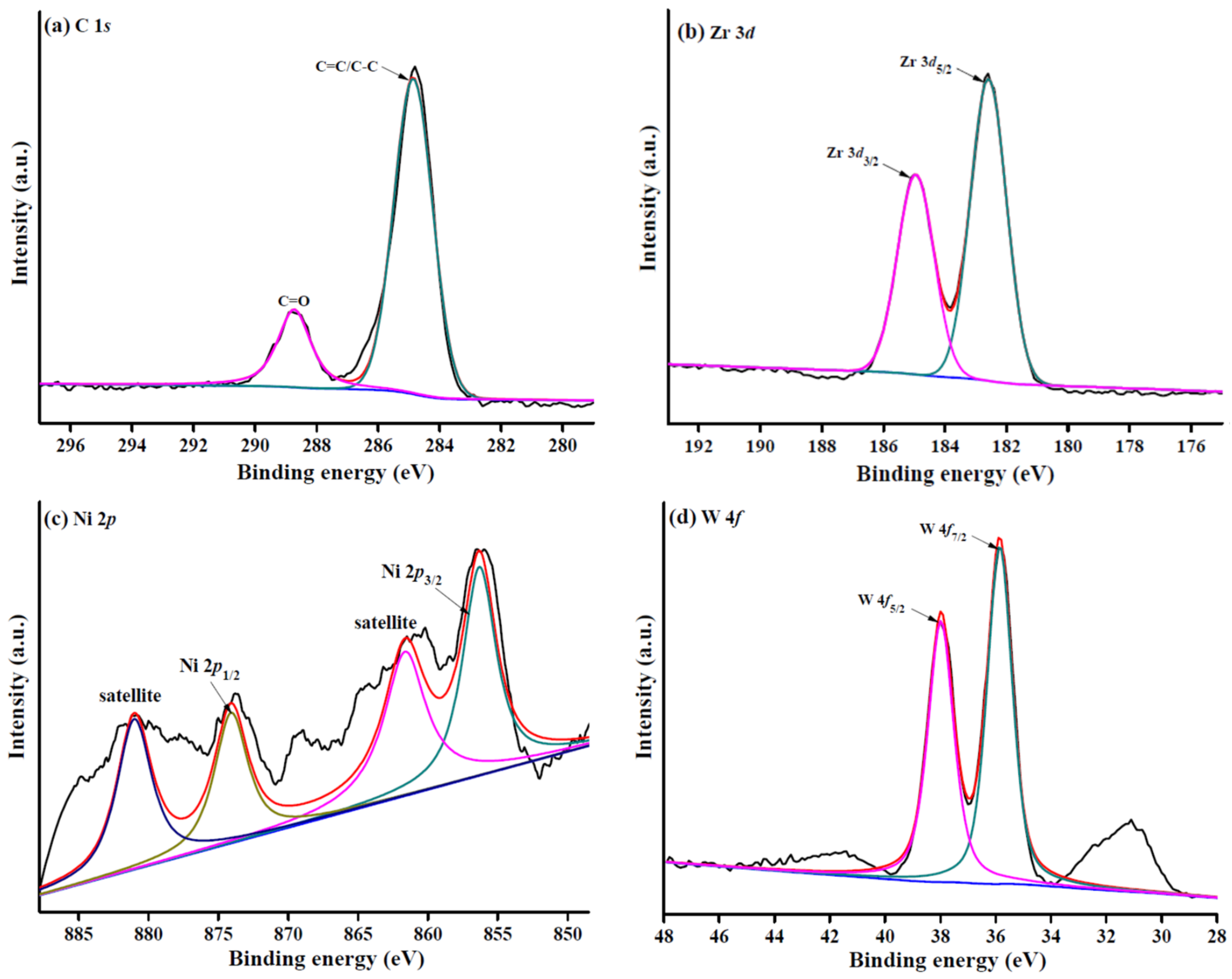
2.1. Catalyst Characterization
2.2. Exploration of the Optimal Esterification Conditions
2.3. Optimum Esterification Conditions of OA and Methanol for HSiW@Ni-Zr-O-1 Catalyst
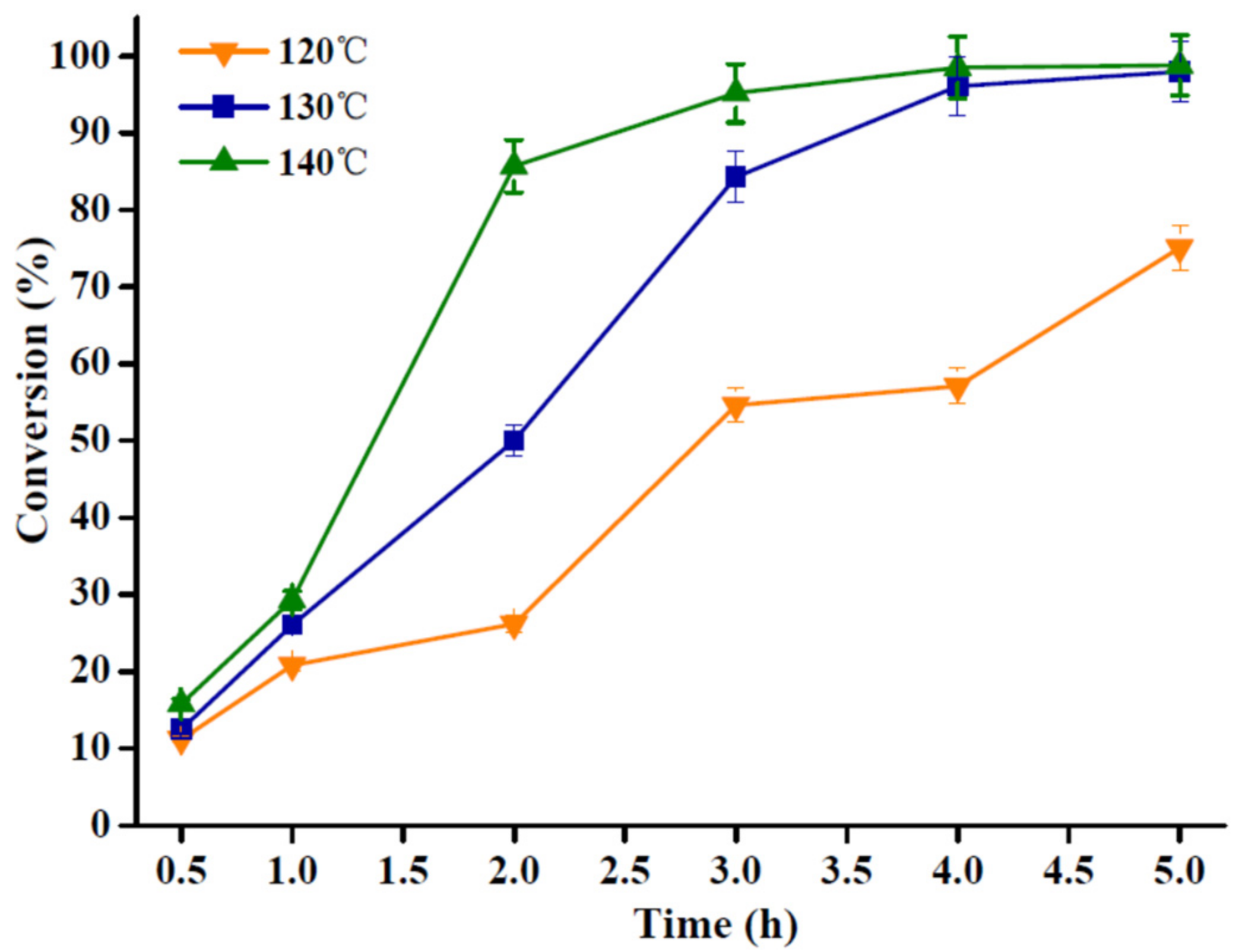
2.3.1. Influence of Reaction Temperature and Time
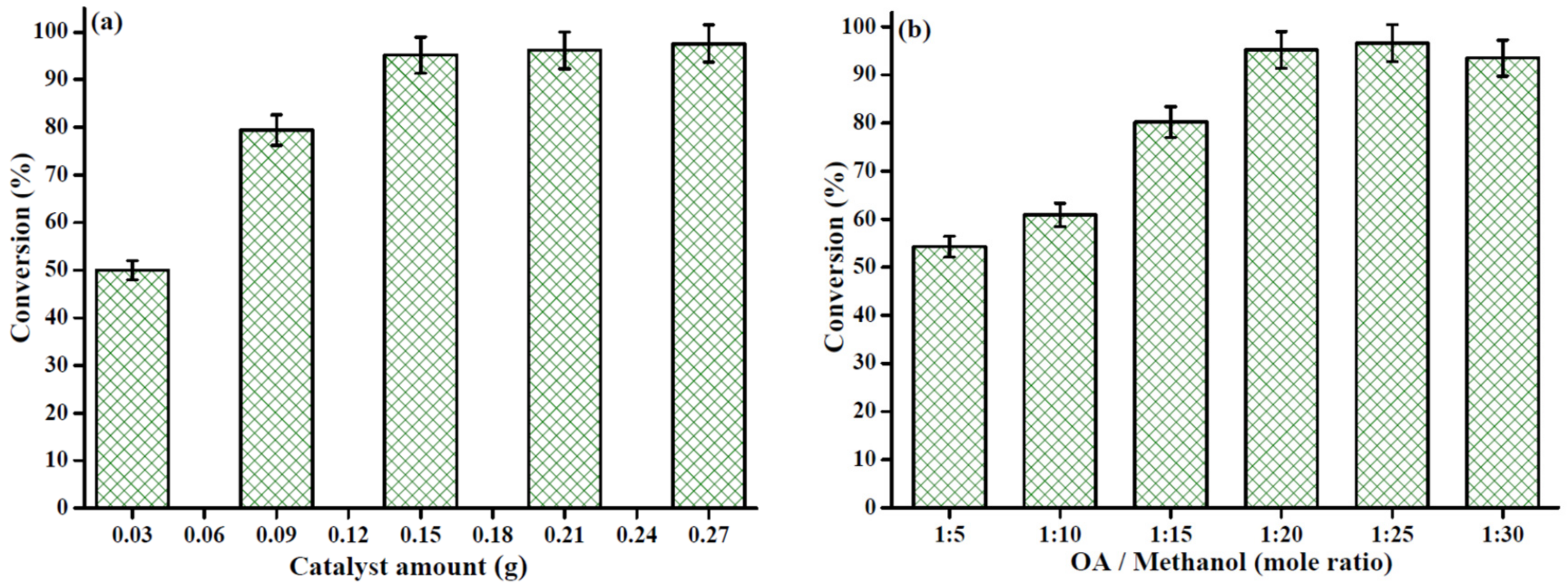
2.3.2. Influence of Catalyst Amount and Substrate Molar Ratio
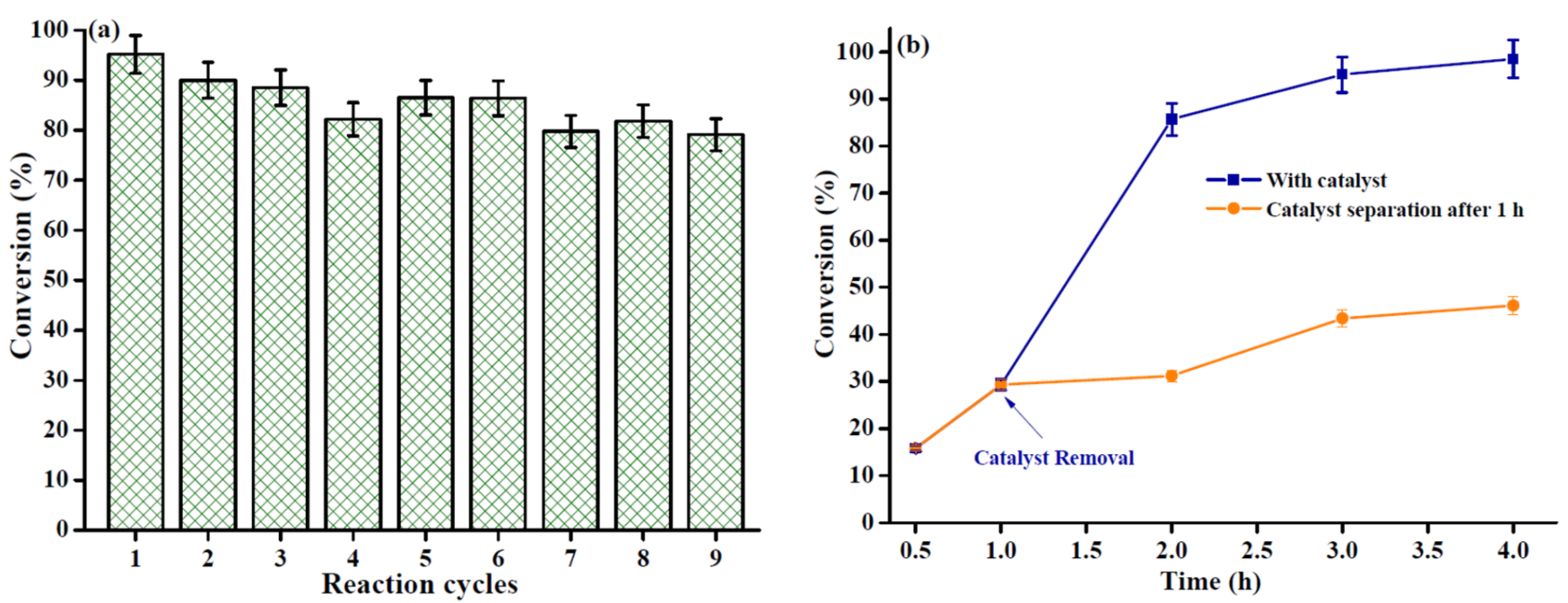
2.4. Reusability and Thermal Filtration Test of the HSiW@Ni-Zr-O-1 Catalyst
2.5. Comparative Study on the Catalytic Activities
3. Materials and Methods
3.1. Chemicals Used
3.2. Catalyst Preparation
3.3. Characterization Techniques
3.4. Catalytic Activity Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meng, Y.; Jian, Y.M.; Li, J.; Wu, H.G.; Zhang, H.; Saravanamurugan, S.; Yang, S.; Li, H. Surface-active site engineering: Synergy of photo- and supermolecular catalysis in hydrogen transfer enables biomass upgrading and H2 evolution. Chem. Eng. J. 2023, 452, 139477. [Google Scholar] [CrossRef]
- Mukhtar, A.; Saqib, S.; Lin, H.; Ul Hassan Shah, M.; Ullah, S.; Younas, M.; Rezakazemi, M.; Ibrahim, M.; Mahmood, A.; Asif, S.; et al. Current status and challenges in the heterogeneous catalysis for biodiesel production. Renew. Sustain. Energy Rev. 2022, 157, 112012. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Lei, D.D.; Luo, Q.Q.; Deng, T.L.; Cheng, J.S.; Zhang, Y.T.; Ma, P.H. Immobilizing Ni (II)-exchanged heteropolyacids on silica as catalysts for acid-catalyzed esterification reactions. Period. Polytech. Chem. Eng. 2021, 65, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.C.; Zhang, S.D.; Li, Z.; Zhang, H.; Li, H.; Yang, S. Green synthesis of heterogeneous polymeric bio-based acid decorated with hydrophobic regulator for efficient catalytic production of biodiesel at low temperatures. Fuel 2022, 329, 125467. [Google Scholar] [CrossRef]
- Welter, R.A.; Santana, H.; de la Torre, L.G.; Robertson, M.; Taranto, O.P.; Oelgemöller, M. Methyl oleate synthesis by TiO2 photocatalytic esterification of oleic acid: Optimisation by response surface quadratic methodology, reaction kinetics and thermodynamics. ChemPhotoChem 2022, 6, e202200007. [Google Scholar] [CrossRef]
- Cao, M.H.; Peng, L.B.; Xie, Q.L.; Xing, K.N.; Lu, M.Z.; Ji, J.B. Sulfonated Sargassum horneri carbon as solid acid catalyst to produce biodiesel via esterification. Bioresour. Technol. 2021, 324, 124614. [Google Scholar] [CrossRef]
- Pan, H.; Xia, Q.N.; Li, H.; Wang, Y.G.; Shen, Z.F.; Wang, Y.Q.; Li, L.F.; Li, X.; Xu, H.Y.; Zhou, Z.M.; et al. Direct production of biodiesel from crude Euphorbia lathyris L. oil catalyzed by multifunctional mesoporous composite materials. Fuel 2022, 309, 122172. [Google Scholar] [CrossRef]
- Bahador, F.; Foroutan, R.; Nourafkan, E.; Peighambardoust, J.S.; Esmaeili, H. Enhancement of biodiesel production from chicken fat using MgO and MgO@Na2O nanocatalysts. Chem. Eng. Technol. 2021, 44, 77–84. [Google Scholar] [CrossRef]
- Farrokheh, A.; Tahvildari, K.; Nozari, M. Biodiesel production from the Chlorella vulgaris and Spirulina platensis microalgae by electrolysis using CaO/KOH-Fe3O4 and KF/KOH-Fe3O4 as magnetic nanocatalysts. Biomass Convers. Bior. 2022, 12, 403–417. [Google Scholar] [CrossRef]
- Gandhi, S.S.; Gogate, P.R. Process intensification of fatty acid ester production using esterification followed by transesterification of high acid value mahua (lluppai ennai) oil: Comparison of the ultrasonic reactors. Fuel 2021, 294, 120560. [Google Scholar] [CrossRef]
- Moradi, P.; Saidi, M. Biodiesel production from Chlorella Vulgaris microalgal-derived oil via electrochemical and thermal processes. Fuel Process. Technol. 2022, 228, 107158. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Luo, Q.Z.; Wu, Y.P.; Yu, R.F.; Cheng, J.S.; Zhang, Y.T. Construction of a Keggin heteropolyacid/Ni-MOF catalyst for esterification of fatty acids. RSC Adv. 2021, 11, 33416–33424. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, B.; Solomon, P.R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production—A review. Clean Energy 2019, 3, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Núñez, F.; Chen, L.; Wang, J.A.; Flores, S.O.; Salmones, J.; Arellano, U.; Noreña, L.E.; Tzompantzi, F. Bifunctional Co3O4/ZSM-5 mesoporous catalysts for biodiesel production via esterification of unsaturated omega-9 oleic acid. Catalysts 2022, 12, 900. [Google Scholar] [CrossRef]
- Woo, J.; Joshi, R.; Park, Y.K.; Jeon, J.K. Biodiesel production from jatropha seeds with bead-type heterogeneous catalyst. Korean J. Chem. Eng. 2021, 38, 763–770. [Google Scholar] [CrossRef]
- Cheng, J.; Mao, Y.X.; Guo, H.; Qian, L.; Shao, Y.; Yang, W.J.; Park, J.Y. Synergistic and efficient catalysis over Brønsted acidic ionic liquid [BSO3HMIm][HSO4]-modified metal-organic framework (IRMOF-3) for microalgal biodiesel production. Fuel 2022, 322, 124217. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Ling, D.; Lei, D.D.; Wan, J.L.; Liu, X.F.; Zhang, Y.T.; Ma, P.H. Green and facile synthesis of metal-organic framework Cu-BTC supported Sn (II)-substituted Keggin heteropoly composites as an esterification nanocatalyst for biodiesel production. Front. Chem. 2020, 8, 129. [Google Scholar] [CrossRef]
- Ul Islam, M.G.; Jan, M.T.; Farooq, M.; Naeem, A.; Khan, I.W.; Khattak, H.U. Biodiesel production from wild olive oil using TPA decorated Cr-Al acid heterogeneous catalyst. Chem. Eng. Res. Des. 2022, 329, 125467. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, W.L.; Guo, L.H. Molybdenum and zirconium oxides supported on KIT-6 silica: A recyclable composite catalyst for oneepot biodiesel production from simulated low-quality oils. Renew. Energy 2022, 187, 907–922. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.M.; Shao, Z.P.; Jiang, S.P. Metal-organic frameworks derived porous carbon, metal oxides and metal sulfides-based compounds for supercapacitors application. Energy Storage Mater. 2020, 26, 1–22. [Google Scholar] [CrossRef]
- Li, W.W.; Gao, G.Q.; Wang, L.L.; Xu, H.M.; Huang, W.J.; Yan, N.Q.; Qu, Z. Dual confinement strategy based on metal-organic frameworks to synthesize MnOx@ZrO2 catalysts for toluene catalytic oxidation. Fuel 2022, 320, 123983. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, S.; Tariq, S.; Luque, R.; Verpoort, F. Self-sacrifice MOFs for heterogeneous catalysis: Synthesis mechanisms and future perspectives. Mater. Today 2022, 55, 137–169. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Wang, J.L.; Zhang, S.Y.; Ma, J.; Cheng, J.S.; Zhang, Y.T. Zr-based metal-organic frameworks for green biodiesel synthesis: A minireview. Bioengineering 2022, 9, 700. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Deng, Z.; Wan, X.Y.; Li, Z.Y.; Ma, X.; Hussain, S.; Ye, Z.Z.; Peng, X.S. Keggin-type polyoxometalates molecularly loaded in Zr-ferrocene metal organic framework nanosheets for solar-driven CO2 cycloaddition. Appl. Catal. B Environ. 2021, 296, 120329. [Google Scholar] [CrossRef]
- Wei, R.P.; Qu, X.M.; Xiao, Y.; Fan, J.D.; Geng, G.L.; Gao, L.J.; Xiao, G.M. Hydrogenolysis of glycerol to propanediols over silicotungstic acid catalysts intercalated with CuZnFe hydrotalcite-like compounds. Catal. Today 2021, 368, 224–231. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.Q.; Han, Y.M.; Zhang, L.L.; Wang, Q.Y.; Wang, G.Y.; Zhang, X.M. UiO-66(Zr/Ti) for catalytic PET polycondensation. Mol. Catal. 2022, 532, 112741. [Google Scholar] [CrossRef]
- Ramasubbu, V.; Kumar, P.R.; Mothi, E.M.; Karuppasamy, K.; Kim, H.S.; Maiyalagan, T.; Shajan, X.S. Highly interconnected porous TiO2-Ni-MOF composite aerogel photoanodes for high power conversion efficiency in quasi-solid dye-sensitized solar cells. Appl. Surf. Sci. 2019, 496, 143646. [Google Scholar] [CrossRef]
- Fan, M.M.; Liu, H.; Zhang, P.B. Ionic liquid on the acidic organic-inorganic hybrid mesoporous material with good acid-water resistance for biodiesel production. Fuel 2018, 215, 541–550. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yang, B.B.; Tian, Y.Y.; Yang, X.J.; Yu, R.F.; Wang, J.L.; Deng, T.L.; Zhang, Y.T. Fabrication of silicotungstic acid immobilized on Ce-based MOF and embedded in Zr-based MOF matrix for green fatty acid esterification. Green Process. Syn. 2022, 11, 184–194. [Google Scholar] [CrossRef]
- Sun, H.; Yu, X.L.; Ma, X.Y.; Yang, X.Q.; Lin, M.Y.; Ge, M.F. MnOx-CeO2 catalyst derived from metal-organic frameworks for toluene oxidation. Catal. Today 2020, 355, 580–586. [Google Scholar] [CrossRef]
- Jeona, Y.; Chia, W.S.; Hwang, J.; Kim, D.H.; Kim, J.H.; Shul, Y.G. Core-shell nanostructured heteropoly acid-functionalized metal-organic frameworks: Bifunctional heterogeneous catalyst for efficient biodiesel production. Appl. Catal. B Environ. 2019, 242, 51–59. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, H.; Li, A.; Li, Z.; Liu, S.; Zhang, Q.; Gong, Y.; Zheng, L.; Zhu, Y.; Chen, C.; et al. Constructing NiCo/Fe3O4 heteroparticles within MOF-74 for efficient oxygen evolution reactions. J. Am. Chem. Soc. 2018, 140, 15336–15341. [Google Scholar] [CrossRef] [PubMed]
- Yassin, J.M.; Taddesse, A.M.; Sánchez-Sánchez, M. Sustainable synthesis of semicrystalline Zr-BDC MOF and heterostructural Ag3PO4/Zr-BDC/g-C3N4 composite for photocatalytic dye degradation. Catal. Today 2022, 390–391, 162–175. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Z.; Liu, H.; Liu, H.; Wang, Y. Cd0.2Zn0.8S@UiO-66-NH2 nanocomposites as efficient and stable visible-light-driven photocatalyst for H2 evolution and CO2 reduction. Appl. Catal. B Environ. 2017, 200, 448–457. [Google Scholar] [CrossRef]
- Wang, M.H.; Wang, C.B.; Zhu, L.; Rong, F.L.; He, L.H.; Lou, Y.F.; Zhang, Z.H. Bimetallic NiCo metal-organic frameworks for efficient non-Pt methanol electrocatalytic oxidation. Appl. Catal. A Gen. 2021, 619, 118159. [Google Scholar] [CrossRef]
- Ji, H.Y.; Sun, J.; Wu, P.W.; Wu, Y.C.; He, J.; Cha, Y.H.; Zhu, W.S.; Li, H.M. Silicotungstic acid immobilized on lamellar hexagonal boron nitride for oxidative desulfurization of fuel components. Fuel 2018, 213, 12–21. [Google Scholar] [CrossRef]
- Zong, M.Y.; Zhao, Y.T.; Fan, C.Z.; Wang, D.H. Synergistic effect between Zr-MOF and phosphotungstic acid for oxidative desulfurization. China Pet. Process. Petrochem. Technol. 2020, 22, 56–62. [Google Scholar]
- Zhang, B.; Gao, M.; Geng, J.; Cheng, Y.; Wang, X.; Wu, C.; Wang, Q.; Liu, S.; Cheung, S.M. Catalytic performance and deactivation mechanism of a one-step sulfonated carbon-based solid-acid catalyst in an esterification reaction. Renew. Energy 2021, 164, 824–832. [Google Scholar] [CrossRef]
- Li, H.; Wang, T.Y.; Wang, Y.Y.; Liu, F.S.; Li, Y.; Wang, Y.P.; Guo, M.; Li, G.N.; Ma, X.L. Catalytic activity enhancement of sulfated metal oxide by doping Co on MIL-100(Fe) for esterification. Fuel 2023, 334, 126631. [Google Scholar] [CrossRef]
- AlKahlaway, A.A.; Betiha, M.A.; Aman, D.; Rabie, A.M. Facial synthesis of ferric molybdate (Fe2(MoO4)3) nanoparticle and its efficiency for biodiesel synthesis via oleic acid esterification. Environ. Technol. Innov. 2021, 22, 101386. [Google Scholar] [CrossRef]
- Mohamed, M.M.; El-Faramawy, H. An innovative nanocatalyst α-Fe2O3/AlOOH processed from gibbsite rubbish ore for efficient biodiesel production via utilizing cottonseed waste oil. Fuel 2021, 297, 120741. [Google Scholar] [CrossRef]
- Guo, M.L.; Jiang, W.Q.; Chen, C.; Qu, S.K.; Lu, J.; Yi, W.M.; Ding, J.C. Process optimization of biodiesel production from waste cooking oil by esterification of free fatty acids using La3+/ZnO-TiO2 photocatalyst. Energy Convers. Manag. 2021, 229, 113745. [Google Scholar] [CrossRef]
- Ibrahim, S.M. Preparation, characterization and application of novel surface-modified ZrSnO4 as Sn-based TMOs catalysts for the stearic acid esterification with methanol to biodiesel. Renew. Energy 2021, 173, 151–163. [Google Scholar] [CrossRef]
- Embong, N.H.; Maniam, G.P.; Mohd, M.H.; Lee, K.T.; Huisingh, D. Utilization of palm fatty acid distillate in methyl esters preparation using SO42−/TiO2-SiO2 as a solid acid catalyst. J. Clean. Product. 2016, 116, 244–248. [Google Scholar] [CrossRef] [Green Version]
- Amouhadi, E.; Fazaeli, R.; Aliyan, H. Biodiesel production via esterification of oleic acid catalyzed by MnO2@Mn(btc) as a novel and heterogeneous catalyst. J. Chin. Chem. Soc. 2019, 66, 608–613. [Google Scholar] [CrossRef]
- Mahmoud, H.R.; El-Molla, S.A.; Ibrahim, M.M. Biodiesel production via stearic acid esterification over mesoporous ZrO2/SiO2 catalysts synthesized by surfactant-assisted sol-gel autocombustion route. Renew. Energy 2020, 160, 42–51. [Google Scholar] [CrossRef]
- Mohebbi, S.; Rostamizadeh, M.; Kahforoushan, D. Effect of molybdenum promoter on performance of high silica MoO3/B-ZSM-5 nanocatalyst in biodiesel production. Fuel 2020, 266, 117063. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Rashid, U.; Choong, T.S.Y.; Nehdi, I.A. Synthesis of nanomagnetic sulphonated impregnated Ni/Mn/Na2SiO3 as catalyst for esterification of palm fatty acid distillate. RSC Adv. 2020, 10, 6098–6108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gecgel, C.; Turabik, M. Synthesis and sulfonation of an aluminum-based metal-organic framework with microwave method and using for the esterification of oleic acid. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4033–4049. [Google Scholar] [CrossRef]
- Silva, J.L., Jr.; Nobre, F.X.; de Freitas, F.A.; de Carvalho, T.A.F.; de Barros, S.S.; Nascimento, M.C.; Manzato, L.; Matos, J.M.E.; Brito, W.R.; Leyet, Y.; et al. Copper molybdate synthesized by sonochemistry route at room temperature as an efficient solid catalyst for esterification of oleic acid. Ultrason. Sonochem. 2021, 73, 105541. [Google Scholar] [CrossRef]

| Catalyst Type | Feedstock | Reaction Conditions | Activity (%) | Ref. | |||
|---|---|---|---|---|---|---|---|
| Temp. (°C) | Alcohol to Fatty Acid Molar Ratio | Catalyst Loading (wt%) | Time (h) | ||||
| SO42−/TiO2-SiO2 | Palm fatty acid distillate | 150 | Methanol (1:5.85) | 2.97 | 3.12 | 93.3 | [44] |
| MnO2@Mn(btc) | Oleic acid | 100 | Ethanol (1:12) | 3 | 12 | 98 | [45] |
| ZrO2/SiO2 | Stearic acid | 120 | Ethanol (1:120) | 10 | 3 | 69.2 | [46] |
| MoO3/B-ZSM-5 | Free fatty acid | 160 | Methanol (1:20) | 3 | 6 | 98 | [47] |
| Ni/Mn/Na2SiO3 | Palm fatty acid distillate | 120 | Methanol (1:16) | 2 | 4 | 96 | [48] |
| SO3-MIL-53(Al) | Oleic acid | 150 | Methanol (1:60) | 3 | 2 | 97.2 | [49] |
| Cu3(MoO4)2(OH)2 | Oleic acid | 140 | Methanol (1:5) | 5 | 5 | 98.38 | [50] |
| HSiW@Ni-Zr-O-1 | Oleic acid | 140 | Methanol (1:20) | 5 | 3 | 95.2 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Hu, M.; Wang, J.; Lei, Y.; Wu, Y.; Liu, Q.; Zhao, Y.; Zhang, Y. Synthesis of Silicotungstic Acid/Ni-Zr-O Composite Nanoparticle by Using Bimetallic Ni-Zr MOF for Fatty Acid Esterification. Catalysts 2023, 13, 40. https://doi.org/10.3390/catal13010040
Zhang Q, Hu M, Wang J, Lei Y, Wu Y, Liu Q, Zhao Y, Zhang Y. Synthesis of Silicotungstic Acid/Ni-Zr-O Composite Nanoparticle by Using Bimetallic Ni-Zr MOF for Fatty Acid Esterification. Catalysts. 2023; 13(1):40. https://doi.org/10.3390/catal13010040
Chicago/Turabian StyleZhang, Qiuyun, Mengmeng Hu, Jialu Wang, Yanting Lei, Yaping Wu, Qing Liu, Yongting Zhao, and Yutao Zhang. 2023. "Synthesis of Silicotungstic Acid/Ni-Zr-O Composite Nanoparticle by Using Bimetallic Ni-Zr MOF for Fatty Acid Esterification" Catalysts 13, no. 1: 40. https://doi.org/10.3390/catal13010040





