Promoting Bifunctional Oxygen Catalyst Activity of Double-Perovskite-Type Cubic Nanocrystallites for Aqueous and Quasi-Solid-State Rechargeable Zinc-Air Batteries
Abstract
:1. Introduction
2. Results and Discussion
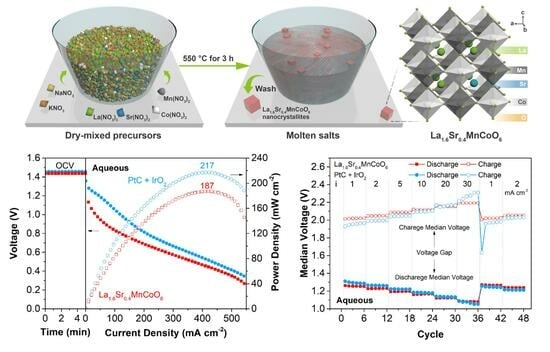
2.1. Synthesis and Characterization of the Perovskite-Type Oxide Nanocrystallites
2.2. Bifunctional Catalytic Activity for ORR and OER
2.3. Performance of the Zn-Air Batteries
2.4. Integration of a Small Amount of Pt/C for Optimization of Oxygen Catalysis
3. Experimental Section
3.1. Synthesis of Perovskite-Type Oxide Nanocrystallites
3.2. Material Characterizations
3.3. Evaluation of Oxygen Catalytic Activity
3.4. Evaluation of Aqueous Zn-Air Batteries
3.5. Evaluation of Coin-Type Zn-Air Batteries with Gel−Polymer Electrolyte
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Lu, J. Metal Air Batteries: Will They Be the Future Electrochemical Energy Storage Device of Choice? ACS Energy Lett. 2017, 2, 1370–1377. [Google Scholar] [CrossRef]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically Rechargeable Zinc-Air Batteries: Progress, Challenges, and Perspectives. Adv. Mater. 2017, 29, 1604685. [Google Scholar] [CrossRef] [PubMed]
- Arafat, Y.; Azhar, M.R.; Zhong, Y.; Abid, H.R.; Tadé, M.O.; Shao, Z. Advances in Zeolite Imidazolate Frameworks (ZIFs) Derived Bifunctional Oxygen Electrocatalysts and Their Application in Zinc–Air Batteries. Adv. Energy Mater. 2021, 11, 2100514. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, T.; Ali, M.; Meng, Y.; Jin, Y.; Cui, Y.; Chen, W. Rechargeable Batteries for Grid Scale Energy Storage. Chem. Rev. 2022, 122, 16610–16751. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Lai, L.; Lin, J.; Shen, Z. Recent Advances in Air Electrodes for Zn-Air Batteries: Electrocatalysis and Structural Design. Mater. Horiz. 2017, 4, 945–976. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, X.; Wang, W.; Shao, Z. Recent Advances in Metal-Organic Framework Derivatives as Oxygen Catalysts for Zinc-Air Batteries. Batter. Supercaps 2019, 2, 272–289. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Tang, C.; Wang, J.; Zhang, Q.; Wang, Y.; Zhang, J. Atomic Modulation and Structure Design of Carbons for Bifunctional Electrocatalysis in Metal-Air Batteries. Adv. Mater. 2019, 31, 1803800. [Google Scholar] [CrossRef]
- Han, C.; Li, W.; Liu, H.-K.; Dou, S.; Wang, J. Design Strategies for Developing Non-Precious Metal Based Bi-Functional Catalysts for Alkaline Electrolyte Based Zinc–Air Batteries. Mater. Horiz. 2019, 6, 1812–1827. [Google Scholar] [CrossRef]
- Hong, W.T.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Shao-Horn, Y. Toward the Rational Design of Non-Precious Transition Metal Oxides for Oxygen Electrocatalysis. Energy Environ. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef]
- Cao, R.; Lee, J.-S.; Liu, M.; Cho, J. Recent Progress in Non-Precious Catalysts for Metal-Air Batteries. Adv. Energy Mater. 2012, 2, 816–829. [Google Scholar] [CrossRef]
- Pan, J.; Tian, X.L.; Zaman, S.; Dong, Z.; Liu, H.; Park, H.S.; Xia, B.Y. Recent Progress on Transition Metal Oxides as Bifunctional Catalysts for Lithium-Air and Zinc-Air Batteries. Batter. Supercaps 2019, 2, 336–347. [Google Scholar] [CrossRef]
- Du, G.; Liu, X.; Zong, Y.; Hor, T.S.A.; Yu, A.; Liu, Z. Co3O4 Nanoparticle-Modified MnO2 Nanotube Bifunctional Oxygen Cathode Catalysts for Rechargeable Zinc-Air Batteries. Nanoscale 2013, 5, 4657–4661. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, B. A Bifunctional Electrocatalyst α-MnO2-LaNiO3/Carbon Nanotube Composite for Rechargeable Zinc-Air Batteries. RSC Adv. 2014, 4, 46084–46092. [Google Scholar] [CrossRef]
- Xu, N.; Liu, Y.; Zhang, X.; Li, X.; Li, A.; Qiao, J.; Zhang, J. Self-Assembly Formation of Bi-Functional Co3O4/MnO2-CNTs Hybrid Catalysts for Achieving Both High Energy/Power Density and Cyclic Ability of Rechargeable Zinc-Air Battery. Sci. Rep. 2016, 6, 33590. [Google Scholar] [CrossRef]
- Luo, Z.; Irtem, E.; Ibanez, M.; Nafria, R.; Marti-Sanchez, S.; Genc, A.; de la Mata, M.; Liu, Y.; Cadavid, D.; Llorca, J.; et al. Mn3O4@CoMn2O4−CoxOy Nanoparticles: Partial Cation Exchange Synthesis and Electrocatalytic Properties toward the Oxygen Reduction and Evolution Reactions. ACS Appl. Mater. Interfaces 2016, 8, 17435–17444. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Shao, Z. Double Perovskites in Catalysis, Electrocatalysis, and Photo(electro)catalysis. Trends Chem. 2019, 1, 410–424. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, A.; Higgins, D.; Li, H.; Wang, H.; Chen, Z. Highly Active and Durable Core-Corona Structured Bifunctional Catalyst for Rechargeable Metal-Air Battery Application. Nano Lett. 2012, 12, 1946–1952. [Google Scholar] [CrossRef]
- Wang, Q.; Xue, Y.; Sun, S.; Li, S.; Miao, H.; Liu, Z. La0.8Sr0.2Co1−xMnxO3 Perovskites as Efficient Bi-Functional Cathode Catalysts for Rechargeable Zinc-Air Batteries. Electrochim. Acta 2017, 254, 14–24. [Google Scholar] [CrossRef]
- Dai, Y.; Yu, J.; Zhang, Z.; Zhai, S.; Cheng, C.; Zhao, S.; Tan, P.; Shao, Z.; Ni, M. Regulating the Interfacial Electron Density of La0.8Sr0.2Mn0.5Co0.5O3/RuOx for Efficient and Low-Cost Bifunctional Oxygen Electrocatalysts and Rechargeable Zn-Air Batteries. ACS Appl. Mater. Interfaces 2021, 13, 61098–61106. [Google Scholar] [CrossRef]
- Bian, J.; Li, Z.; Li, N.; Sun, C. Oxygen Deficient LaMn0.75Co0.25O3−δ Nanofibers as an Efficient Electrocatalyst for Oxygen Evolution Reaction and Zinc-Air Batteries. Inorg. Chem. 2019, 58, 8208–8214. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Lee, D.U.; Zamani, P.; Seo, M.H.; Nazar, L.F.; Chen, Z. Electrospun Porous Nanorod Perovskite Oxide/Nitrogen-Doped Graphene Composite as a Bi-Functional Catalyst for Metal Air Batteries. Nano Energy 2014, 10, 192–200. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Zhong, Y.; Shi, C.; Guan, D.; Ge, L.; Hu, Z.; Chin, Y.-Y.; Lin, H.-J.; Chen, C.-T.; et al. New Undisputed Evidence and Strategy for Enhanced Lattice-Oxygen Participation of Perovskite Electrocatalyst through Cation Deficiency Manipulation. Adv. Sci. 2022, 9, 2200530. [Google Scholar] [CrossRef]
- Wang, X.; Sunarso, J.; Lu, Q.; Zhou, Z.; Dai, J.; Guan, D.; Zhou, W.; Shao, Z. High-Performance Platinum-Perovskite Composite Bifunctional Oxygen Electrocatalyst for Rechargeable Zn-Air Battery. Adv. Energy Mater. 2020, 10, 1903271. [Google Scholar] [CrossRef]
- Bhardwaj, U.; Sharma, A.; Mathur, A.; Halder, A.; Kushwaha, H.S. Synthesis of a Novel Sr2TiMnO6 Double Perovskite Electrocatalyst for Rechargeable Zinc–Air Batteries. Energy Storage 2022, 4, e293. [Google Scholar] [CrossRef]
- Mondal, S.; Majee, R.; Arif Islam, Q.; Bhattacharyya, S. 2D Heterojunction Between Double Perovskite Oxide Nanosheet and Layered Double Hydroxide to Promote Rechargeable Zinc-Air Battery Performance. ChemElectroChem 2020, 7, 5005–5012. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, Z.; Chen, Z.; Luo, X.; Hou, B.; Gholizadeh, M.; Gao, X.; Fan, X.; Tan, Z. Enhancement on PrBa0.5Sr0.5Co1.5Fe0.5O5 Electrocatalyst Performance in the Application of Zn-Air Battery. Catalysts 2022, 12, 800. [Google Scholar] [CrossRef]
- Bu, Y.; Gwon, O.; Nam, G.; Jang, H.; Kim, S.; Zhong, Q.; Cho, J.; Kim, G. A Highly Efficient and Robust Cation Ordered Perovskite Oxide as a Bifunctional Catalyst for Rechargeable Zinc-Air Batteries. ACS Nano 2017, 11, 11594–11601. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design Principles for Oxygen-Reduction Activity on Perovskite Oxide Catalysts for Fuel Cells and Metal-Air Batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef]
- Zhu, Z.; Song, Q.; Xia, B.; Jiang, L.; Duan, J.; Chen, S. Perovskite Catalysts for Oxygen Evolution and Reduction Reactions in Zinc-Air Batteries. Catalysts 2022, 12, 1490. [Google Scholar] [CrossRef]
- Majee, R.; Islam, Q.A.; Bhattacharyya, S. Surface Charge Modulation of Perovskite Oxides at the Crystalline Junction with Layered Double Hydroxide for a Durable Rechargeable Zinc-Air Battery. ACS Appl. Mater. Interfaces 2019, 11, 35853–35862. [Google Scholar] [CrossRef]
- Peng, S.; Han, X.; Li, L.; Chou, S.; Ji, D.; Huang, H.; Du, Y.; Liu, J.; Ramakrishna, S. Electronic and Defective Engineering of Electrospun CaMnO3 Nanotubes for Enhanced Oxygen Electrocatalysis in Rechargeable Zinc-Air Batteries. Adv. Energy Mater. 2018, 8, 1800612. [Google Scholar] [CrossRef]
- Ishihara, T.; Guo, L.M.; Miyano, T.; Inoishi, Y.; Kaneko, K.; Ida, S. Mesoporous La0.6Ca0.4CoO3 Perovskites with Large Surface Areas as Stable Air Electrodes for Rechargeable Zn-Air Batteries. J. Mater. Chem. A 2018, 6, 7686–7692. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Tao, H.-B.; Chen, Z.; Li, M.; Sun, Y.-F.; Hua, B.; Luo, J.-L. In Situ Grown Cobalt Phosphide (CoP) on Perovskite Nanofibers as an Optimized Trifunctional Electrocatalyst for Zn-Air Batteries and Overall Water Splitting. J. Mater. Chem. A 2019, 7, 26607–26617. [Google Scholar] [CrossRef]
- Kuai, L.; Kan, E.; Cao, W.; Huttula, M.; Ollikkala, S.; Ahopelto, T.; Honkanen, A.-P.; Huotari, S.; Wang, W.; Geng, B. Mesoporous LaMnO3+δ Perovskite from Spray−Pyrolysis with Superior Performance for Oxygen Reduction Reaction and Zn−Air Battery. Nano Energy 2018, 43, 81–90. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, H.; Chen, X.; Fu, X.; Chen, C.; Cheng, F.; Chen, J. Rapid Low-Temperature Synthesis of Perovskite/Carbon Nanocomposites as Superior Electrocatalysts for Oxygen Reduction in Zn-Air Batteries. Nano Res. 2018, 11, 3282–3293. [Google Scholar] [CrossRef]
- Arafat, Y.; Azhar, M.R.; Zhong, Y.; Xu, X.; Tadé, M.O.; Shao, Z. A Porous Nano-Micro-Composite as a High-Performance Bi-Functional Air Electrode with Remarkable Stability for Rechargeable Zinc–Air Batteries. Nano-Micro Lett. 2020, 12, 130. [Google Scholar] [CrossRef]
- Wang, C.; Hou, B.; Wang, X.; Yu, Z.; Luo, D.; Gholizadeh, M.; Fan, X. High-Performance A-Site Deficient Perovskite Electrocatalyst for Rechargeable Zn–Air Battery. Catalysts 2022, 12, 703. [Google Scholar] [CrossRef]
- Grimaud, A.; May, K.J.; Carlton, C.E.; Lee, Y.-L.; Risch, M.; Hong, W.T.; Zhou, J.; Shao-Horn, Y. Double Perovskites as a Family of Highly Active Catalysts for Oxygen Evolution in Alkaline Solution. Nat. Commun. 2013, 4, 2439. [Google Scholar] [CrossRef]
- Kleibeuker, J.E.; Choi, E.-M.; Jones, E.D.; Yu, T.-M.; Sala, B.; MacLaren, B.A.; Kepaptsoglou, D.; Hernandez-Maldonado, D.; Ramasse, Q.M.; Jones, L.; et al. Route to Achieving Perfect B-Site Ordering in Double Perovskite Thin Films. NPG Asia Mater. 2017, 9, e406. [Google Scholar] [CrossRef]
- Xue, P.; Wu, H.; Lu, Y.; Zhu, X. Recent Progress in Molten Salt Synthesis of Low-Dimensional Perovskite Oxide Nanostructures, Structural Characterization, Properties, and Functional Applications: A Review. J. Mater. Sci. Technol. 2018, 34, 914–930. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, D.; Jiao, X. La1−xSrxMnO3 (x = 0, 0.3, 0.5, 0.7) Nanoparticles Nearly Freestanding in Water: Preparation and Magnetic Properties. Chem. Mater. 2006, 18, 6088–6090. [Google Scholar] [CrossRef]
- Li, L.H.; Deng, J.X.; Chen, J.; Xing, X.R. Topochemical Molten Salt Synthesis for Functional Perovskite Compounds. Chem. Sci. 2016, 7, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Androulakis, J.; Katsarakis, N.; Giapintzakis, J.; Vouroutzis, N.; Pavlidou, E.; Chrissafis, K.; Polychroniadis, E.K.; Perdikatsis, V. LaSrMnCoO6: A New Cubic Double Perovskite Oxide. J. Solid State Chem. 2003, 173, 350–354. [Google Scholar] [CrossRef]
- Tang, T.; Jiang, W.-J.; Liu, X.-Z.; Deng, J.; Niu, S.; Wang, B.; Jin, S.-F.; Zhang, Q.; Gu, L.; Hu, J.-S.; et al. Metastable Rock Salt Oxide-Mediated Synthesis of High-Density Dual-Protected M@NC for Long-Life Rechargeable Zinc–Air Batteries with Record Power Density. J. Am. Chem. Soc. 2020, 142, 7116–7127. [Google Scholar] [CrossRef]
- Chen, C.-F.; King, G.; Dickerson, R.M.; Papin, P.A.; Gupta, S.; Kellogg, W.R.; Wu, G. Oxygen-Deficient BaTiO3−x Perovskite as an Efficient Bifunctional Oxygen Electrocatalyst. Nano Energy 2015, 13, 423–432. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.; Fan, B.; Zhang, J.; Zhou, M.; Yang, W.; Hu, X.; Wang, H.; Pan, B.; Xie, Y. Ultrathin Spinel-Structured Nanosheets Rich in Oxygen Deficiencies for Enhanced Electrocatalytic Water Oxidation. Angew. Chem. Int. Ed. 2015, 54, 7399–7404. [Google Scholar] [CrossRef]
- Ji, Q.; Bi, L.; Zhang, J.; Cao, H.; Zhao, X.S. The Role of Oxygen Vacancies of ABO3 Perovskite Oxides in the Oxygen Reduction Reaction. Energy Environ. Sci. 2020, 13, 1408–1428. [Google Scholar] [CrossRef]
- She, S.; Yu, J.; Tang, W.; Zhu, Y.; Chen, Y.; Sunarso, J.; Zhou, W.; Shao, Z. Systematic Study of Oxygen Evolution Activity and Stability on La1−xSrxFeO3−δ Perovskite Electrocatalysts in Alkaline Media. ACS Appl. Mater. Interfaces 2018, 10, 11715–11721. [Google Scholar] [CrossRef]
- Yu, J.; Sunarso, J.; Zhu, Y.; Xu, X.; Ran, R.; Zhou, W.; Shao, Z. Activity and Stability of Ruddlesden–Popper-Type Lan+1NinO3n+1 (n = 1, 2, 3, and ∞) Electrocatalysts for Oxygen Reduction and Evolution Reactions in Alkaline Media. Chem. Eur. J. Chem. 2016, 22, 2719–2727. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Zhong, Y.; Bu, Y.; Chen, X.; Zhong, Q.; Liu, M.; Shao, Z. A Perovskite Nanorod as Bifunctional Electrocatalyst for Overall Water Splitting. Adv. Energy Mater. 2017, 7, 1602122. [Google Scholar] [CrossRef]
- Merino, N.A.; Barbero, B.P.; Eloy, P.; Cadús, L.E. La1−xCaxCoO3 Perovskite-Type Oxides: Identification of the Surface Oxygen Species by XPS. Appl. Surf. Sci. 2006, 253, 1489–1493. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, X.; Liu, P.; Ran, R.; Jiang, S.P.; Wu, H.; Shao, Z. A Function-Separated Design of Electrode for Realizing High-Performance Hybrid Zinc Battery. Adv. Energy Mater. 2020, 10, 2002992. [Google Scholar] [CrossRef]
- Kim, H.-I.; Kim, E.-J.; Kim, S.-J.; Shin, H.-C. Influence of ZnO Precipitation on the Cycling Stability of Rechargeable Zn–Air Batteries. J. Appl. Electrochem. 2015, 45, 335–342. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, S.T.; Cao, R.; Choi, N.-S.; Liu, M.; Lee, K.T.; Cho, J. Metal-Air Batteries with High Energy Density: Li-Air versus Zn-Air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Miao, H.; Wang, Z.; Wang, Q.; Sun, S.; Xue, Y.; Wang, F.; Zhao, J.; Liu, Z.; Yuan, J. A New Family of Mn-Based Perovskite (La1−xYxMnO3) with Improved Oxygen Electrocatalytic Activity for Metal-Air Batteries. Energy 2018, 154, 561–570. [Google Scholar] [CrossRef]
- Seong, A.; Kim, J.; Kwon, O.; Jeong, H.Y.; Gorte, R.J.; Vohs, J.M.; Kim, G. Self-Reconstructed Interlayer Derived by In-Situ Mn Diffusion from La0.5Sr0.5MnO3 via Atomic Layer Deposition for an Efficient Bi-Functional Electrocatalyst. Nano Energy 2020, 71, 104564. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Xu, X.; Su, C.; Tadé, M.O.; Shao, Z. Promoting Bifunctional Oxygen Catalyst Activity of Double-Perovskite-Type Cubic Nanocrystallites for Aqueous and Quasi-Solid-State Rechargeable Zinc-Air Batteries. Catalysts 2023, 13, 1332. https://doi.org/10.3390/catal13101332
Zhong Y, Xu X, Su C, Tadé MO, Shao Z. Promoting Bifunctional Oxygen Catalyst Activity of Double-Perovskite-Type Cubic Nanocrystallites for Aqueous and Quasi-Solid-State Rechargeable Zinc-Air Batteries. Catalysts. 2023; 13(10):1332. https://doi.org/10.3390/catal13101332
Chicago/Turabian StyleZhong, Yijun, Xiaomin Xu, Chao Su, Moses Oludayo Tadé, and Zongping Shao. 2023. "Promoting Bifunctional Oxygen Catalyst Activity of Double-Perovskite-Type Cubic Nanocrystallites for Aqueous and Quasi-Solid-State Rechargeable Zinc-Air Batteries" Catalysts 13, no. 10: 1332. https://doi.org/10.3390/catal13101332