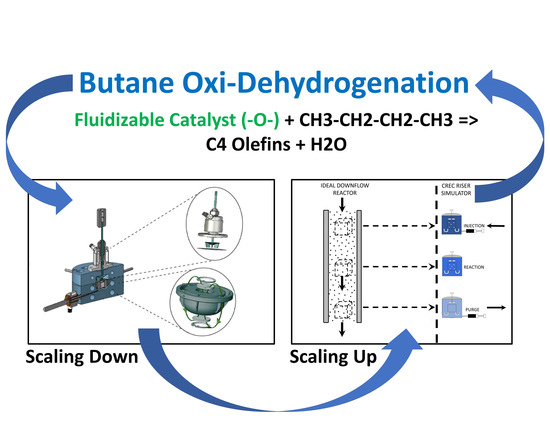
A Fluidizable Catalyst for N-Butane Oxidative Dehydrogenation under Oxygen-Free Reaction Conditions
Abstract
:1. Introduction
2. Results and Discussions
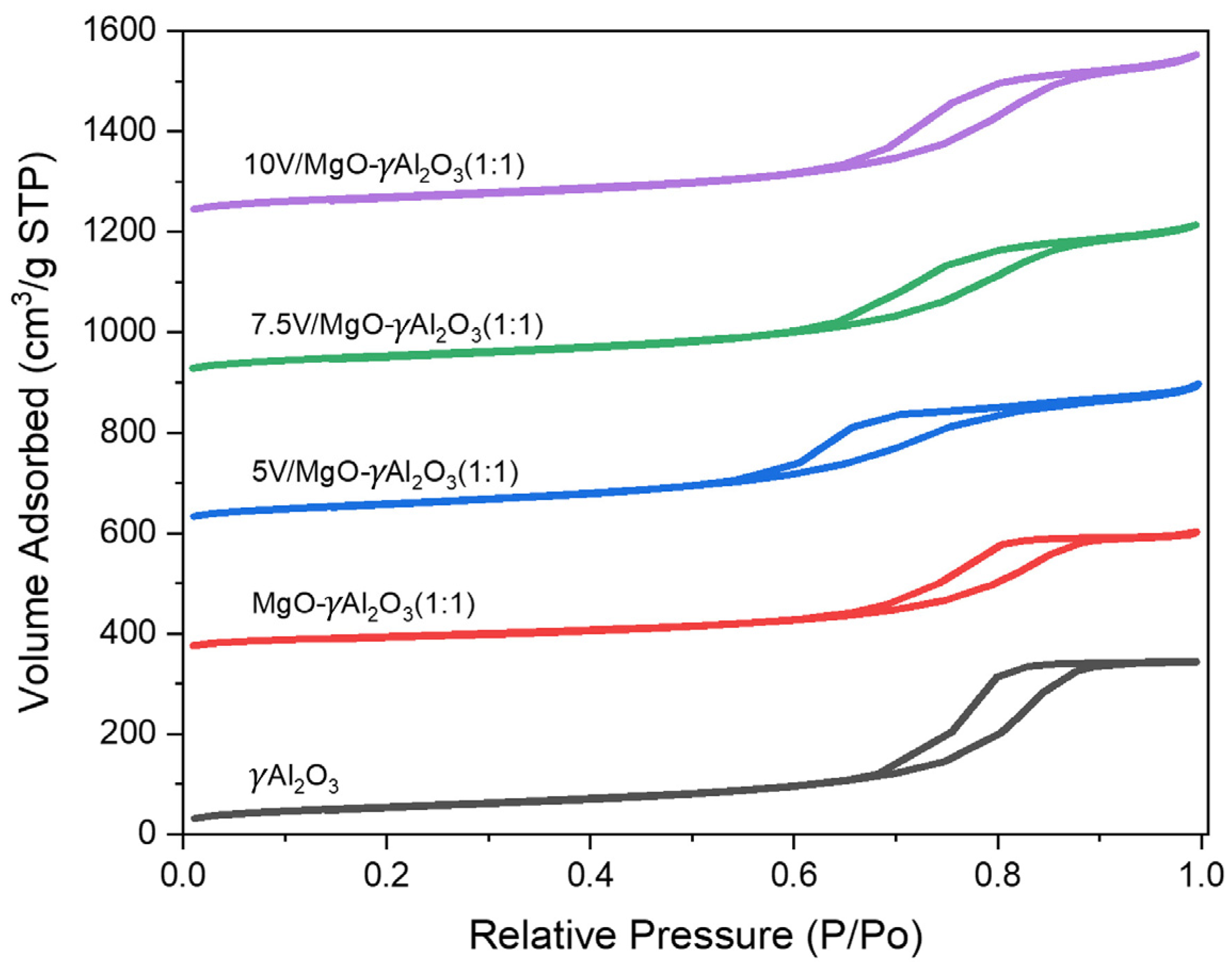
2.1. Specific Surface Area (BET Method)
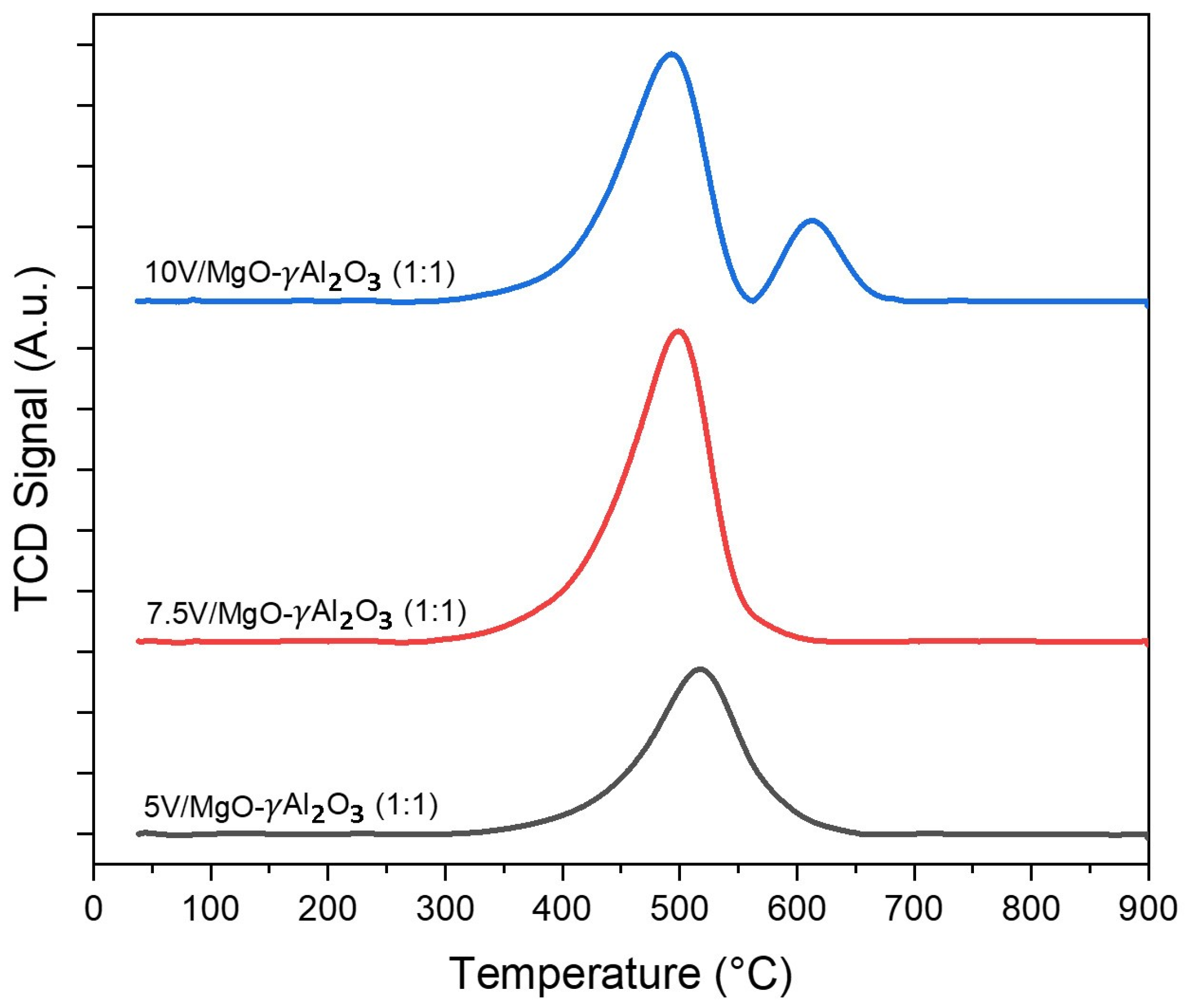
2.2. H2-TPR and Degree of Reduction
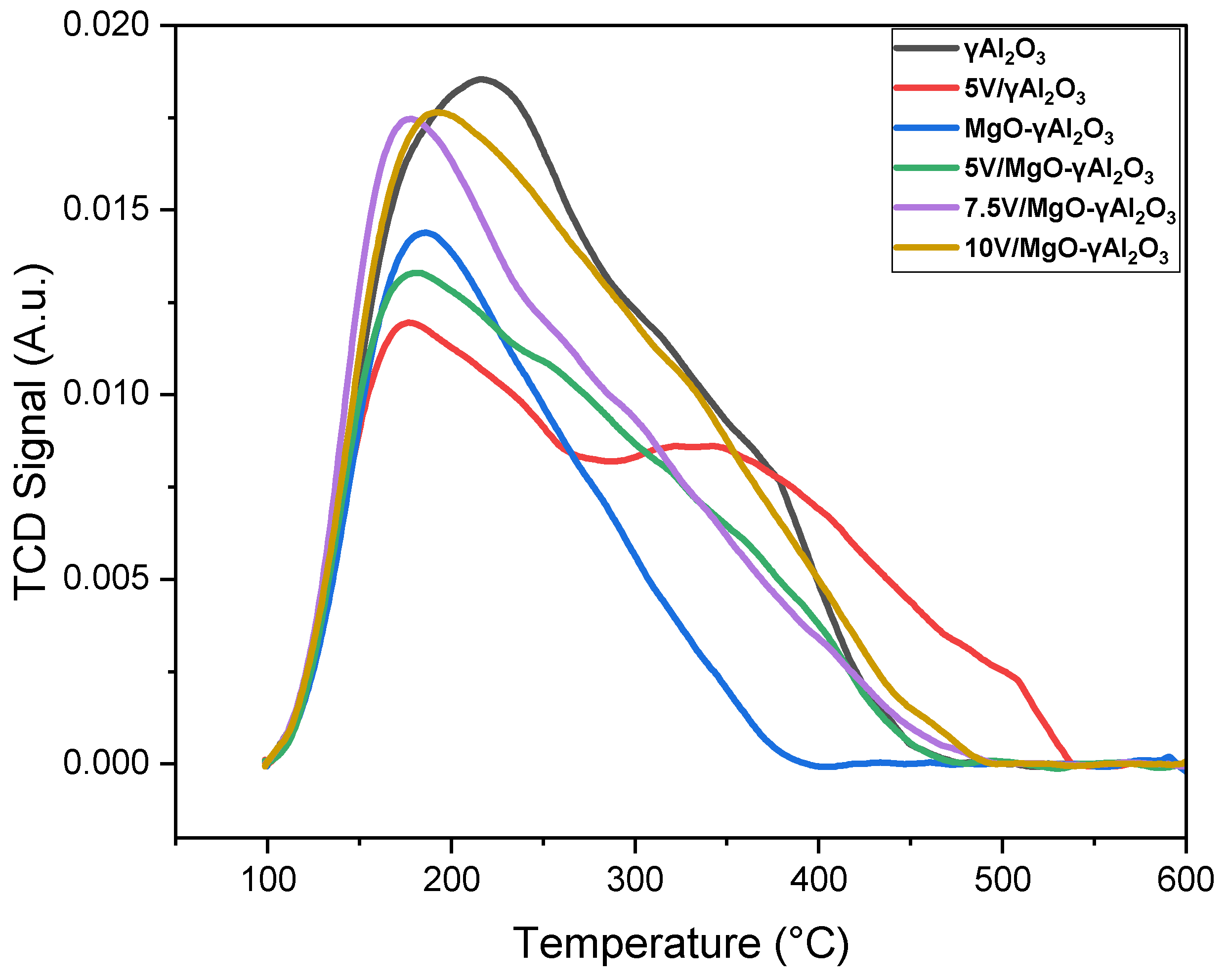
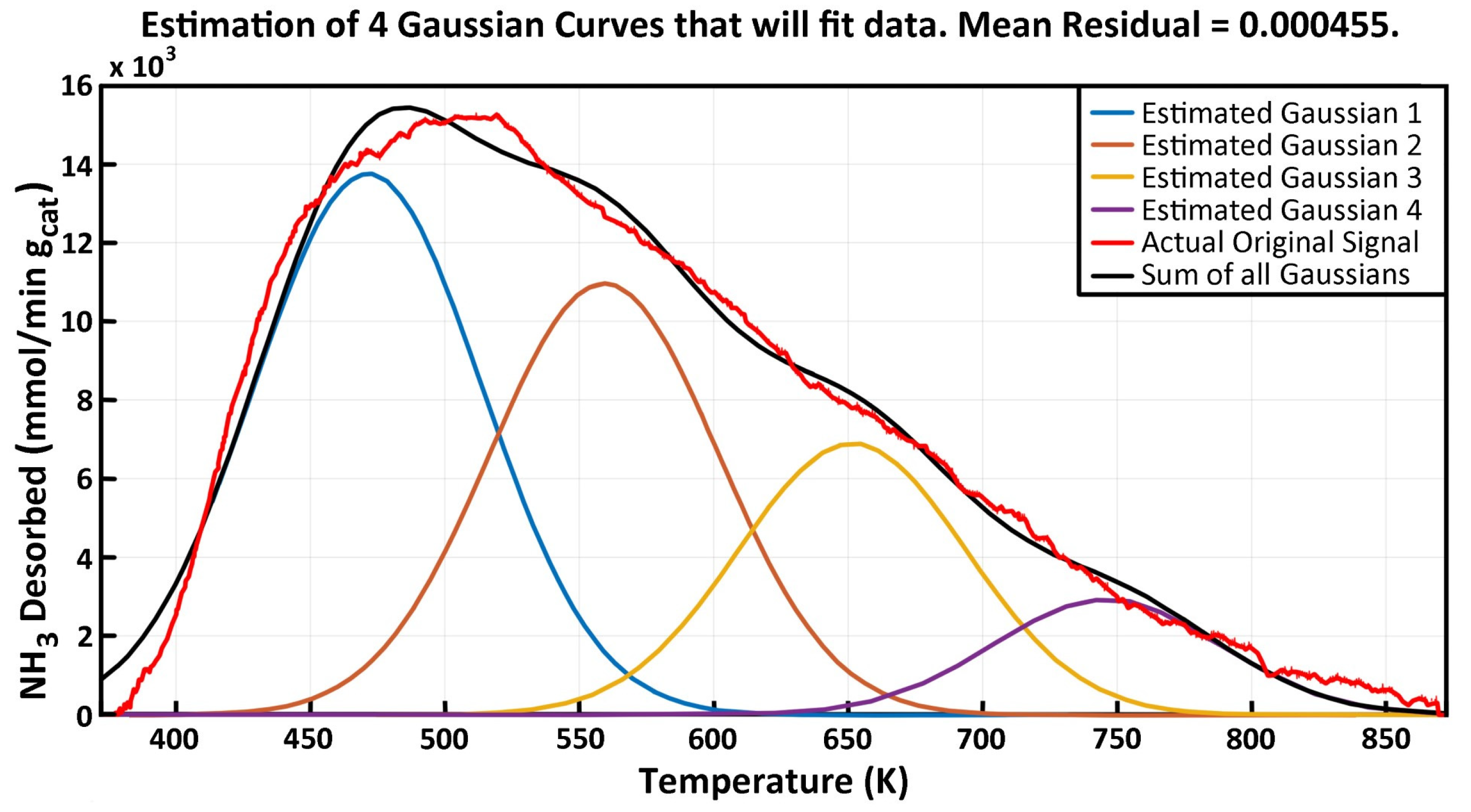
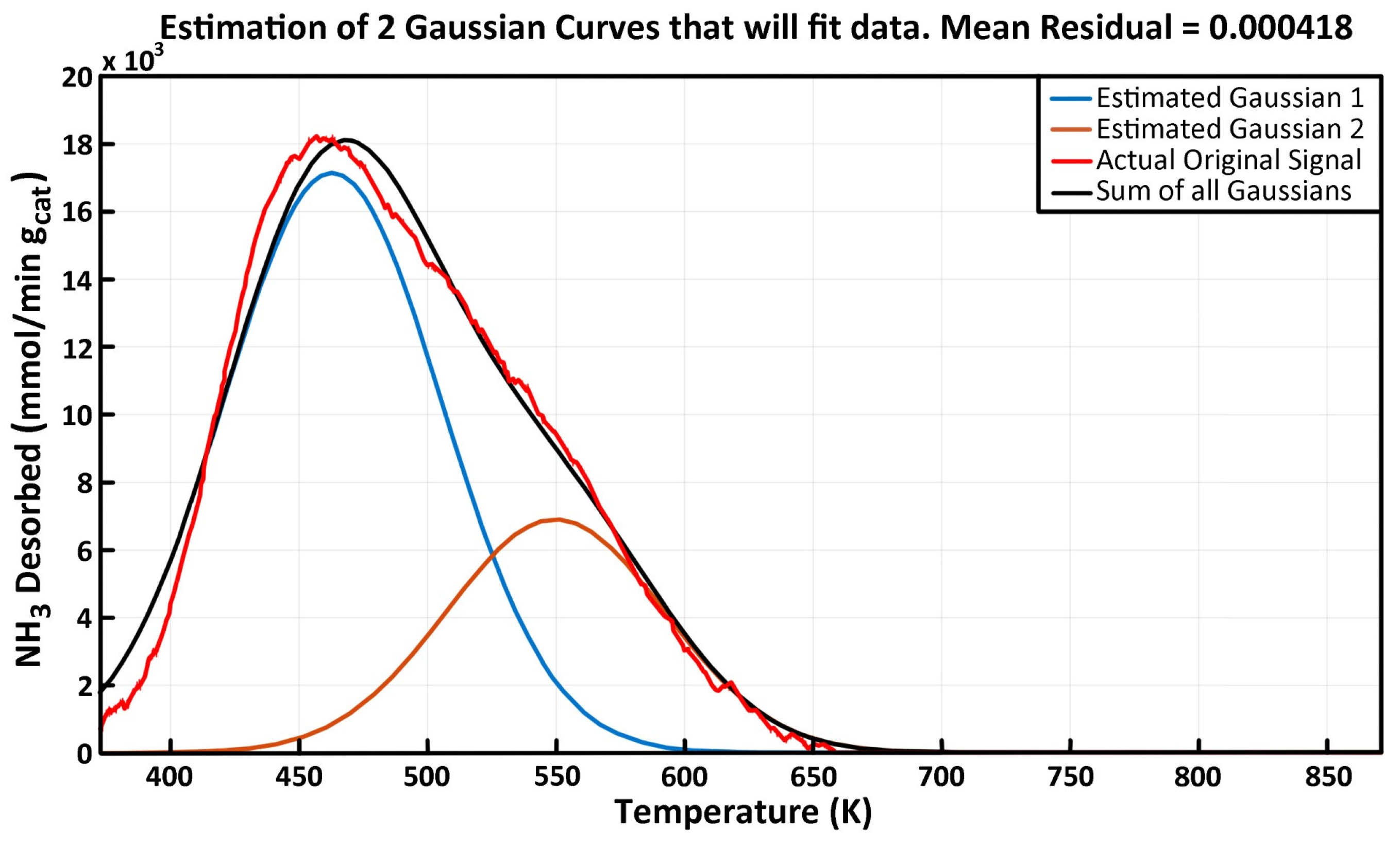
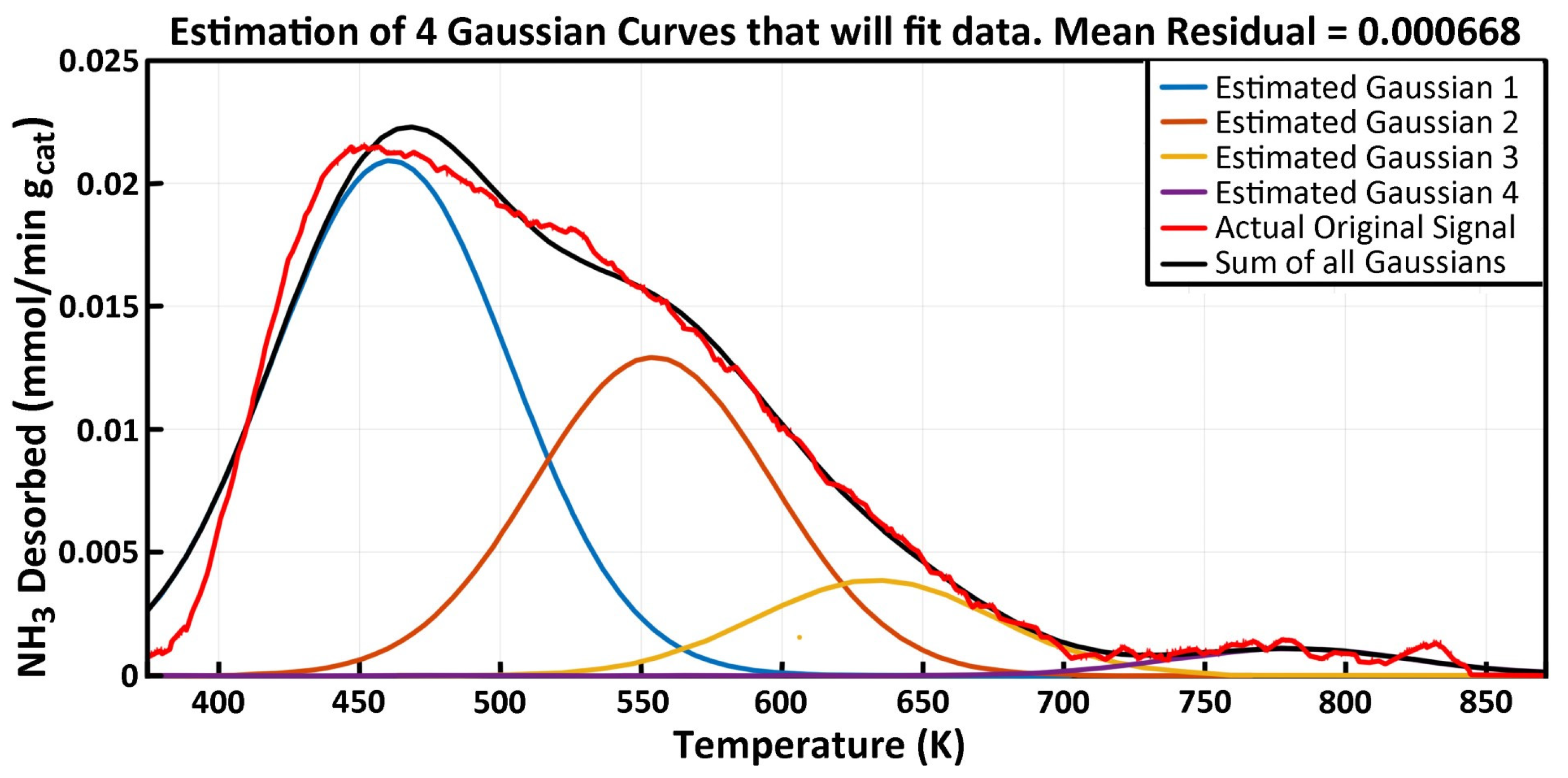
2.3. NH3-TPD Analysis
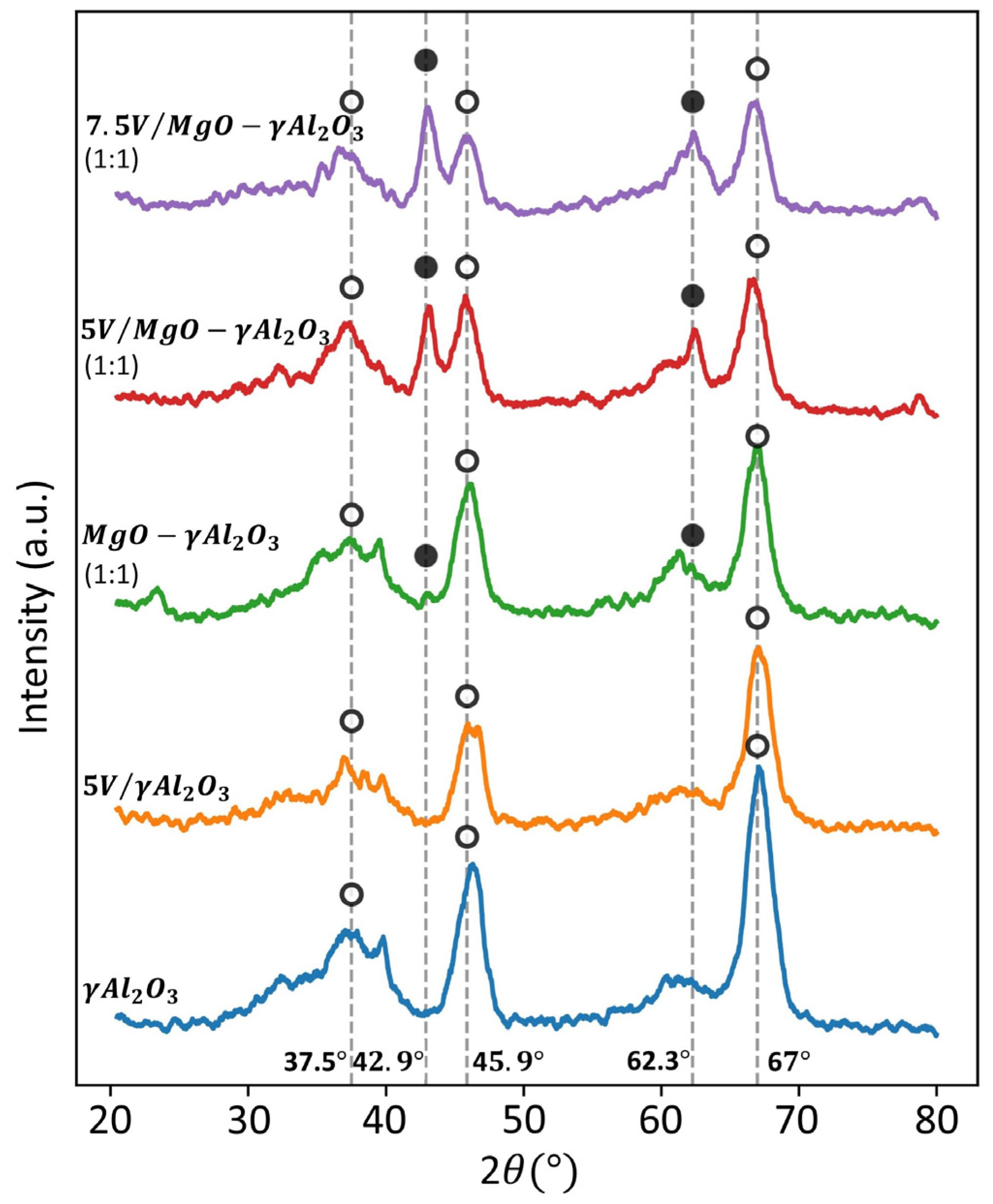
2.4. X-ray Diffraction (XRD)
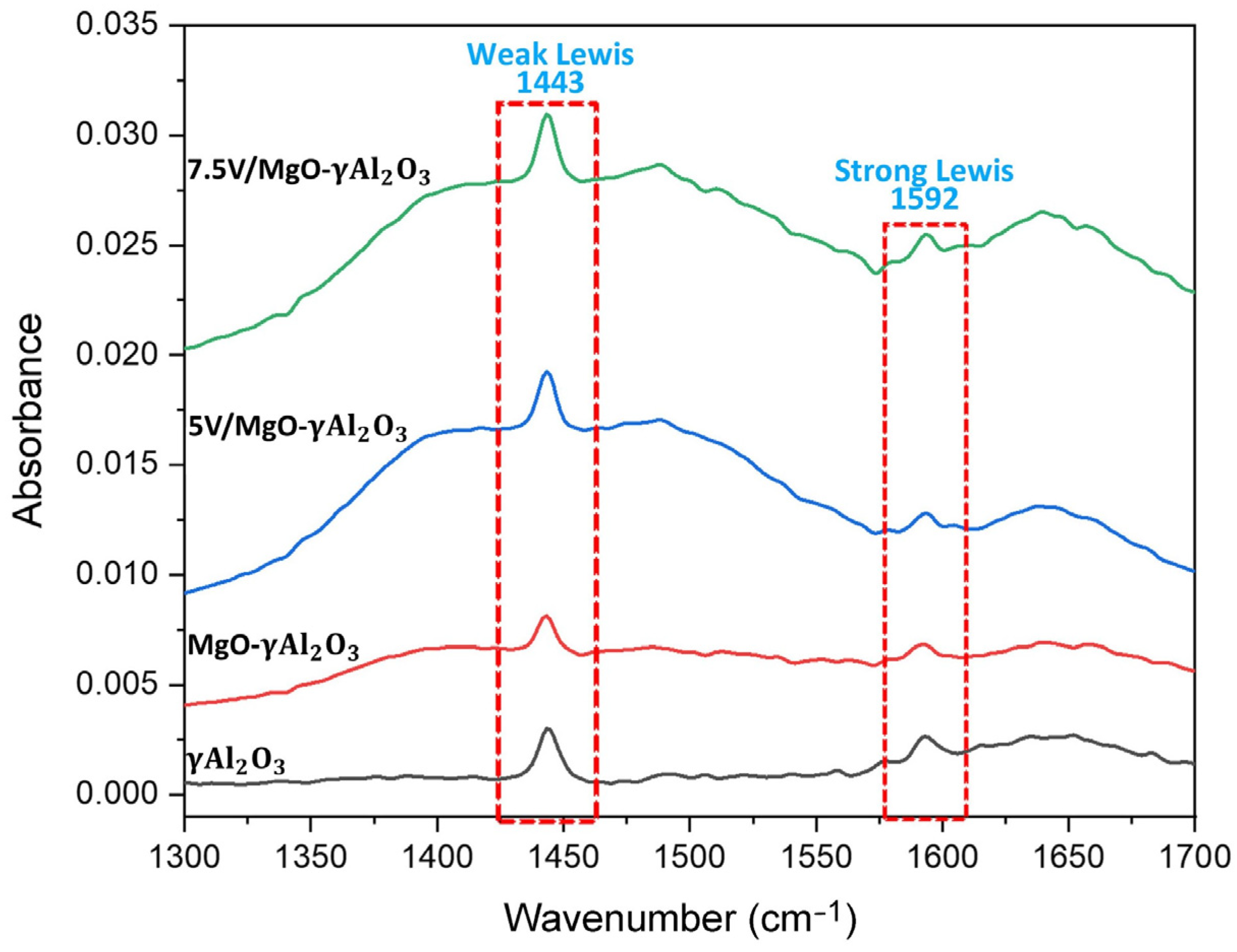
2.5. Pyridine-FTIR
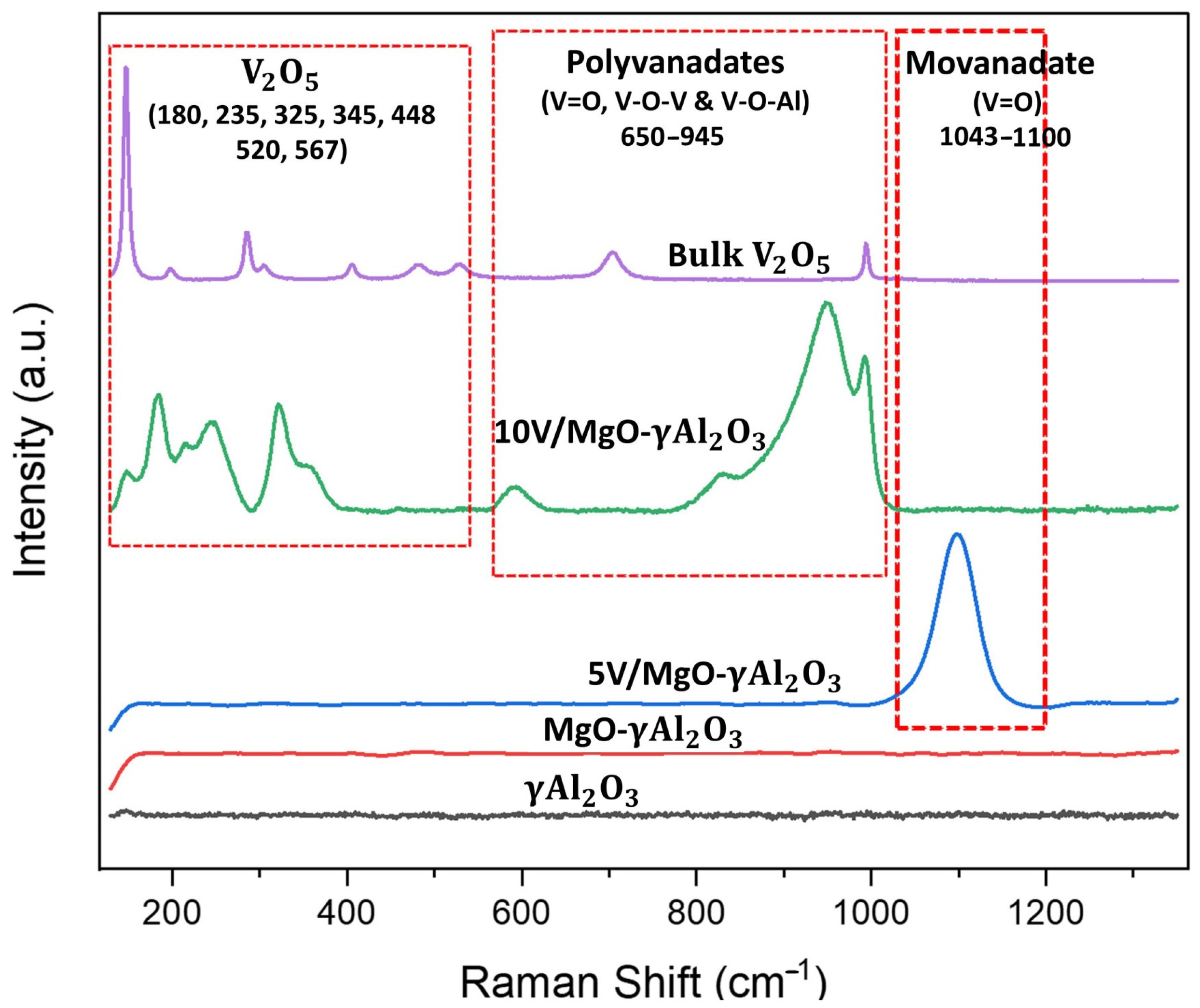
2.6. Laser Raman Spectroscopy (LRS)
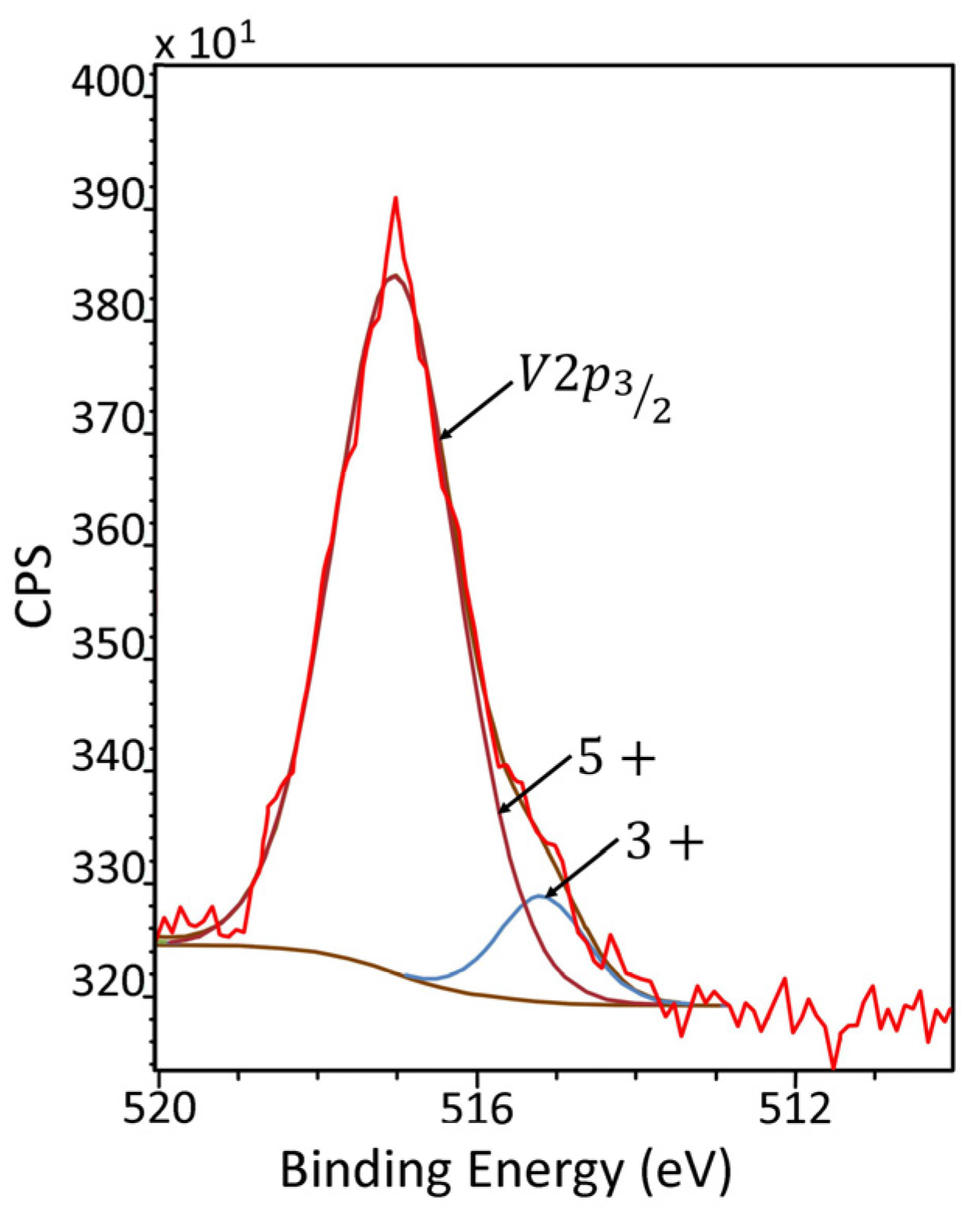
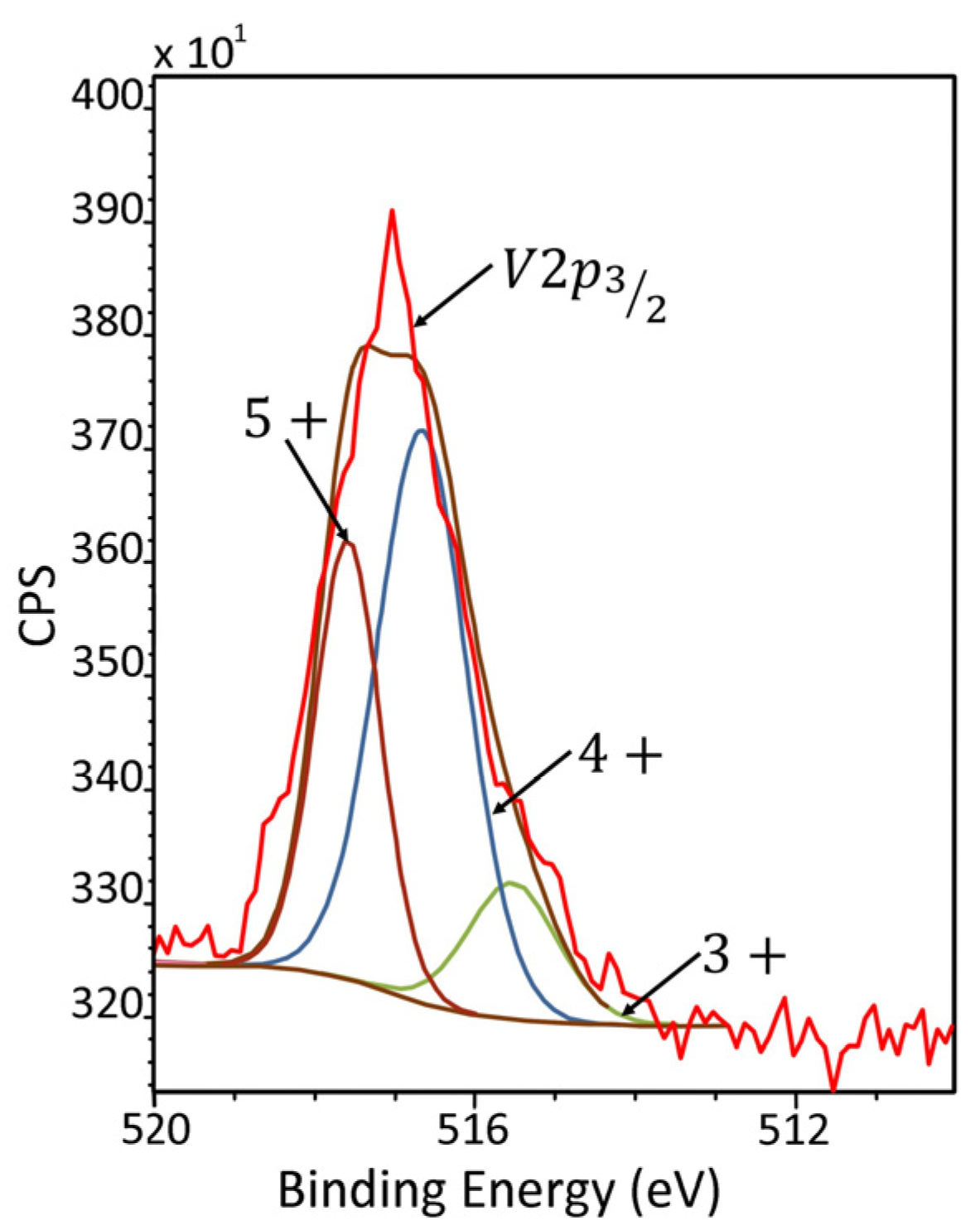
2.7. X-ray Photoelectron Spectroscopy (XPS)
2.8. BODH in the CREC Riser Simulator
2.9. Thermal Run
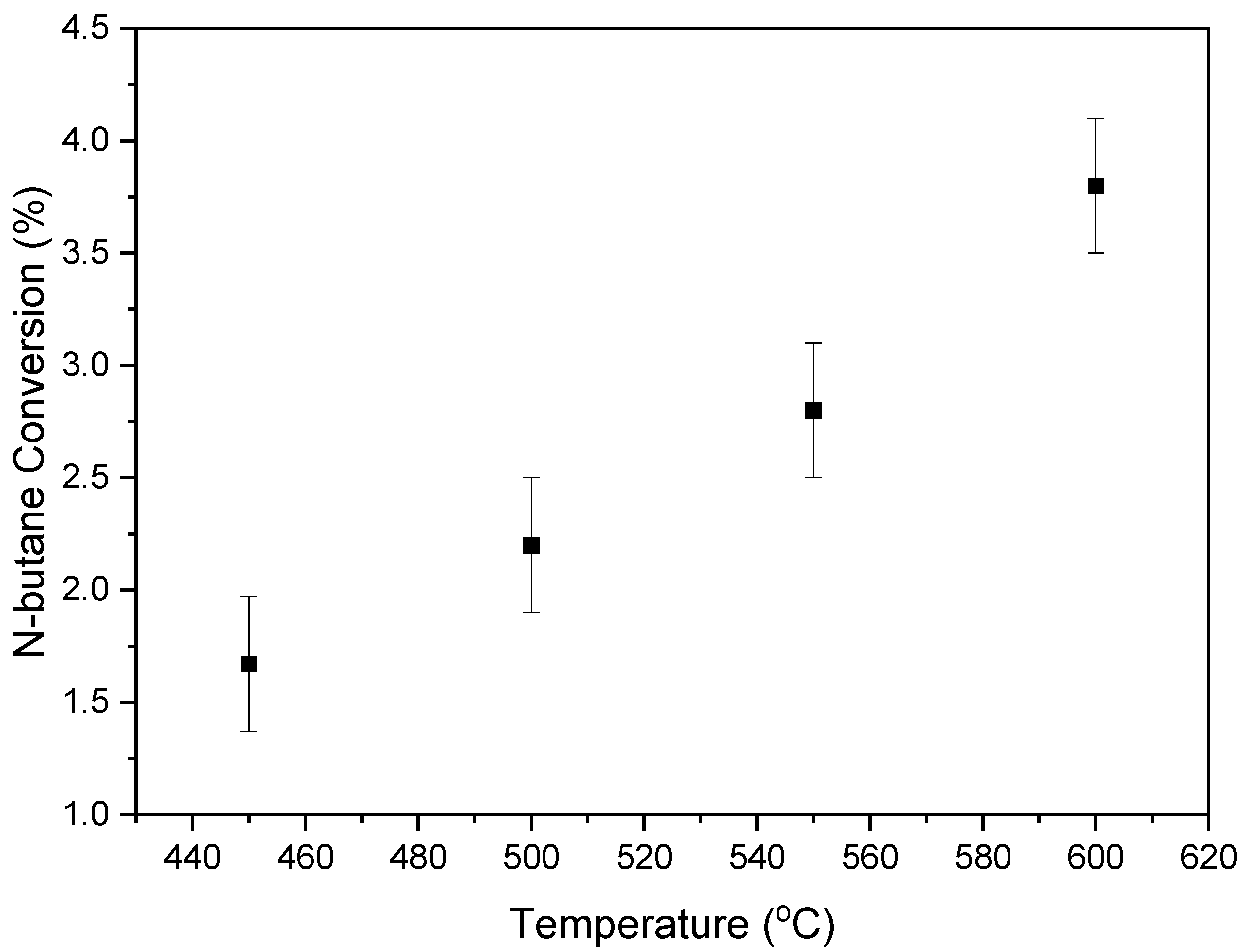
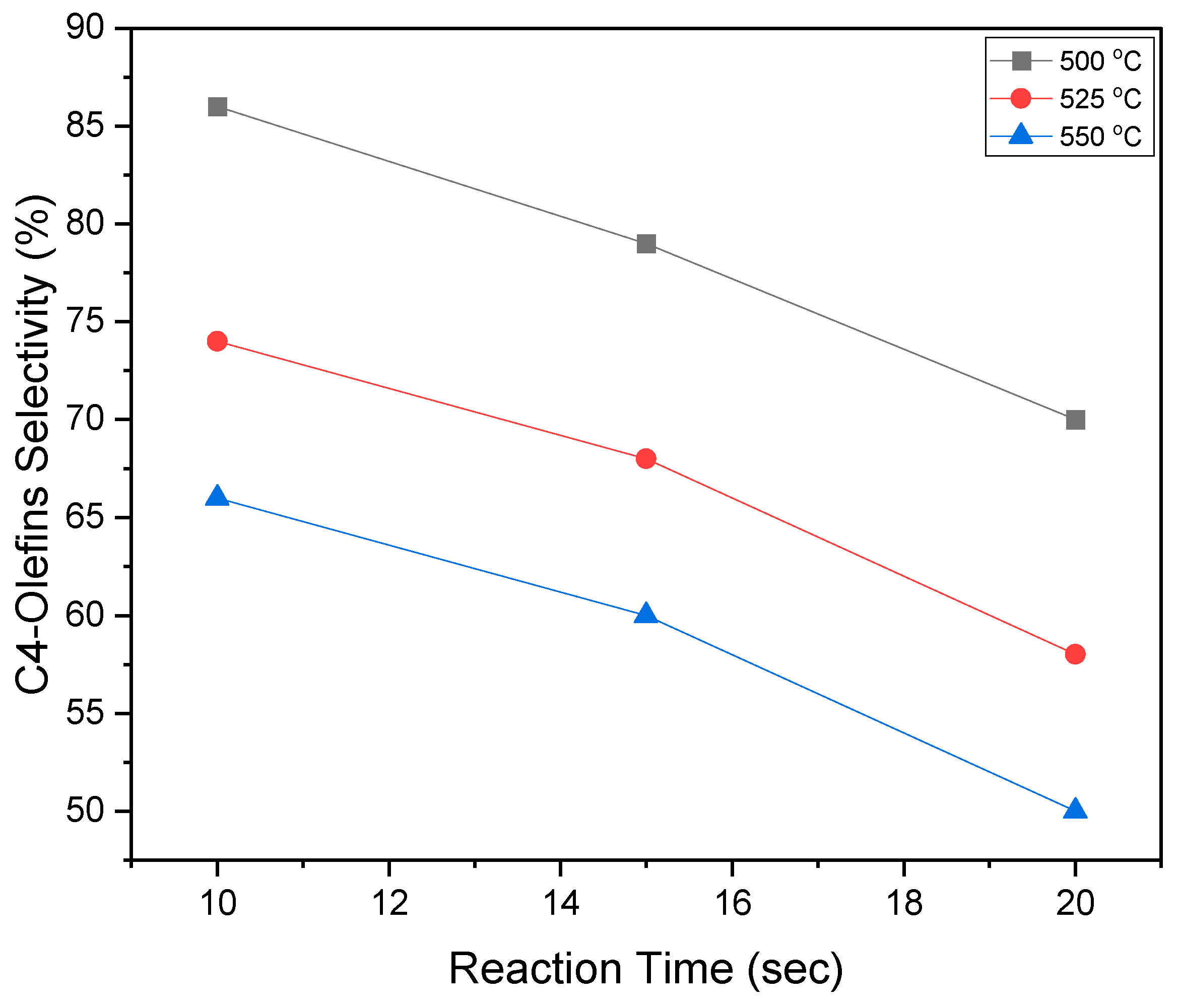
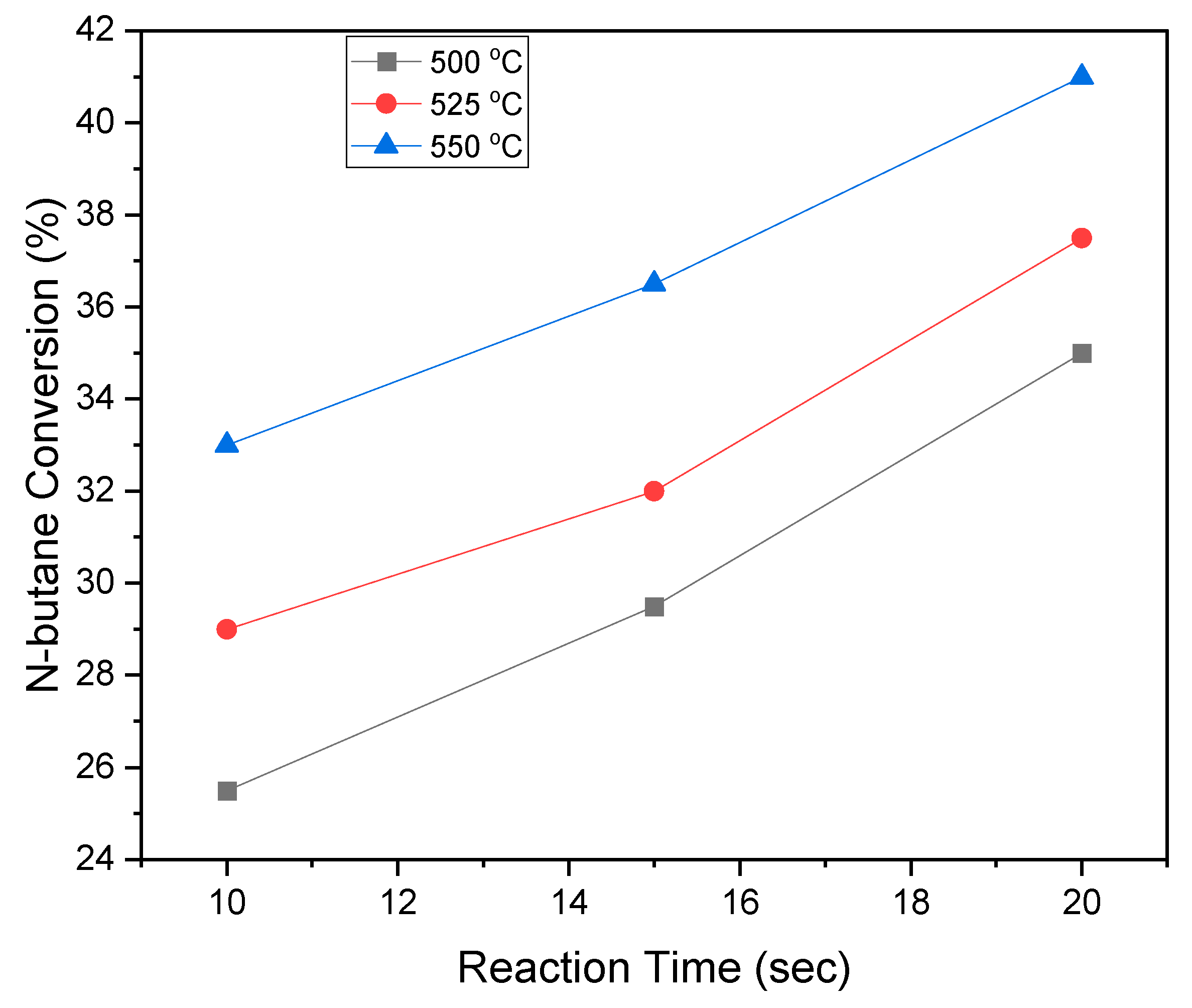
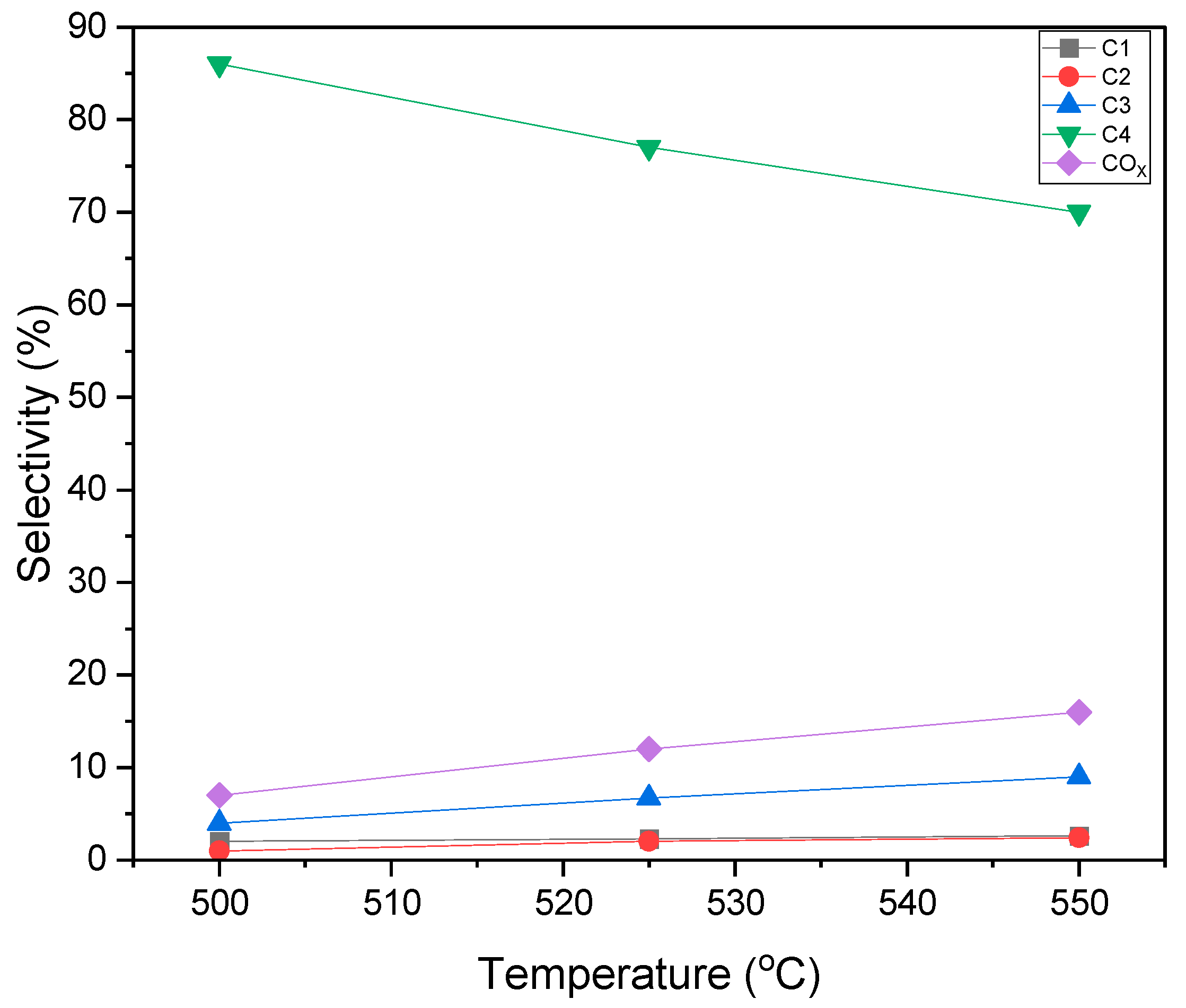
2.10. BODH Experiments: Catalyst Evaluation and Product Distribution
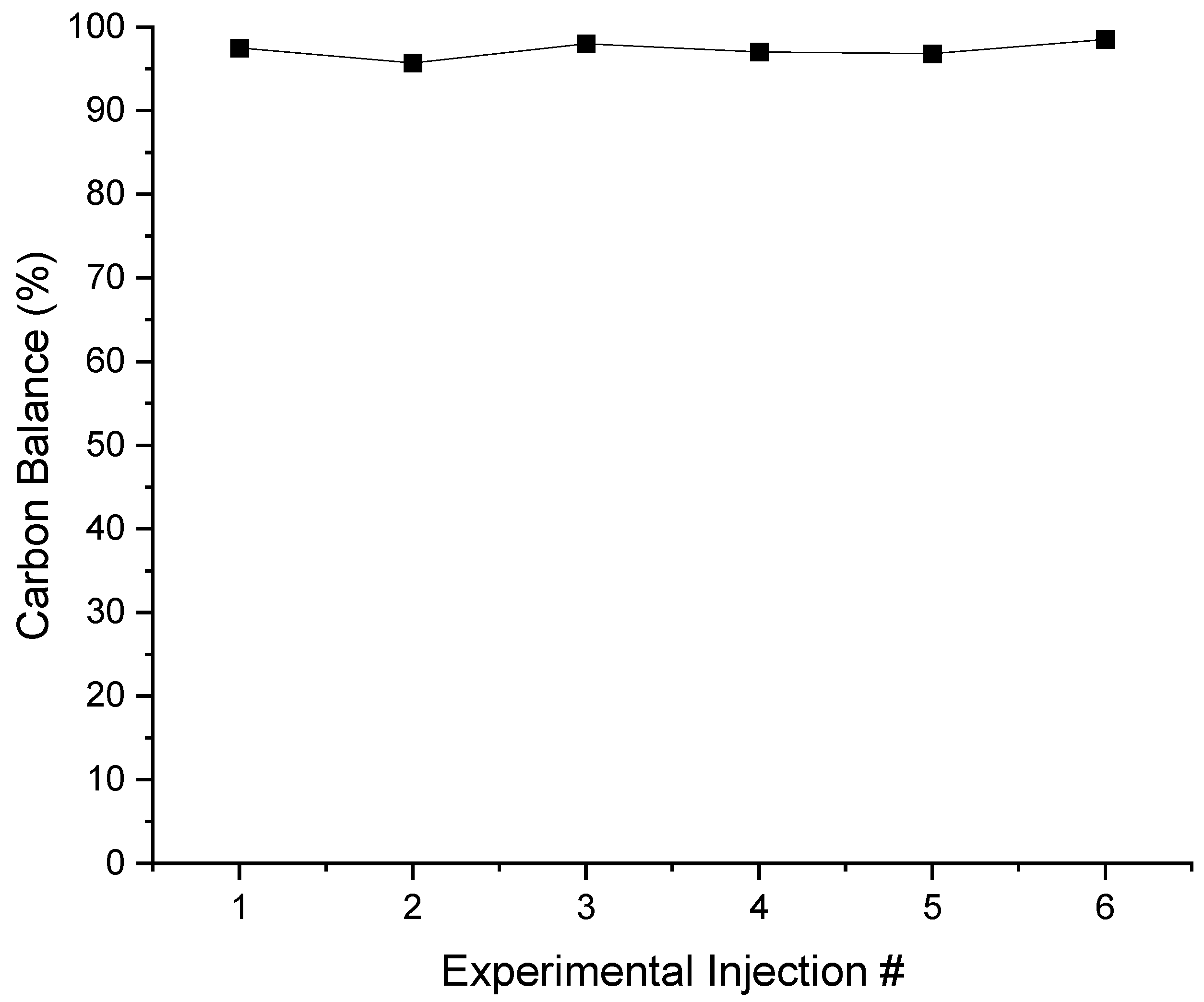
2.11. Coke Formed Analysis Using Total Organic Carbon (TOC)
2.12. Hydrogen (H2) and C4-Olefin Ratios and BODH Mechanism Confirmation
3. Experimental Section
3.1. Catalyst Synthesis
3.2. Catalyst Characterization
3.2.1. BET Surface Area and Pore Size Distribution
3.2.2. Temperature-programmed Reduction/Oxidation (TPR/TPO)
3.2.3. Temperature-Programmed Desorption of Ammonia (NH3-TPD)
3.2.4. X-ray Diffraction (XRD)
3.2.5. Pyridine-Fourier Transform Infrared Spectroscopy (FTIR)
3.2.6. Laser Raman Spectroscopy (LRS)
3.2.7. X-ray Photoelectron Spectroscopy (XPS)
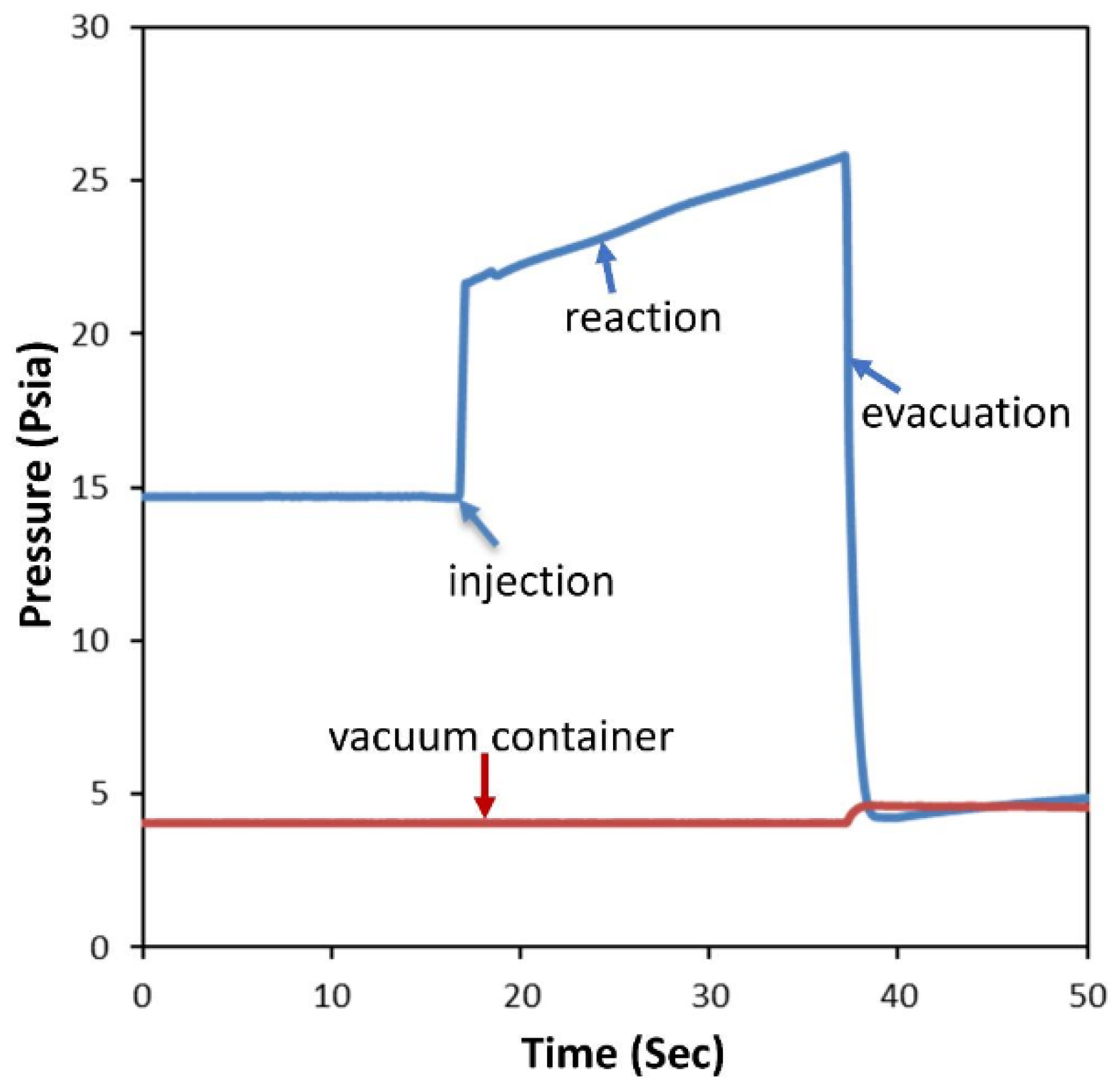
3.3. Reactor System-the CREC Riser Simulator
Experimental Procedures in the CREC Riser Simulator
4. Conclusions
- A VOx/MgO-γAl2O3 fluidizable catalyst for n-butane oxidative dehydrogenation (BODH) was prepared and characterized successfully using BET, XRD LRS, XPS, TPR/TPO, NH3-TPD, and pyridine-FTIR.
- The various surface science techniques used confirmed the catalyst mesoporous structure, its high surface area, its amorphous VOx phase, its dominant Lewis moderate acidity, and its weak metal–support interactions.
- The effectiveness of the VOx/MgO-γAl2O3 catalyst for C4-olefin production was demonstrated in a fluidized CREC Riser Simulator, operated under gas-phase oxygen-free conditions at 5 to 20 s reaction times and within a 450 °C to 600 °C temperature range.
- In particular, the 5 wt% VOx-doped MgO-γAl2O3 catalyst yielded promising selectivities for C4-olefin production, ranging from 82% to 86%, alongside a butane conversion rate of 24% to 27% at 500 °C and at 10 s reaction time.
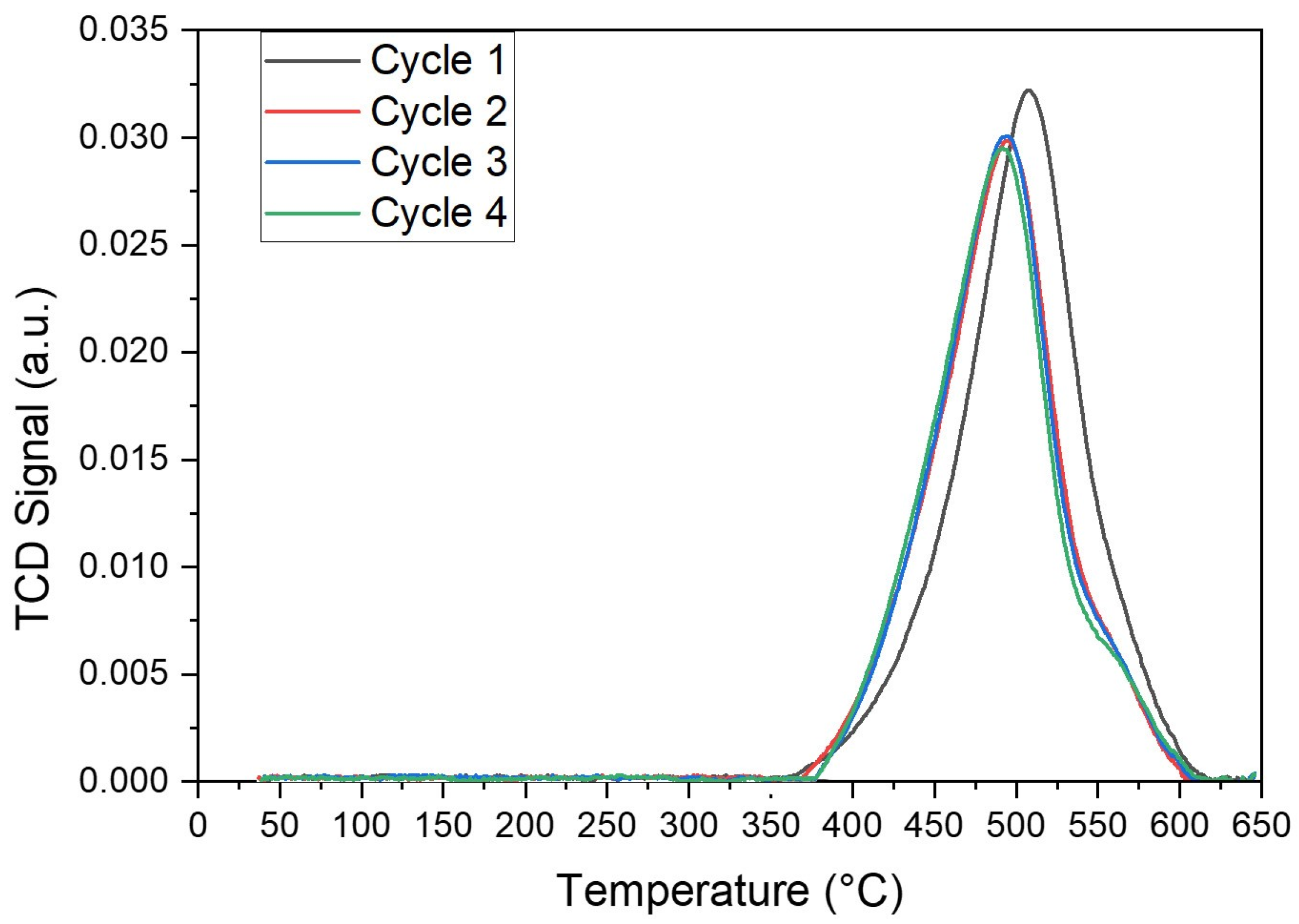
- The developed VOx/MgO-γAl2O3 catalysts were shown to be stable over multiple injections of butane feed, with catalyst regeneration being performed after each six consecutive BODH runs and the coke formed being very low (0.022%/injection).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclatures and Abbreviations
| Nomenclatures | |
| COx | Carbon Oxides |
| Edes | Activation Energy of Desorption (kJ/mol) |
| Ei | Activation energy (kJ/mol) |
| Kd | Desorption Constant, (cm3 gcat × min) |
| kdes0 | Pre-Exponential Factor, (cm3 gcat × min) |
| ki | Reaction rate constant (mol/gcat.s) |
| ni | Moles of gaseous carbon-containing product ‘i’. |
| N-n-butane | Moles of unconverted n-butane in the product stream. |
| NC4H10 | Number of moles of n-butane injected (mole) |
| pg | Gas pressure (Pa) |
| Pi | Partial pressure of species “i” (atm) |
| R | Universal gas constant Re Reynolds number (dimensionless) |
| SBET | Brunauer–Emmet–Teller Specific Surface area (m2/g) |
| Si | Selectivity of component i (%) based on n-butane conversion into gas-phase carbon-containing products |
| Tm | Centering temperature which minimizes the cross-correlation between parameters (k) |
| Vdes | Volume of ammonia desorbed (cm3/gcat) |
| Vm | Volume of monolayer coverage (cm3/gcat) |
| Vpore | Pore volume (cm3/g) |
| Vox | Vanadium oxide surface species V 2p3/2 XPS spectra for vanadium 2p3/2 |
| XC4H10 | N-butane conversion (%) based on gas-phase carbon-containing products |
| YC4-Olefins | C4-olefins yield (%) based on n-butane conversion into gas-phase carbon-containing products |
| Abbreviations | |
| DH | Dehydrogenation |
| BODH | N-butane oxidative dehydrogenation |
| CREC | Chemical Reactor Engineering Center |
| FCC | Fluid catalytic cracking |
| TCD | Thermal conductivity detector |
| FID | Flame ionization detector |
| FTIR | Fourier transform infrared spectroscopy |
| LRS | Laser Raman spectroscopy |
| XRD | X-ray diffraction |
| TPD | Temperature-programmed desorption |
| TPO | Temperature-programmed oxidation |
| TPR | Temperature-programmed reduction |
| XPS | X-ray photoelectron spectroscopy |
Appendix A. Modeling TPD Desorption Process
- The catalyst surface is assumed to be homogeneous, with the desorption rate constant (Kdes0) utilizing the Arrhenius equation, exp((−Edes)/(RT)), where Edes represents the desorption energy and R is the gas constant. The surface coverage (θads) is considered independent, in this context.
- The desorption process is considered irreversible. Once ammonia is desorbed, it does not reabsorb onto the catalyst surface during the TPD experiments.
- The concentration of adsorbed ammonia remains constant throughout the TPD experiments, even as the gas flow removes it.
- The rate of ammonia desorption is hypothesized to be of first order with respect to the surface coverage. This assumption implies that the desorption rate is directly proportional to the amount of ammonia adsorbed on the catalyst surface.
- The temperature is assumed to increase linearly during the TPD experiments.
Appendix B. Conversion and Products Distribution Results
| Temperature (°C) | Time (s) | Injection | Selectivity (%) | X.C4H10 (%) | Y.C4H8 (%) | |||||||
| CO | CH4 | CO2 | C2H4 | C2H6 | C3H6 | C3H8 | C4H8 | |||||
| 500 | 10 | 1 | 6.35 | 0.32 | 10.62 | 1.30 | 0.07 | 5.31 | 0.64 | 75.39 | 32.96 | 24.85 |
| 2 | 2.24 | 0.41 | 7.22 | 1.36 | 0.07 | 5.57 | 0.55 | 82.58 | 27.51 | 22.72 | ||
| 3 | 1.56 | 0.46 | 6.07 | 1.69 | 0.10 | 6.01 | 0.38 | 83.73 | 26.06 | 21.82 | ||
| 4 | 1.42 | 0.52 | 4.62 | 1.91 | 0.10 | 6.06 | 0.32 | 85.04 | 26.10 | 22.20 | ||
| 5 | 1.28 | 0.51 | 4.00 | 1.92 | 0.10 | 5.97 | 0.31 | 85.91 | 26.05 | 22.38 | ||
| 6 | 1.15 | 0.49 | 3.55 | 1.90 | 0.11 | 5.76 | 0.29 | 86.74 | 25.76 | 22.34 | ||
| Temperature (°C) | Time (s) | Injection | Selectivity (%) | X.C4H10 (%) | Y.C4H8 (%) | |||||||
| CO | CH4 | CO2 | C2H4 | C2H6 | C3H6 | C3H8 | C4H8 | |||||
| 525 | 10 | 1 | 7.19 | 0.37 | 12.03 | 1.80 | 0.08 | 6.88 | 0.72 | 70.92 | 33.51 | 23.77 |
| 2 | 2.75 | 0.50 | 8.87 | 1.67 | 0.09 | 9.05 | 0.68 | 76.38 | 29.06 | 22.20 | ||
| 3 | 2.02 | 0.59 | 7.82 | 2.18 | 0.12 | 9.36 | 0.50 | 77.41 | 28.47 | 22.04 | ||
| 4 | 1.90 | 0.70 | 6.20 | 2.57 | 0.13 | 10.10 | 0.43 | 77.97 | 27.69 | 21.59 | ||
| 5 | 1.77 | 0.71 | 5.56 | 2.66 | 0.15 | 10.50 | 0.43 | 78.22 | 27.35 | 21.39 | ||
| 6 | 1.63 | 0.70 | 5.03 | 2.70 | 0.16 | 10.68 | 0.41 | 78.69 | 27.85 | 21.92 | ||
| Temperature (°C) | Time (s) | Injection | Selectivity (%) | X.C4H10 (%) | Y.C4H8 (%) | |||||||
| CO | CH4 | CO2 | C2H4 | C2H6 | C3H6 | C3H8 | C4H8 | |||||
| 550 | 10 | 1 | 7.61 | 0.33 | 14.38 | 1.99 | 0.07 | 10.34 | 0.66 | 64.62 | 37.36 | 24.14 |
| 2 | 3.28 | 0.47 | 9.36 | 2.53 | 0.08 | 11.95 | 0.64 | 71.69 | 32.79 | 23.51 | ||
| 3 | 2.42 | 0.57 | 8.74 | 2.70 | 0.12 | 12.53 | 0.48 | 72.43 | 31.17 | 22.58 | ||
| 4 | 2.14 | 0.66 | 7.64 | 2.81 | 0.12 | 12.98 | 0.40 | 73.24 | 30.84 | 22.59 | ||
| 5 | 1.93 | 0.68 | 6.37 | 3.03 | 0.14 | 13.55 | 0.41 | 73.89 | 30.21 | 22.32 | ||
| 6 | 1.83 | 0.68 | 5.31 | 3.23 | 0.16 | 13.95 | 0.41 | 74.44 | 29.65 | 22.07 | ||
Appendix C. C4-Olefin/(H2) Molar Ratio Calculations Including Coke Formation
| Inject. | Selectivity (%) | X.C4H10 (%) | Y.C4H8 (%) | C4-Olefin/H2 (Molar Ratio) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CO | CH4 | CO2 | C2H4 | C2H6 | C3H6 | C3H8 | C4H8 | ||||
| 1 | 6.23 | 0.33 | 10.41 | 1.29 | 0.09 | 5.22 | 0.65 | 73.76 | 32.26 | 24.33 | 7.34 |
| 2 | 2.21 | 0.42 | 7.08 | 1.35 | 0.09 | 5.47 | 0.56 | 80.79 | 26.93 | 22.24 | 5.69 |
| 3 | 1.55 | 0.47 | 5.96 | 1.67 | 0.12 | 5.90 | 0.39 | 81.92 | 25.51 | 21.36 | 6.31 |
| 4 | 1.41 | 0.53 | 4.54 | 1.89 | 0.12 | 5.95 | 0.33 | 83.20 | 25.55 | 21.74 | 6.13 |
| 5 | 1.27 | 0.52 | 3.93 | 1.90 | 0.12 | 5.86 | 0.33 | 84.05 | 25.50 | 21.91 | 6.21 |
| 6 | 1.15 | 0.50 | 3.49 | 1.88 | 0.13 | 5.66 | 0.31 | 84.86 | 25.22 | 21.87 | 6.36 |
Appendix D. Comparison of the Performance of the BODH Catalysts of the Present Study
| Catalyst | Selectivity (%) | X.C4H10 (%) | Y.C4H8 (%) | C4-Olefin/H2 (Molar Ratio) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CO | CH4 | CO2 | C2H4 | C2H6 | C3H6 | C3H8 | C4H8 | ||||
| 5% V/MgO-γAl2O3 | 1.15 | 0.49 | 3.55 | 1.91 | 0.11 | 5.76 | 0.29 | 86.74 | 25.76 | 22.34 | 3.46 |
| 7.5% V/MgO-γAl2O3 | 1.85 | 0.79 | 5.72 | 3.07 | 0.18 | 12.13 | 0.47 | 75.81 | 25.36 | 19.22 | 2.45 |
| 10% V/MgO-γAl2O3 | 1.87 | 0.71 | 6.22 | 2.71 | 0.16 | 17.88 | 0.42 | 70.04 | 24.91 | 17.44 | 1.89 |
References
- Park, J.H.; Noh, H.; Park, J.W.; Row, K.; Jung, K.D.; Shin, C.H. Effects of iron content on bismuth molybdate for the oxidative dehydrogenation of n-butenes to 1,3-butadiene. Appl. Catal. A Gen. 2012, 431–432, 137–143. [Google Scholar] [CrossRef]
- White, W.C. Butadiene production process overview. Chem. Biol. Interact. 2007, 166, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef]
- Kharlamova, T.; Sushchenko, E.; Izaak, T.; Vodyankina, O. Phase composition, structural peculiarities and catalytic properties of supported MgO-V2O5/Al2O3 catalysts for oxidative dehydrogenation of propane: Insight into formation of surface Mg-V-O phase. Catal. Today 2016, 278, 174–184. [Google Scholar] [CrossRef]
- Atanga, M.A.; Rezaei, F.; Jawad, A.; Fitch, M.; Rownaghi, A.A. Oxidative dehydrogenation of propane to propylene with carbon dioxide. Appl. Catal. B Environ. 2018, 220, 429–445. [Google Scholar] [CrossRef]
- Madeira, L.M. Catalytic oxidative dehydrogenation of n-butane. Catal. Rev.-Sci. Eng. 2002, 44, 247–286. [Google Scholar] [CrossRef]
- Sheng, J.; Yan, B.; Lu, W.D.; Qiu, B.; Gao, X.Q.; Wang, D.; Lu, A.H. Oxidative dehydrogenation of light alkanes to olefins on metal-free catalysts. Chem. Soc. Rev. 2021, 50, 1438–1468. [Google Scholar] [CrossRef] [PubMed]
- Jermy, B.R.; Ajayi, B.P.; Abussaud, B.A.; Asaoka, S.; Al-Khattaf, S. Oxidative dehydrogenation of n-butane to butadiene over Bi-Ni-O/γ-alumina catalyst. J. Mol. Catal. A Chem. 2015, 400, 121–131. [Google Scholar] [CrossRef]
- Blasco, T.; López Nieto, J.M. Oxidative dehydrogenation of short chain alkanes on supported vanadium oxide catalysts. Appl. Catal. A Gen. 1997, 157, 117–142. [Google Scholar] [CrossRef]
- Khan, M.Y.; Adamu, S.; Lucky, R.A.; Razzak, S.A.; Hossain, M.M. Oxidative Dehydrogenation of n-Butane to C4-Olefins Using Lattice Oxygen of VOx/Ce-meso-Al2O3 under Gas-Phase Oxygen-Free Conditions. Energy Fuels 2020, 34, 7410–7421. [Google Scholar] [CrossRef]
- Xu, B.; Zhu, X.; Cao, Z.; Yang, L.; Yang, W. Catalytic oxidative dehydrogenation of n-butane over V2O5/MO-Al2O3 (M = Mg, Ca, Sr, Ba) catalysts. Cuihua Xuebao/Chin. J. Catal. 2015, 36, 1060–1067. [Google Scholar] [CrossRef]
- Madaan, N.; Haufe, R.; Shiju, N.R.; Rothenberg, G. Oxidative Dehydrogenation of n-Butane: Activity and Kinetics over VOx/Al2O3 Catalysts. Top. Catal. 2014, 57, 1400–1406. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, G.; Chen, Z.; Jiang, W.; Zhou, H. In-situ synthesis and characterization of V-MCM-41 for oxidative dehydrogenation of n-butane. Microporous Mesoporous Mater. 2016, 223, 261–267. [Google Scholar] [CrossRef]
- Lee, K.J.; Lee, H.; Gi, U.; Yoo, Y.; Cho, Y.; Lee, J.; Chang, H.; Kyu, I. Journal of Industrial and Engineering Chemistry Oxidative dehydrogenation of n-butane over Mg3(VO4)2/MgO–ZrO2 catalysts: Effect of oxygen capacity and acidity of the catalysts. J. Ind. Eng. Chem. 2012, 18, 1758–1763. [Google Scholar] [CrossRef]
- Garcia, E.M.; Sanchez, M.D.; Tonetto, G.; Volpe, M.A. Preparation of USY zeolite VOx supported catalysts from V(AcAc)3 and NH4VO3. Catalytic properties for the dehydrogenation of n-butane in oxygen-free atmosphere. J. Colloid Interface Sci. 2005, 292, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Rubio, O.; Herguido, J.; Menéndez, M. Oxidative dehydrogenation of n-butane on V/MgO catalysts-kinetic study in anaerobic conditions. Chem. Eng. Sci. 2003, 58, 4619–4627. [Google Scholar] [CrossRef]
- Corrna, A.; Nieto, J.M.L.; Parades, N.; Dejoz, A.; Vazquez, I. Oxidative dehydrogenation of propane and n-butane on V-Mg based catalysts. Stud. Surf. Sci. Catal. 1994, 82, 113–123. [Google Scholar]
- Liu, X.; Duan, L.; Yang, W.; Zhu, X. Oxidative dehydrogenation of n-butane to butenes on Mo-doped VMgO catalysts. RSC Adv. 2017, 7, 34131–34137. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Keller, D.E. Chemistry, spectroscopy, and the role of supported vanadium oxides in heterogeneous catalysis. Catal. Today 2003, 78, 25–26. [Google Scholar] [CrossRef]
- Cavani, F.; Ballarini, N.; Cericola, A. Oxidative dehydrogenation of ethane and propane: How far from commercial implementation? Catal. Today 2007, 127, 113–131. [Google Scholar] [CrossRef]
- Lemonidou, A.A.; Nalbandian, L.; Vasalos, I.A. Oxidative dehydrogenation of propane over vanadium oxide based catalysts. Effect of support and alkali promoter. Catal. Today 2000, 61, 333–341. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Xu, X.; Marcel Veder, J.P.; Shao, Z. Recent advances in anion-doped metal oxides for catalytic applications. J. Mater. Chem. A 2019, 7, 7280–7300. [Google Scholar] [CrossRef]
- Van Assche, A.; Especel, C.; Le Valant, A.; Epron, F. Effect of potassium and platinum contents on catalytic performance of Pt/Al2O3 monometallic catalysts for propane dehydrogenation. Mol. Catal. 2022, 517, 112059. [Google Scholar] [CrossRef]
- Jermy, B.R.; Asaoka, S.; Al-Khattaf, S. Influence of calcination on performance of Bi-Ni-O/gamma-alumina catalyst for n-butane oxidative dehydrogenation to butadiene. Catal. Sci. Technol. 2015, 5, 4622–4635. [Google Scholar] [CrossRef]
- Vedrine, J.C. Heterogeneous catalytic partial oxidation of lower alkanes (C1–C6) on mixed metal oxides. J. Energy Chem. 2016, 25, 936–946. [Google Scholar] [CrossRef]
- Wang, C.; Chen, J.G.; Xing, T.; Liu, Z.T.; Liu, Z.W.; Jiang, J.; Lu, J. Vanadium oxide supported on titanosilicates for the oxidative dehydrogenation of n-butane. Ind. Eng. Chem. Res. 2015, 54, 3602–3610. [Google Scholar] [CrossRef]
- Rischard, J.; Antinori, C.; Maier, L.; Deutschmann, O. Oxidative dehydrogenation of n-butane to butadiene with Mo-V-MgO catalysts in a two-zone fluidized bed reactor. Appl. Catal. A Gen. 2016, 511, 23–30. [Google Scholar] [CrossRef]
- Valverde, J.A.; Echavarría, A.; Eon, J.-G.; Faro, A.C., Jr.; Palacio, L.A. V–Mg–Al catalyst from hydrotalcite for the oxidative dehydrogenation of propane. React. Kinet. Mech. Catal. 2014, 111, 679–696. [Google Scholar] [CrossRef]
- Schacht, P.; Herna, I.; Co, I.R. Influence of reducibility of vanadium–magnesium mixed oxides on the oxidative dehydrogenation of propane. Catal. Today 2005, 108, 371–376. [Google Scholar]
- de Lasa, H.I. Multifunctional Riser and Downer Simulator. USA Pat. 2019, 10, 1–9. [Google Scholar]
- Zhu, H.; Dong, H.; Laveille, P.; Saih, Y.; Caps, V.; Basset, J.M. Metal oxides modified NiO catalysts for oxidative dehydrogenation of ethane to ethylene. Catal. Today 2014, 228, 58–64. [Google Scholar] [CrossRef]
- Do, D.D. Fundamentals of Diffusion and Adsorption in Porous Media. Ser. Chem. Eng. 1998, 2, 337–414. [Google Scholar]
- Meira, D.M.; Cortez, G.G.; Monteiro, W.R.; Rodrigues, J.A.J. Vanadium oxides supported on hydrotalcite-type precursors: The effect of acid-base properties on the oxidation of isopropanol. Braz. J. Chem. Eng. 2006, 23, 351–358. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.; Volpe, M.; Hossain, M.M.; de Lasa, H. VOx/c-Al2O3 catalyst for oxidative dehydrogenation of ethane to ethylene: Desorption kinetics and catalytic activity. Appl. Catal. A Gen. 2013, 450, 120–130. [Google Scholar] [CrossRef]
- Bakare, I.A.; Mohamed, S.A.; Al-Ghamdi, S.; Razzak, S.A.; Hossain, M.M.; de Lasa, H.I. Fluidized bed ODH of ethane to ethylene over VOx-MoOx/γ-Al2O3 catalyst: Desorption kinetics and catalytic activity. Chem. Eng. J. 2015, 278, 207–216. [Google Scholar] [CrossRef]
- Elbadawi AA, H.; Ba-Shammakh, M.S.; Al-Ghamdi, S.; Razzak, S.A.; Hossain, M.M. Reduction kinetics and catalytic activity of VOx/γ-Al2O3-ZrO2 for gas phase oxygen free ODH of ethane. Chem. Eng. J. 2016, 284, 448–457. [Google Scholar] [CrossRef]
- Tonetto, G.; Atias, J.; de Lasa, H. FCC catalysts with different zeolite crystallite sizes: Acidity, structural properties and reactivity. Appl. Catal. A Gen. 2004, 270, 9–25. [Google Scholar] [CrossRef]
- Rostom, S.; de Lasa, H. Propane oxidative dehydrogenation on vanadium-based catalysts under oxygen-free atmospheres. Catalysts 2020, 10, 418. [Google Scholar] [CrossRef]
- Boullosa-Eiras, S.; Vanhaecke, E.; Zhao, T.; Chen, D.; Holmen, A. Raman spectroscopy and X-ray diffraction study of the phase transformation of ZrO2-Al2O3 and CeO2-Al2O3 nanocomposites. Catal. Today 2011, 166, 10–17. [Google Scholar] [CrossRef]
- Khodakov, A.; Olthof, B.; Bell, A.T.; Iglesia, E. Structure, and catalytic properties of supported vanadium oxides: Support effects on oxidative dehydrogenation reactions. J. Catal. 1999, 181, 205–216. [Google Scholar] [CrossRef]
- Liu, Y.; McGill, C.J.; Green, W.H.; Deshlahra, P. Effects of surface species and homogeneous reactions on rates and selectivity in ethane oxidation on oxide catalysts. AIChE J. 2021, 67, e17483. [Google Scholar] [CrossRef]
- Gao, X.; Bare, S.R.; Weckhuysen, B.M.; Wachs, I.E. In situ spectroscopic investigation of molecular structures of highly dispersed vanadiumoxide on silica under various conditions. J. Phys. Chem. B 1998, 102, 10844. [Google Scholar] [CrossRef]
- Wachs, I.E.; Weckhuysen, B.M. Structure and reactivity of surface vanadium oxide species on oxide supports. Appl. Catal. A Gen. 1997, 157, 67–90. [Google Scholar] [CrossRef]
- Kharlamova, T.S.; Timofeev, K.L.; Salaev, M.A.; Svetlichnyi, V.A.; Vodyankina, O.V. Monolayer MgVOx/Al2O3 catalysts for propane oxidative dehydrogenation: Insights into a role of structural, redox, and acid-base properties in catalytic performance. Appl. Catal. A Gen. 2020, 598, 117574. [Google Scholar] [CrossRef]
- Sanchez, J.C.; Livage, G.L. Infrared and Raman study of amorphous V2O5. J. Raman Spectrosc. 1982, 12, 68–72. [Google Scholar] [CrossRef]
- Murgia, V.; Sham, E.; Gottifredi, J.C.; Farfan Torres, E.M. Oxidative dehydrognation of propane and n-butane over alumina supported vanadium catalysts. Lat. Am. Appl. Res. 2004, 34, 75. [Google Scholar]
- de Lasa, H. The CREC Fluidized Riser Simulator a Unique Tool for Catalytic Process Development. Catalysts 2022, 12, 888. [Google Scholar] [CrossRef]
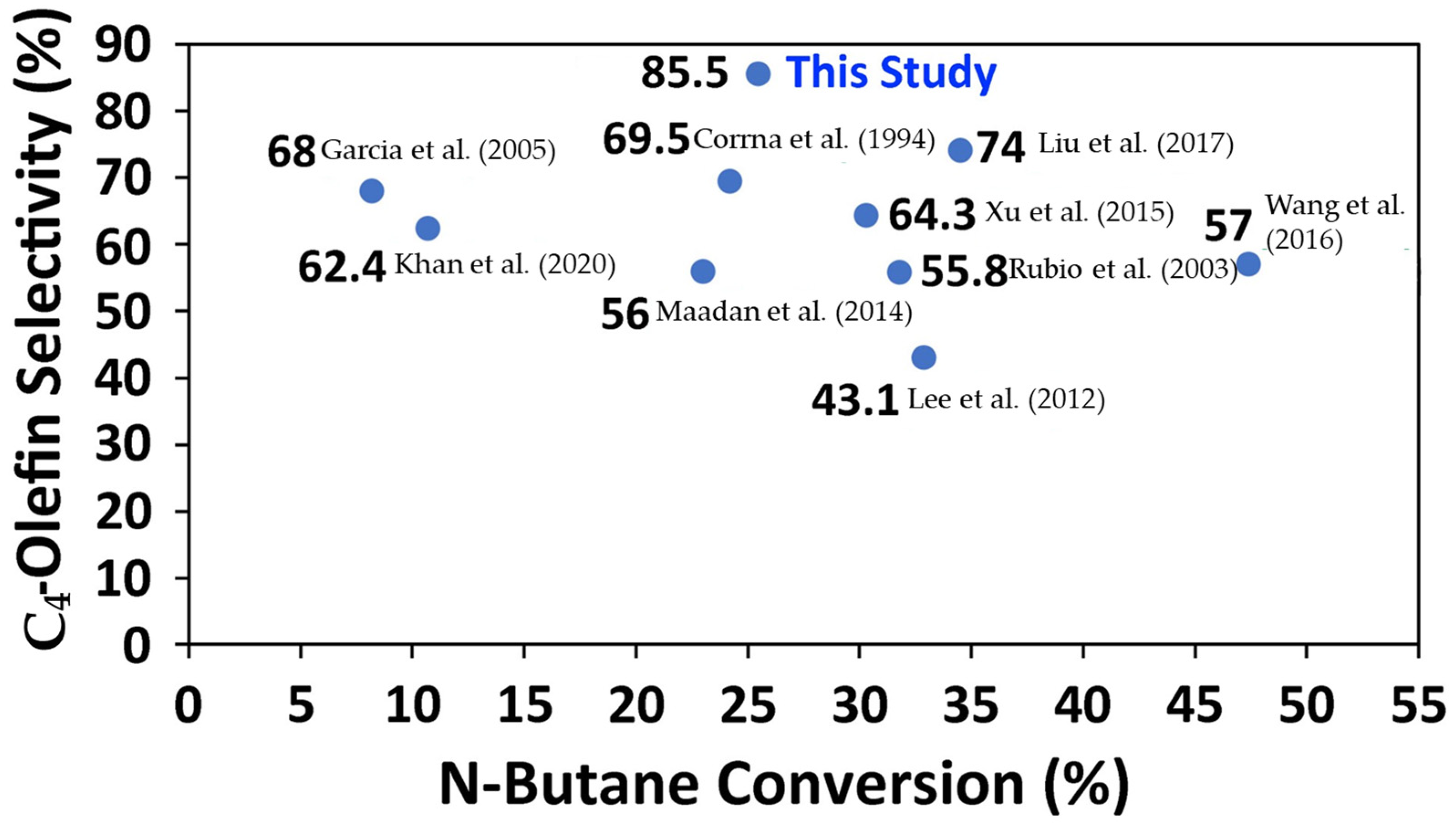
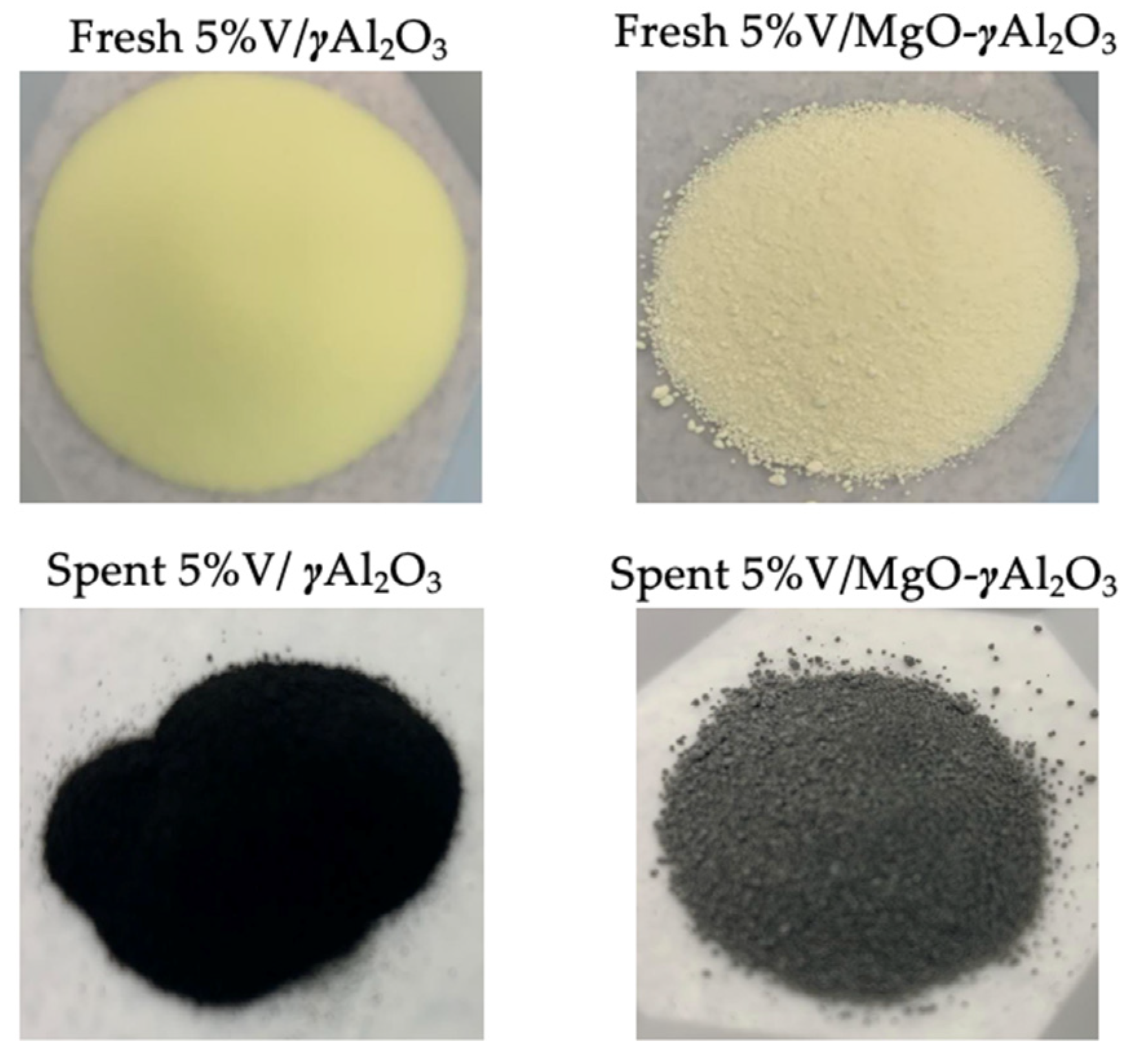
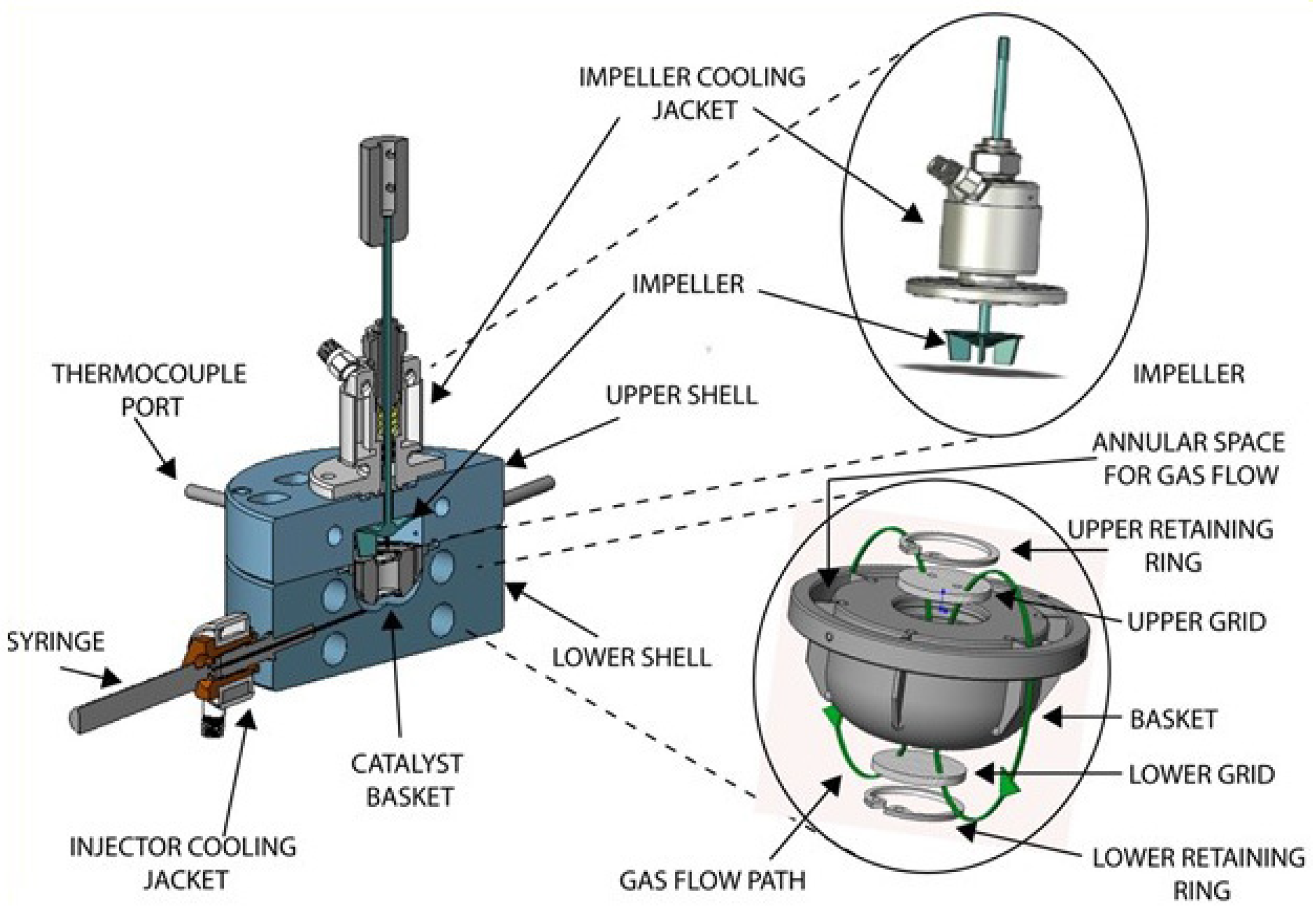
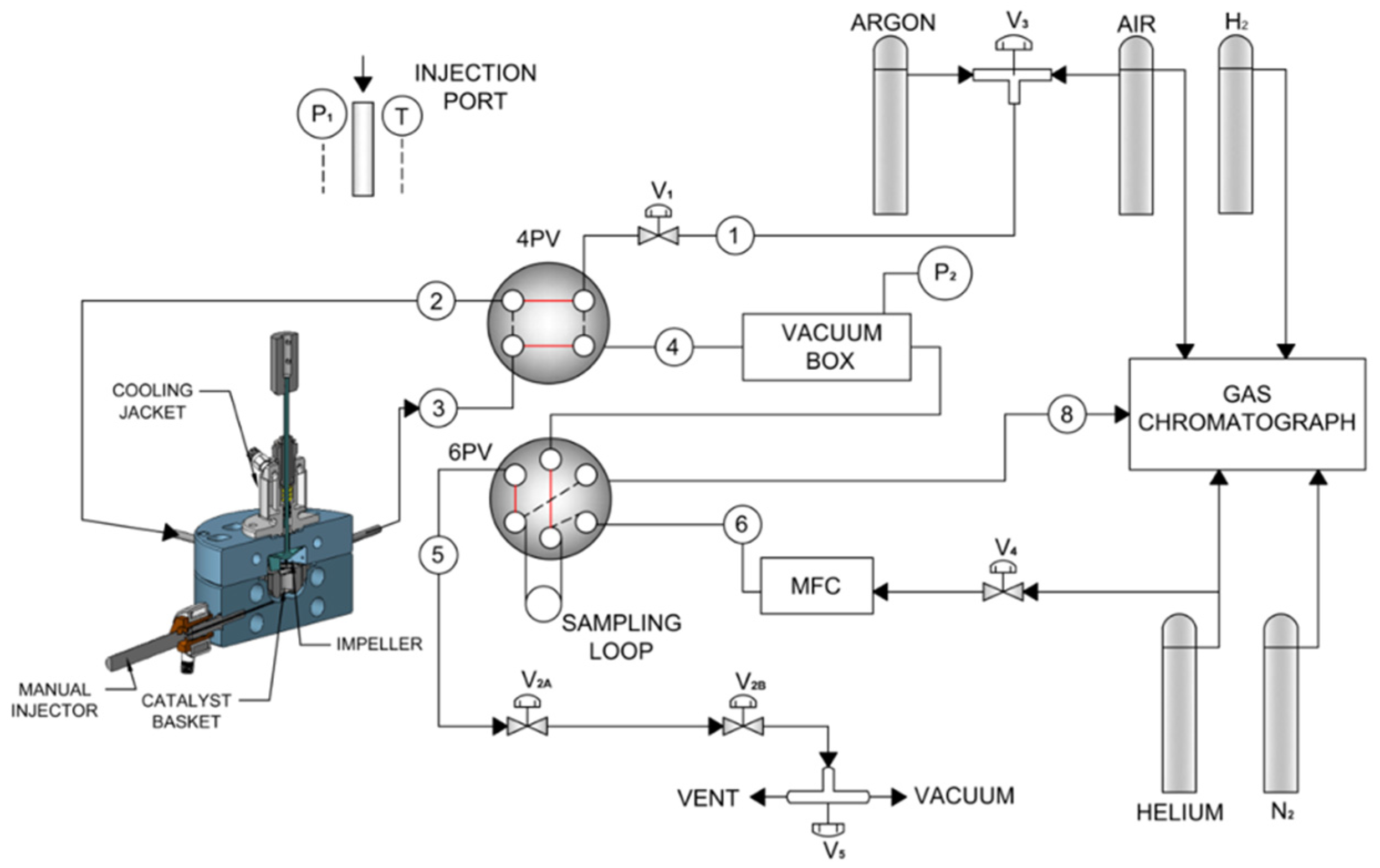
| Catalyst | X (%) | T (°C) | Time | S (%) | Y (%) | Reactor System | Reference |
|---|---|---|---|---|---|---|---|
| VOx/Ce-Al2O3 | 10.7 | 450 | 5 s | 62.4 | 6.7 | Fluidized bed | Khan et al. [10] |
| V2O5/MgO-Al2O3 | 30.3 | 600 | NA | 64.3 | 19.4 | Fixed bed | Xu et al. [11] |
| VOx/Al2O3 | 23.0 | 600 | 1 h | 56.0 | 12.9 | Fixed bed | Maadan et al. [12] |
| VOx/MCM-41 | 47.4 | 550 | 1 h | 57.0 | 27.0 | Fixed bed | Wang et al. [13] |
| V2O5/MgO-ZrO2 | 32.9 | 500 | 6 h | 43.1 | 13.8 | Fixed bed | Lee et al. [14] |
| VOx/USY | 8.2 | 520 | 4 min | 68.0 | 5.6 | Fixed bed | Garcia et al. [15] |
| V2O5/MgO | 31.8 | 500 | NA | 55.8 | 17.7 | Fixed bed | Rubio et al. [16] |
| MoO3-V2O5/MgO | 24.2 | 550 | NA | 69.5 | 17.3 | Fixed bed | Corrna et al. [17] |
| Mo/VMgO | 34.5 | 620 | 2 min | 74.0 | 25.2 | Fixed bed | Liu et al. [18] |
| Catalyst | SBET (m2/g) | Vpore (cm3/g) | Dpore (Å) |
|---|---|---|---|
| γAl2O3 | 208 | 0.56 | 109 |
| MgO-γAl2O3 (1:1) | 152 | 0.38 | 164 |
| 5% V/MgO-γAl2O3 (1:1) | 198 | 0.44 | 89 |
| 7.5% V/MgO-γAl2O3 (1:1) | 192 | 0.47 | 94 |
| 10% V/MgO-γAl2O3 (1:1) | 187 | 0.50 | 97 |
| Catalyst | Tmax1, (°C) | Tmax2, (°C) | Total H2 Consumption (cm3/g) | Nominal V (%) | Reducible V (%) |
|---|---|---|---|---|---|
| 5% V/γAl2O3 | 480 | - | 9 | 5.0 | 3.02 |
| 5% V/MgO-γAl2O3 (1:1) | 518 | - | 12 | 5.0 | 3.63 |
| 7.5% V/MgO-γAl2O3 (1:1) | 501 | - | 18 | 7.5 | 5.44 |
| 10% V/MgO-γAl2O3 (1:1) | 497 | 613 | 21 | 10.0 | 7.31 |
| Area under the Peak (mmol K/gcat min) | ||||||
|---|---|---|---|---|---|---|
| Acidity Type | γAl2O3 | 5% V/γAl2O3 | MgO-γAl2O3 | 5% V/MgO-γAl2O3 | 7.5% V/MgO-γAl2O3 | 10% V/MgO-γAl2O3 |
| Weak | 3.45 | 4.81 | 4.30 | 5.25 | 5.42 | 5.96 |
| Medium | 2.75 | 2.58 | 1.73 | 3.24 | 3.32 | 3.16 |
| Strong | 1.73 | 2.17 | -- | 0.97 | 1.68 | 0.80 |
| Very Strong | 0.73 | 0.82 | -- | 0.27 | 0.21 | 0.31 |
| Peak #1 (Low Temp) | Peak #2 | Peak #3 | Peak #4 (High Temp) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experiment | Tmax | Kdeso | Edes | Tmax | Kdeso | Edes | Tmax | Kdeso | Edes | Tmax | Kdeso | Edes |
| γAl2O3 | 471.3 | 1.90 | 36.43 | 559.2 | 1.41 | 62.09 | 651.4 | 0.84 | 86.05 | 745.9 | 0.32 | 113.15 |
| 5% V-γAl2O3 | 461.2 | 2.15 | 30.04 | 556.8 | 1.13 | 63.47 | 622.7 | 1.01 | 79.29 | 723.3 | 0.32 | 105.00 |
| MgO-γAl2O3 | 462.9 | 2.32 | 35.23 | 549.7 | 0.91 | 61.12 | -- | -- | -- | -- | -- | -- |
| 5% V/MgO-γAl2O3 | 460.9 | 2.91 | 34.26 | 554.2 | 1.28 | 59.52 | 633.1 | 0.37 | 80.87 | 780.6 | 0.10 | 133.25 |
| 7.5% V/MgO-γAl2O3 | 466.6 | 2.48 | 33.03 | 554.9 | 1.41 | 60.38 | 624.7 | 0.74 | 81.65 | 743.8 | 0.06 | 110.32 |
| 10% V/MgO-γAl2O3 | 460.5 | 3.18 | 32.77 | 561.0 | 1.29 | 59.80 | 649.1 | 0.32 | 87.06 | 789.4 | 0.12 | 140.29 |
| Catalyst Type | gCoke/gcat./Injection | gCoke/gbutane./Injection |
|---|---|---|
| 5% V/γAl2O3 | 0.00038 | 0.0377 |
| 5% V/MgO-γAl2O3 | 0.00022 | 0.0219 |
| Inject. | Selectivity (%) | X.C4H10 (%) | Y.C4H8 (%) | C4-Olefin/H2 (molar ratio) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CO | CH4 | CO2 | C2H4 | C2H6 | C3H6 | C3H8 | C4H8 | ||||
| 1 | 6.35 | 0.32 | 10.62 | 1.30 | 0.07 | 5.31 | 0.64 | 75.39 | 32.96 | 24.85 | 3.99 |
| 2 | 2.24 | 0.41 | 7.22 | 1.36 | 0.07 | 5.57 | 0.55 | 82.58 | 27.51 | 22.72 | 3.09 |
| 3 | 1.56 | 0.46 | 6.07 | 1.69 | 0.10 | 6.01 | 0.38 | 83.73 | 26.06 | 21.82 | 3.42 |
| 4 | 1.42 | 0.52 | 4.62 | 1.91 | 0.10 | 6.06 | 0.32 | 85.04 | 26.10 | 22.20 | 3.33 |
| 5 | 1.28 | 0.51 | 4.00 | 1.92 | 0.10 | 5.97 | 0.31 | 85.91 | 26.05 | 22.38 | 3.38 |
| 6 | 1.15 | 0.49 | 3.55 | 1.90 | 0.11 | 5.76 | 0.29 | 86.74 | 25.76 | 22.34 | 3.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bin Sulayman, A.; Torres Brauer, N.; de Lasa, H. A Fluidizable Catalyst for N-Butane Oxidative Dehydrogenation under Oxygen-Free Reaction Conditions. Catalysts 2023, 13, 1462. https://doi.org/10.3390/catal13121462
Bin Sulayman A, Torres Brauer N, de Lasa H. A Fluidizable Catalyst for N-Butane Oxidative Dehydrogenation under Oxygen-Free Reaction Conditions. Catalysts. 2023; 13(12):1462. https://doi.org/10.3390/catal13121462
Chicago/Turabian StyleBin Sulayman, Abdulhamid, Nicolas Torres Brauer, and Hugo de Lasa. 2023. "A Fluidizable Catalyst for N-Butane Oxidative Dehydrogenation under Oxygen-Free Reaction Conditions" Catalysts 13, no. 12: 1462. https://doi.org/10.3390/catal13121462