TiO2-Based Photocatalytic Building Material for Air Purification in Sustainable and Low-Carbon Cities: A Review
Abstract
:1. Introduction
2. Working Principles and Properties of TiO2-Based Photocatalytic Building Materials
2.1. The Basic Principal Mechanism of Photocatalysts
2.2. The Mechanism of Photocatalysts for Air Purification and Deodorization
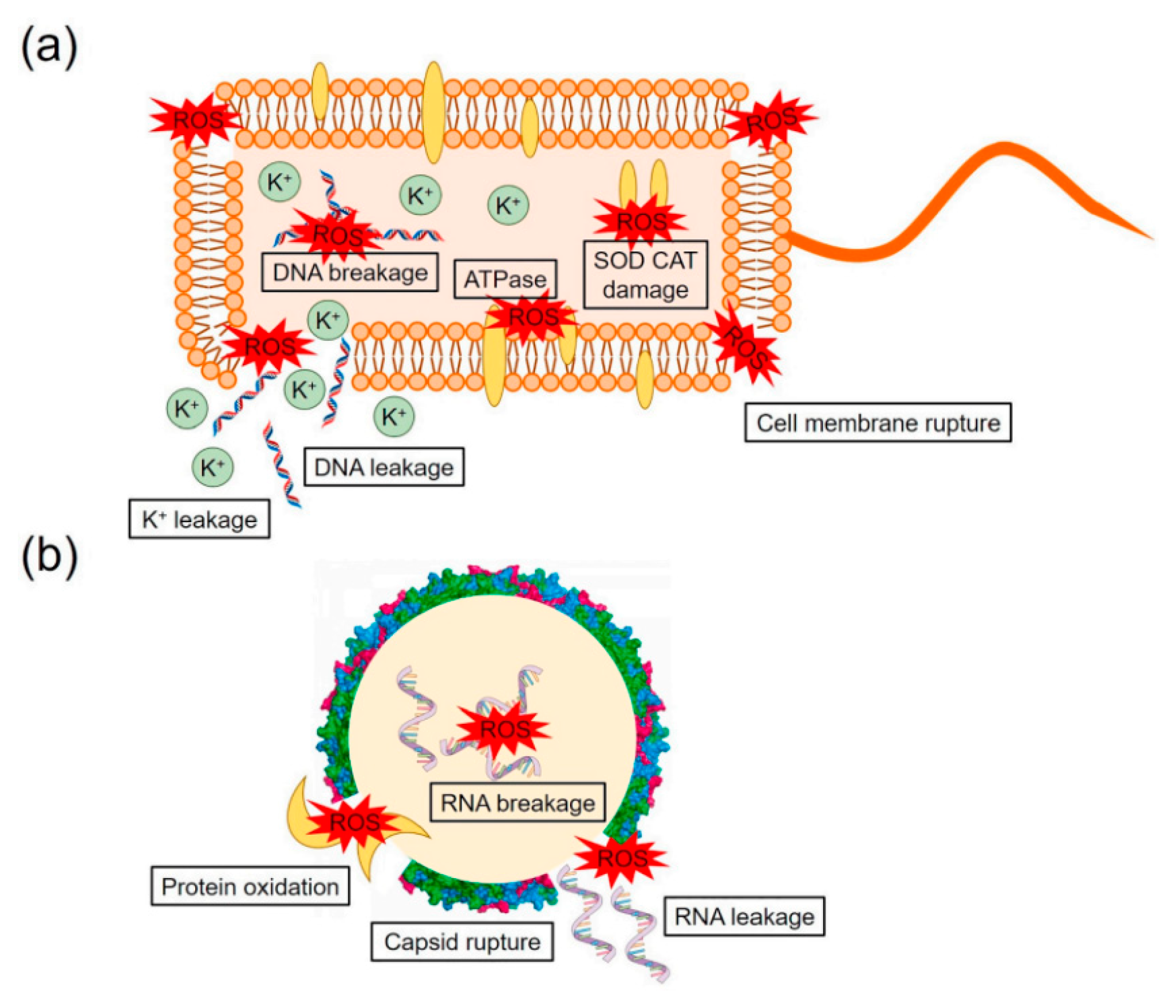
2.3. The Photocatalytic Mechanism of Disinfection
3. The Status of TiO2-Based Photocatalytic Building Materials
| Building Material | Method | Light Source | Efficiency | Reference/Year |
|---|---|---|---|---|
| cement mortar | Mixing with cement mortar | UV | The degradation rate of NOX can reach 40.0% | 2009 [27] |
| cement mortar | Mix with mortar (2 and 5 wt%) | UV | NO (400 ppb) removal rate: 90 μ·mol/(m2·h) Toluene (200 ppb) removal: 100% | 2011 [28] |
| ceramic tiles | Photocatalyst brushing on the top surface of tiles | UV | Toluene (17–35 ppbv) removal rate up to 512 μ·g/(m2·h) | 2008 [26] |
| cement mortar | Mix with mortar (1–10% wt%) | UV | Formaldehyde (20 ppm) removal rate up to 65% | 2011 [29] |
| portland cement | Mix with cement slurry (0.5–5 wt%.) | UV | NOX (1 ppmv) removal amount: 120 μmol/m2, 65 h | 2012 [24] |
| Wall paint and plaster | Mixing with 2 wt% TiO2 | UV | NOX (400 ppb) conversion range ranges from 80% of 50 days samples to 30% of 1-year samples | 2011 [30] |
| cement mortar | Mixed cement (0.5–2.5 wt%) | Simulated sunlight | The removal rate of NO (1 ppm) can reach 15% | 2014 [31] |
| cement mortar | Mixing with cement mortar | UV | The degradation efficiency of NOX (1000 ppb) can reach 60.4% | 2015 [32] |
| cement mortar | Combine photocatalytic materials with building materials using mixing and spraying methods, respectively | UV | NO (1000 ppb) removal condition: Material for spraying method: 220 μ·Mol/(m2·Å·h), mixed material: 80 μ·mol/(m2·h) | 2017 [33] |
| cement mortar | Mix with cement mortar (0.5~2.5 wt%) | UV Sunlight Visible light | The highest conversion rates of NO (500 ppb) are 38% (P25), 15% (P25), and 5.5% (Fe TiO2 and V-TiO2), respectively | 2017 [34] |
| cement mortar | Mixing with cement mortar (1–10 wt%) | UV | NO (1 ppm) removal rate: 72% | 2017 [35] |
| Concrete and gypsum | Coating deposited on the test concrete wall | Sunlight | Efficient removal of NOX from polluted air. | 2017 [36] |
| White cement (WC) and ordinary Portland cement paste | Mixed cement slurry (2–5 wt%) | UV | NO (1000 ppb) removal condition: OPC is 380 μ·mol/(m2·h) and WC at 500 μ·mol/(m2·h) | 2018 [37] |
4. Preparation of TiO2 Photocatalytic Building Materials
4.1. Sol–Gel Method
4.2. Hydrothermal Method
4.3. Spray Drying Method
4.4. Anodic Oxidation Method
4.5. Microwave-Assisted Method
5. Strategies for Improving TiO2’s Photocatalytic Efficiency
5.1. Strategies for Reducing Aggregation of TiO2
5.2. Strategies for Improving the Photocatalytic Efficiency of TiO2
6. Application Status and Prospects of TiO2-Based Building Materials
6.1. Application Status and Key Influencing Factors of Photocatalytic Building Materials
6.2. Future Perspective and Related Problem Discussions
- Enhanced stability of the photocatalytic materials: The stability of the photocatalytic materials is crucial for their practical use, necessitating a comprehensive investigation into the effects of prolonged exposure to light and environmental factors on their lifetime. Humidity, temperature, pH, pollutants, and microorganisms can significantly impact the stability of these materials. Additionally, it is important to consider that photocatalytic materials may also induce degradation in the substrates or binders they are attached to, leading to reduced mechanical strength and durability [80]. Therefore, it is imperative to develop strategies aimed at augmenting both the stability of photocatalytic materials themselves and that of their associated substrates or binders to ensure long-term performance.
- Photocatalytic reaction rate: The photocatalytic reaction rate is a crucial factor influencing the real-world use of photocatalytic building materials. It is imperative to ensure that the reaction rate is sufficiently rapid to degrade the desired harmful substances. Therefore, it becomes necessary to explore diverse mechanisms governing photocatalytic reactions to enhance the reaction rate. Several factors such as the light intensity, wavelength, catalyst loading, surface area, morphology, crystallinity, doping, and co-catalysts may influence the reaction rate [4]. Additionally, the type and concentration of pollutant along with other interfering substances also impacts the reaction rate. Consequently, optimizing these parameters becomes indispensable for achieving high efficiency and selectivity in photocatalysis.
- Selectivity of photocatalytic materials: The selectivity of photocatalytic materials refers to their capacity for the targeted oxidation or reduction of specific pollutants in the presence of other substances [81]. Achieving high selectivity is crucial for enhancing efficiency and minimizing unwanted by-products or secondary pollution during photocatalysis. However, most existing photocatalytic materials exhibit low selectivity and tend to react with a variety of organic and inorganic compounds present in the air [82]. This can lead to reduced photocatalytic activity and increased energy consumption. Therefore, a key challenge lies in designing and modifying photocatalytic materials with enhanced selectivity.
- The economics of photocatalytic materials encompass their cost-effectiveness and feasibility in terms of production and application. Factors influencing the cost include the type and quantity of raw materials, synthesis method, fabrication process, scale-up potential, and maintenance expenses. The benefits are contingent upon the photocatalytic material’s performance, durability, environmental impact, and social acceptance [58]. Therefore, it is crucial to evaluate and optimize the economic aspects of photocatalytic materials so they can be widely adopted and used for air pollution prevention.
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kilian, J.; Kitazawa, M. The emerging risk of exposure to air pollution on cognitive decline and Alzheimer’s disease—Evidence from epidemiological and animal studies. Biomed. J. 2018, 41, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Stanaszek-Tomal, E. Anti-smog building and civil engineering structures. Processes 2021, 9, 1446. [Google Scholar] [CrossRef]
- Majbauddin, A.; Onishi, K.; Otani, S.; Kurosaki, Y.; Kurozawa, Y. Association between asian dust-Borne air pollutants and daily symptoms on healthy subjects: A web-based pilot study in Yonago, Japan. J. Environ. Public. Health 2016, 2016, 8280423. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Jeon, W.; Choi, W. Photocatalytic air purification mimicking the self-cleaning process of the atmosphere. Nat. Commun. 2021, 12, 2528. [Google Scholar] [CrossRef] [PubMed]
- Saputera, W.H.; Amri, A.F.; Daiyan, R.; Sasongko, D. Photocatalytic technology for Palm Oil Mill Effluent (POME) wastewater treatment: Current Progress and Future Perspective. Materials 2021, 14, 2846. [Google Scholar] [CrossRef]
- Al-Nuaim, M.A.; Alwasiti, A.A.; Shnain, Z.Y. The photocatalytic process in the treatment of polluted water. Chem. Pap. 2023, 77, 677–701. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C.-s. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Gopalan, A.I.; Lee, J.C.; Saianand, G.; Lee, K.P.; Sonar, P.; Dharmarajan, R.; Hou, Y.L.; Ann, K.Y.; Kannan, V.; Kim, W.J. Recent progress in the abatement of hazardous pollutants using photocatalytic TiO2-based building materials. Nanomaterials 2020, 10, 1854. [Google Scholar] [CrossRef]
- Domínguez-Espíndola, R.B.; Arias, D.M.; Rodríguez-González, C.; Sebastian, P.J. A critical review on advances in TiO2-based photocatalytic systems for CO2 reduction. Appl. Therm. Eng. 2022, 216, 119009. [Google Scholar] [CrossRef]
- Poudel, M.B.; Yu, C.; Kim, H.J. Synthesis of conducting bifunctional polyaniline@Mn-TiO2 nanocomposites for supercapacitor electrode and visible light driven photocatalysis. Catalysts 2020, 10, 546. [Google Scholar] [CrossRef]
- Liu, C.; Bai, J.; Zhang, S.; Yang, Z.; Luo, M. Applications and advances in TiO2 based photocatalytic building materials. J. Phys. Conf. Ser. 2021, 2011, 012049. [Google Scholar] [CrossRef]
- Yuan, T.; Yao, W. Preparation and properties of g-C3N4-TiO2 cement-based materials supported by recycled concrete pow. Catalysts 2023, 13, 312. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, Q.; Meng, H.; Zhang, Y.; Cao, C. Recent advances in photocatalytic self-cleaning performances of TiO2-based building materials. RSC Adv. 2023, 13, 20584–20597. [Google Scholar] [CrossRef]
- Kumer, A.; Chakma, U. Developing the amazing photocatalyst of ZnAg2GeSe4, ZnAg2Ge0.93Fe0.07Se4 and ZnAg2Ge0.86Fe0.14Se4 through the computational explorations by four DFT functionals. Heliyon 2021, 7, e07467. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2017, 334, 2408–2439. [Google Scholar] [CrossRef]
- Ma, J.; Wang, C.; He, H. Enhanced photocatalytic oxidation of NO over g-C3N4-TiO2 under UV and visible light. Appl. Catal. B Environ. 2016, 184, 28–34. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, D.; Zhang, W. Effects of substrates on N2O emissions in an anaerobic ammonium oxidation (anammox) reactor. SpringerPlus 2016, 5, 741. [Google Scholar] [CrossRef]
- Razavi, Z.; Mirghaffari, N.; Alemrajabi, A.A.; Davar, F.; Soleimani, M. Adsorption and photocatalytic removal of SO2 using natural and synthetic zeolites-supported TiO2 in a solar parabolic trough collector. J. Clean. Prod. 2021, 310, 127376. [Google Scholar] [CrossRef]
- Mendoza, J.A.; Lee, D.H.; Kim, L.H.; Kim, I.H.; Kang, J.H. Photocatalytic performance of TiO2 and WO3/TiO2 nanoparticles coated on urban green infrastructure materials in removing nitrogen oxide. Int. J. Environ. Sci. Technol. 2017, 15, 581–592. [Google Scholar] [CrossRef]
- Yousefi, A.; Allahverdi, A.; Hejazi, P. Effective dispersion of nano-TiO2 powder for enhancement of photocatalytic properties in cement mixes. Constr. Build. Mater. 2013, 41, 224–230. [Google Scholar] [CrossRef]
- Igenepo John, K.; Abdul Adenle, A.; Timothy Adeleye, A.; Pearl Onyia, I.; Amune-Matthews, C.; Omorogie, M.O. Unravelling the effect of crystal dislocation density and microstrain of titanium dioxide nanoparticles on tetracycline removal performance. Chem. Phys. Lett. 2021, 776, 138725. [Google Scholar] [CrossRef]
- Cárdenas, C.; Tobón, J.I.; García, C.; Vila, J. Functionalized building materials: Photocatalytic abatement of NOX by cement pastes blended with TiO2 nanoparticles. Constr. Build. Mater. 2012, 36, 820–825. [Google Scholar] [CrossRef]
- Poon, C.S.; Cheung, E. NO removal efficiency of photocatalytic paving blocks prepared with recycled materials. Constr. Build. Mater. 2007, 21, 1746–1753. [Google Scholar] [CrossRef]
- Demeestere, K.; Dewulf, J.; De Witte, B.; Beeldens, A.; Van Langenhove, H. Heterogeneous photocatalytic removal of toluene from air on building materials enriched with TiO2. Build. Environ. 2008, 43, 406–414. [Google Scholar] [CrossRef]
- Hüsken, G.; Hunger, M.; Brouwers, H.J.H. Experimental study of photocatalytic concrete products for air purification. Build. Environ. 2009, 44, 2463–2474. [Google Scholar] [CrossRef]
- Chen, J.; Kou, S.-c.; Poon, C.-s. Photocatalytic cement-based materials: Comparison of nitrogen oxides and toluene removal potentials and evaluation of self-cleaning performance. Build. Environ. 2011, 46, 1827–1833. [Google Scholar] [CrossRef]
- Aïssa, A.H.; Puzenat, E.; Plassais, A.; Herrmann, J.-M.; Haehnel, C.; Guillard, C. Characterization and photocatalytic performance in air of cementitious materials containing TiO2. Case study of formaldehyde removal. Appl. Catal. B Environ. 2011, 107, 1–8. [Google Scholar] [CrossRef]
- Pirola, C.; Boffito, D.C.; Vitali, S.; Bianchi, C.L. Photocatalytic coatings for building industry: Study of 1 year of activity in the NO x degradation. J. Coat. Technol. Res. 2011, 9, 453–458. [Google Scholar] [CrossRef]
- Karapati, S.; Giannakopoulou, T.; Todorova, N.; Boukos, N.; Antiohos, S.; Papageorgiou, D.; Chaniotakis, E.; Dimotikali, D.; Trapalis, C. TiO2 functionalization for efficient NOX removal in photoactive cement. Appl. Surf. Sci. 2014, 319, 29–36. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Balbuena, J.; Cruz-Yusta, M.; Sánchez, L.; Navarro-Blasco, I.; Fernández, J.M.; Alvarez, J.I. Photocatalytic NOX abatement by calcium aluminate cements modified with TiO2: Improved NO2 conversion. Cem. Concr. Res. 2015, 70, 67–76. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Ling, T.-C.; Poon, C.S. Photocatalytic NOX degradation of concrete surface layers intermixed and spray-coated with nano-TiO2: Influence of experimental factors. Cem. Concr. Compos. 2017, 83, 279–289. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Navarro-Blasco, I.; Fernández, J.M.; Alvarez, J.I. Atmospheric NOX removal: Study of cement mortars with iron- and vanadium-doped TiO2 as visible light–sensitive photocatalysts. Constr. Build. Mater. 2017, 149, 257–271. [Google Scholar] [CrossRef]
- Seo, D.; Yun, T.S. NOX removal rate of photocatalytic cementitious materials with TiO2 in wet condition. Build. Environ. 2017, 112, 233–240. [Google Scholar] [CrossRef]
- Zouzelka, R.; Rathousky, J. Photocatalytic abatement of NOX pollutants in the air using commercial functional coating with porous morphology. Appl. Catal. B Environ. 2017, 217, 466–476. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Poon, C.S. Superior photocatalytic NOX removal of cementitious materials prepared with white cement over ordinary Portland cement and the underlying mechanisms. Cem. Concr. Compos. 2018, 90, 42–49. [Google Scholar] [CrossRef]
- Cano-Casanova, L.; Amoros-Perez, A.; Lillo-Rodenas, M.A.; Roman-Martinez, M.D.C. Effect of the preparation method (sol-gel or hydrothermal) and conditions on the TiO2 properties and activity for propene oxidation. Materials 2018, 11, 2227. [Google Scholar] [CrossRef]
- Macwan, D.P.; Dave, P.N.; Chaturvedi, S. A review on nano-TiO2 sol–gel type syntheses and its applications. J. Mater. Sci. 2011, 46, 3669–3686. [Google Scholar] [CrossRef]
- Huang, L.; Liu, T.; Zhang, H.; Guo, W.; Zeng, W. Hydrothermal synthesis of different TiO2 nanostructures: Structure, growth and gas sensor properties. J. Mater. Sci. Mater. Electron. 2012, 23, 2024–2029. [Google Scholar] [CrossRef]
- Zeng, B.; Zeng, W. Hydrothermal synthesis and gas sensing property of titanium dioxide regular nano-polyhedron with reactive (001) facets. J. Mater. Sci. Mater. Electron. 2017, 28, 13821–13828. [Google Scholar] [CrossRef]
- Jaworski, R.; Pawlowski, L.; Pierlot, C.; Roudet, F.; Kozerski, S.; Petit, F. Recent developments in suspension plasma sprayed titanium oxide and hydroxyapatite coatings. J. Therm. Spray Technol. 2009, 19, 240–247. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, L.; Shao, M.; Huang, J.; Ding, M.; Deng, X.; Wei, X.; Xu, X. Anodic oxidation synthesis of one-dimensional TiO2 nanostructures for photocatalytic and field emission properties. J. Nanomater. 2014, 2014, 831752. [Google Scholar] [CrossRef]
- Ge, M.; Li, Q.; Cao, C.; Huang, J.; Li, S.; Zhang, S.; Chen, Z.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. One-dimensional TiO2 nanotube photocatalysts for solar water splitting. Adv. Sci. 2017, 4, 1600152. [Google Scholar] [CrossRef] [PubMed]
- Falk, G.S.; Borlaf, M.; López-Muñoz, M.J.; Fariñas, J.C.; Rodrigues Neto, J.B.; Moreno, R. Microwave-assisted synthesis of TiO2 nanoparticles: Photocatalytic activity of powders and thin films. J. Nanopart. Res. 2018, 20, 23. [Google Scholar] [CrossRef]
- Yu, Y.-H.; Chen, Y.-P.; Cheng, Z. Microwave-assisted synthesis of rod-like CuO/TiO2 for high-efficiency photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2015, 40, 15994–16000. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Recent progress in enhancing photocatalytic efficiency of TiO2-based materials. Appl. Catal. A Gen. 2015, 495, 131–140. [Google Scholar] [CrossRef]
- Liao, C.; Wu, Q.; Su, T.; Zhang, D.; Wu, Q.; Wang, Q. Nanocomposite gels via in situ photoinitiation and disassembly of TiO2-clay composites with polymers applied as UV protective films. ACS Appl. Mater. Interfaces 2014, 6, 1356–1360. [Google Scholar] [CrossRef]
- Ibusuki, T.; Takeuchi, K. Removal of low concentration nitrogen oxides through photoassisted heterogeneous catalysis. J. Mol. Catal. 1994, 88, 93–102. [Google Scholar] [CrossRef]
- Resende, S.F.; Nunes, E.H.M.; Houmard, M.; Vasconcelos, W.L. Simple sol-gel process to obtain silica-coated anatase particles with enhanced TiO2-SiO2 interfacial area. J. Colloid. Interface Sci. 2014, 433, 211–217. [Google Scholar] [CrossRef]
- Ghedini, E.; Menegazzo, F.; Manzoli, M.; Di Michele, A.; Puglia, D.; Signoretto, M. Multifunctional and environmentally friendly TiO2-SiO2 mesoporous materials for sustainable green buildings. Molecules 2019, 24, 4226. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Luo, Y.; Hooper, M.; Hu, E. Removal of VOCs by photocatalysis process using adsorption enhanced TiO2–SiO2 catalyst. Chem. Eng. Process. Process Intensif. 2006, 45, 959–964. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, S.; Yang, S.; Wu, L. Fabrication of all-water-based self-Repairing superhydrophobic coatings based on UV-responsive microcapsules. Adv. Funct. Mater. 2015, 25, 1035–1041. [Google Scholar] [CrossRef]
- Joo, J.B.; Zhang, Q.; Lee, I.; Dahl, M.; Zaera, F.; Yin, Y. Mesoporous anatase titania hollow nanostructures though silica-protected calcination. Adv. Funct. Mater. 2012, 22, 166–174. [Google Scholar] [CrossRef]
- Yang, C.C.; Wang, C.X.; Kuan, C.Y.; Chi, C.Y.; Chen, C.Y.; Lin, Y.Y.; Chen, G.S.; Hou, C.H.; Lin, F.H. Using C-doped TiO2 nanoparticles as a novel sonosensitizer for cancer treatment. Antioxidants 2020, 9, 880. [Google Scholar] [CrossRef]
- Zhao, X.; Ju, W.; Zhang, J.; Liu, B.; Zhang, J.; Yi, X. Mesoporous TiO2/SiO2/Ag ternary composite aerogels for high photocatalysis. New J. Chem. 2019, 43, 6234–6241. [Google Scholar] [CrossRef]
- Raza, N.; Raza, W.; Gul, H.; Azam, M.; Lee, J.; Vikrant, K.; Kim, K.H. Solar-light-active silver phosphate/titanium dioxide/silica heterostructures for photocatalytic removal of organic dye. J. Clean. Prod. 2020, 254, 120031. [Google Scholar] [CrossRef]
- Zhang, W.; Li, G.; Liu, H.; Chen, J.; Ma, S.; An, T. Micro/nano-bubble assisted synthesis of Au/TiO2@CNTs composite photocatalyst for photocatalytic degradation of gaseous styrene and its enhanced catalytic mechanism. Environ. Sci. Nano 2019, 6, 948–958. [Google Scholar] [CrossRef]
- Liang, Z.; Bai, X.; Hao, P.; Guo, Y.; Xue, Y.; Tian, J.; Cui, H. Full solar spectrum photocatalytic oxygen evolution by carbon-coated TiO2 hierarchical nanotubes. Appl. Catal. B Environ. 2019, 243, 711–720. [Google Scholar] [CrossRef]
- Nguyen, K.C.; Ngoc, M.P.; Nguyen, M.V. Enhanced photocatalytic activity of nanohybrids TiO2/CNTs materials. Mater. Lett. 2016, 165, 247–251. [Google Scholar] [CrossRef]
- Wang, S.G.; Du, Z.R.; Kong, C.X.; Li, P.F.; Xu, J.L.; Wang, C.X.; Wang, J.H. Photocatalytic properties of TiO2/CNTs films with different morphology on stainless steel substrates. Nano 2014, 9, 1450003. [Google Scholar] [CrossRef]
- Olana, M.H.; Sabir, F.K.; Bekele, E.T.; Gonfa, B.A. Citrus sinensis and musa acuminata peel waste extract mediated synthesis of TiO2/rGO nanocomposites for photocatalytic degradation of methylene blue under visible light irradiation. Bioinorg. Chem. Appl. 2022, 2022, 5978707. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhao, Y.; Liu, S.; Korzeniewski, C.L.; Wang, S.; Fan, Z. Comparing graphene-TiO2 nanowire and graphene-TiO2 nanoparticle composite photocatalysts. ACS Appl. Mater. Interfaces 2012, 4, 3944–3950. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Luo, S.; Teng, Y.; Liu, C.; Xu, X.; Zhang, X.; Chen, L. Efficient removal of herbicide 2,4-dichloropheNOXyacetic acid from water using Ag/reduced graphene oxide co-decorated TiO2 nanotube arrays. J. Hazard. Mater. 2012, 241–242, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Lee, C.W.; Lu, X.; Sun, Y.; Hua, W.; Zhuang, G.; Zhang, S.; Chen, J.; Hou, H.; Zhao, D. Synchronous role of coupled adsorption and photocatalytic oxidation on ordered mesoporous anatase TiO2–SiO2 nanocomposites generating excellent degradation activity of RhB dye. Appl. Catal. B Environ. 2010, 95, 197–207. [Google Scholar] [CrossRef]
- Dholam, R.; Patel, N.; Adami, M.; Miotello, A. Hydrogen production by photocatalytic water-splitting using Cr- or Fe-doped TiO2 composite thin films photocatalyst. Int. J. Hydrogen Energy 2009, 34, 5337–5346. [Google Scholar] [CrossRef]
- Liu, M.; Qiu, X.; Miyauchi, M.; Hashimoto, K. Energy-level matching of Fe(III) ions grafted at surface and doped in bulk for efficient visible-light photocatalysts. J. Am. Chem. Soc. 2013, 135, 10064–10072. [Google Scholar] [CrossRef]
- Liu, M.; Inde, R.; Nishikawa, M.; Qiu, X.; Atarashi, D.; Sakai, E.; Nosaka, Y.; Hashimoto, K.; Miyauchi, M. Enhanced photoactivity with nanocluster-grafted titanium dioxide photocatalysts. ACS Nano 2014, 8, 7229–7238. [Google Scholar] [CrossRef]
- Milan Babu Poudel, Allison A Kim, Silver nanoparticles decorated TiO2 nanoflakes for antibacterial properties. Appl. Inorg. Chem. Commun. 2023, 152, 110675. [CrossRef]
- Hajjaji, A.; Labidi, A.; Gaidi, M.; Rabha, M.B.; Smirani, R.; Bejaoui, A.; Bessais, B.; Khakani, M.A.E. Structural, optical and sensing properties of Cr-doped TiO2 thin films. Sens. Lett. 2011, 9, 1697–1703. [Google Scholar] [CrossRef]
- Preethi, L.K.; Antony, R.P.; Mathews, T.; Walczak, L.; Gopinath, C.S. A study on doped heterojunctions in TiO2 nanotubes: An efficient photocatalyst for solar water splitting. Sci. Rep. 2017, 7, 14314. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Baglee, D.; Chu, J.W.; Du, D.; Guo, X. Photocatalytic oxidation of NOX under visible light on asphalt-pavement surface. J. Mater. Civ. Eng. 2017, 29, 04017133. [Google Scholar] [CrossRef]
- Khan, T.; Bari, G.; Kang, H.-J.; Lee, T.-G.; Park, J.-W.; Hwang, H.; Hossain, S.; Mun, J.; Suzuki, N.; Fujishima, A.; et al. Synthesis of N-doped TiO2 for efficient photocatalytic degradation of atmospheric NOX. Catalysts 2021, 11, 109. [Google Scholar] [CrossRef]
- Barolo, G.; Livraghi, S.; Chiesa, M.; Paganini, M.C.; Giamello, E. Mechanism of the photoactivity under visible light of N-doped titanium dioxide. Charge carriers migration in irradiated N-TiO2 investigated by electron paramagnetic resonance. J. Phys. Chem. C 2012, 116, 20887–20894. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Xing, M.; Leghari, S.A.K.; Sajjad, S. Development of modified N doped TiO2 photocatalyst with metals, nonmetals and metal oxides. Energy Environ. Sci. 2010, 3, 715–726. [Google Scholar] [CrossRef]
- Mancuso, A.; Sacco, O.; Sannino, D.; Pragliola, S.; Vaiano, V. Enhanced visible-light-driven photodegradation of Acid Orange 7 azo dye in aqueous solution using Fe-N co-doped TiO2. Arab. J. Chem. 2020, 13, 8347–8360. [Google Scholar] [CrossRef]
- Weerasinghe, R.; Li, Y.; Fu, Q.; Yu, J.; Fang, C. Study on the effect of Fe and N co-doped supported TiO2/GF photocatalytic oxidation of nitrobenzene wastewater. E3S Web Conf. 2021, 237, 01036. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Y.; Zhu, S.; Li, D. TiN nanoparticles hybridized with Fe, N co-doped carbon nanosheets composites as highly efficient electrocatalyst for oxygen reduction reaction. Chem. Eng. J. 2020, 400, 125968. [Google Scholar] [CrossRef]
- Hayati, F.; Khodabakhshi, M.R.; Isari, A.A.; Moradi, S.; Kakavandi, B. LED-assisted sonocatalysis of sulfathiazole and pharmaceutical wastewater using N,Fe co-doped TiO2@SWCNT: Optimization, performance and reaction mechanism studies. J. Water Process Eng. 2020, 38, 101693. [Google Scholar] [CrossRef]
- Chew, M.Y.L.; Conejos, S.; Law, J.S.L. Green maintainability design criteria for nanostructured titanium dioxide (TiO2) façade coatings. Int. J. Build. Pathol. Adapt. 2017, 35, 139–158. [Google Scholar] [CrossRef]
- Tsukamoto, D.; Ikeda, M.; Shiraishi, Y.; Hara, T.; Ichikuni, N.; Tanaka, S.; Hirai, T. Selective photocatalytic oxidation of alcohols to aldehydes in water by TiO2 partially coated with WO3. Chemistry 2011, 17, 9816–9824. [Google Scholar] [CrossRef] [PubMed]
- Pastor, A.; Balbuena, J.; Cruz-Yusta, M.; Pavlovic, I.; Sánchez, L. ZnO on rice husk: A sustainable photocatalyst for urban air purification. Chem. Eng. J. 2019, 368, 659–667. [Google Scholar] [CrossRef]



| Building Name | Location | Building Material | Benefits | Difficulties |
|---|---|---|---|---|
| Palazzo Italia | Milan, Italy | TiO2-based photocatalytic coating on façade | Purifies air, reduces carbon emissions, energy-efficient design, use of renewable energy sources | Cost of installation and maintenance |
| Jubilee Church | Rome, Italy | TiO2-coated façade | Reduces air pollution, improves air quality by breaking down harmful pollutants | Limited effectiveness in high-traffic areas |
| Palazzo Lombardia | Milan, Italy | TiO2-coated façade | Purifies air, reduces energy consumption by reflecting sunlight and reducing need for air conditioning | Cost of installation and maintenance |
| Bullitt Center | Seattle, USA | TiO2-coated roof | Purifies air, reduces air pollution by breaking down harmful pollutants | Limited effectiveness in high-traffic areas |
| Denby Dale Passivhaus | Yorkshire, UK | TiO2-coated façade | Purifies air, reduces air pollution, reduces energy consumption for heating and cooling | Cost of installation and maintenance |
| Edificio Malecon | Mexico City, Mexico | TiO2-coated façade | Reduces air pollution, improves air quality, self-cleaning properties, reduces energy consumption | Cost of installation and maintenance |
| Haze-Free Tower | Beijing, China | TiO2-coated façade | Reduces air pollution, improves air quality, enhances aesthetics, self-cleaning properties | Limited effectiveness in high-traffic areas |
| Queen’s Building | Bristol, UK | TiO2-coated façade | Purifies air, reduces air pollution, self-cleaning properties | Limited effectiveness in shaded areas |
| Nanjing Green Lighthouse | Nanjing, China | TiO2-coated façade | Purifies air, reduces energy consumption, improves air quality, self-cleaning properties | Cost of installation and maintenance |
| LaFargeHolcim Headquarters | Switzerland | TiO2-coated façade | Reduces air pollution, self-cleaning properties, improves energy efficiency | Limited effectiveness in high-pollution areas |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Meng, H.; Wu, Q.; Bai, X.; Zhang, Y. TiO2-Based Photocatalytic Building Material for Air Purification in Sustainable and Low-Carbon Cities: A Review. Catalysts 2023, 13, 1466. https://doi.org/10.3390/catal13121466
Wei Y, Meng H, Wu Q, Bai X, Zhang Y. TiO2-Based Photocatalytic Building Material for Air Purification in Sustainable and Low-Carbon Cities: A Review. Catalysts. 2023; 13(12):1466. https://doi.org/10.3390/catal13121466
Chicago/Turabian StyleWei, Yuanchen, Hong Meng, Que Wu, Xiaoyu Bai, and Yongqing Zhang. 2023. "TiO2-Based Photocatalytic Building Material for Air Purification in Sustainable and Low-Carbon Cities: A Review" Catalysts 13, no. 12: 1466. https://doi.org/10.3390/catal13121466





