Catalytic Reduction of N2O by CO on Single-Atom Catalysts Au/C2N and Cu/C2N: A First-Principles Study
Abstract
:1. Introduction
2. Results and Discussion
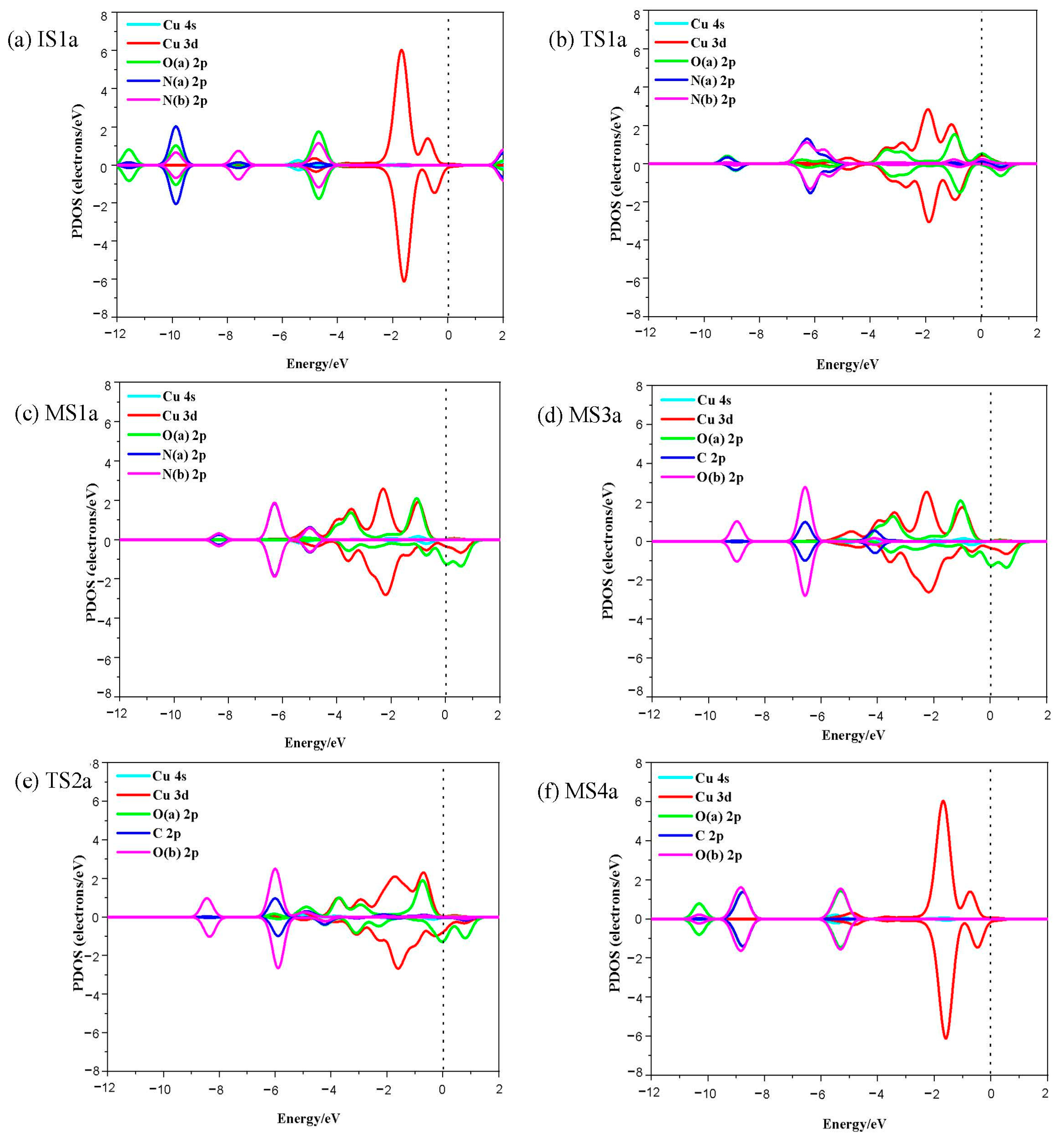
2.1. Geometric and Electronic Properties of Cu/C2N and Au/C2N
2.2. Mechanism of Catalytic N2O Reduction
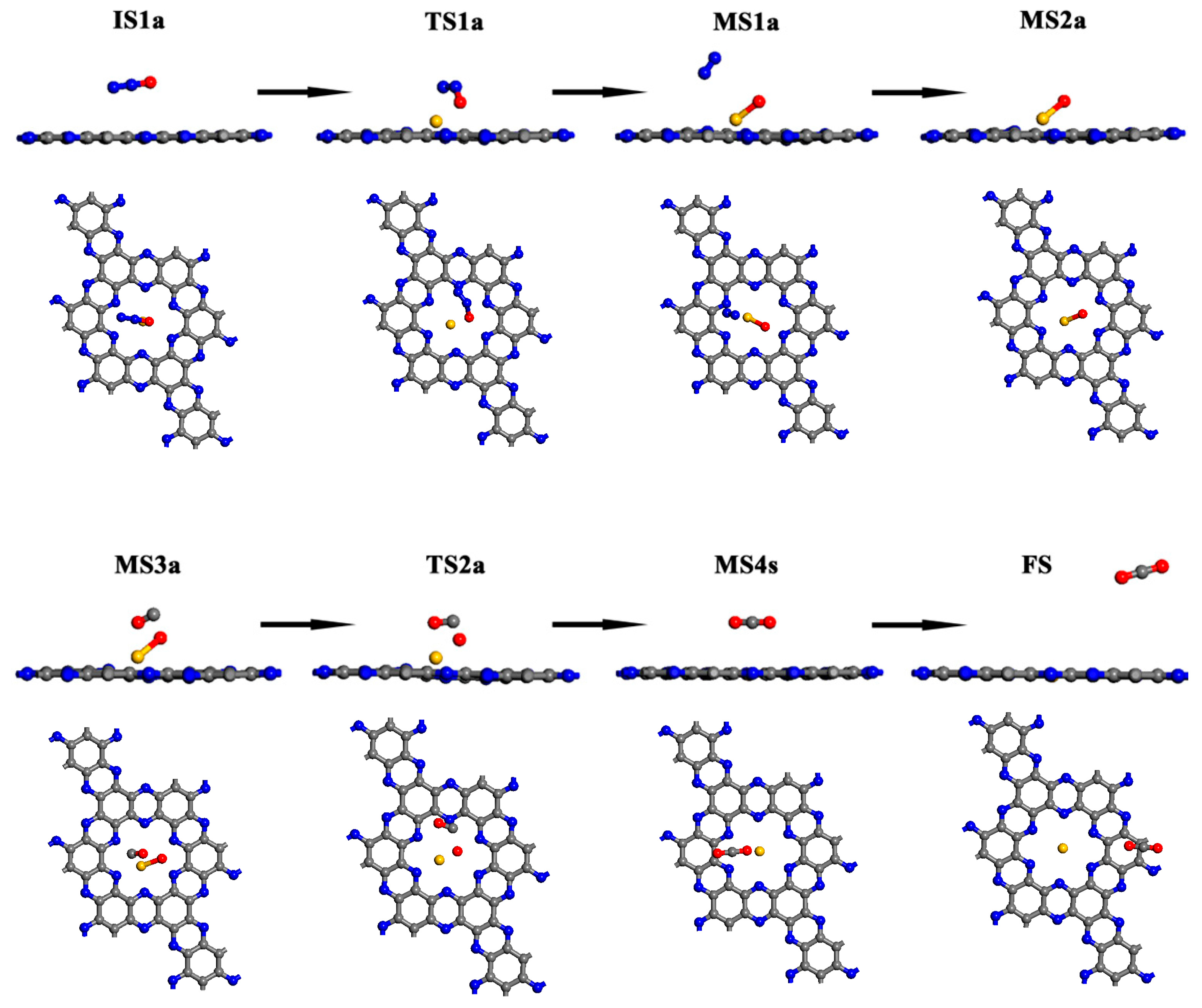
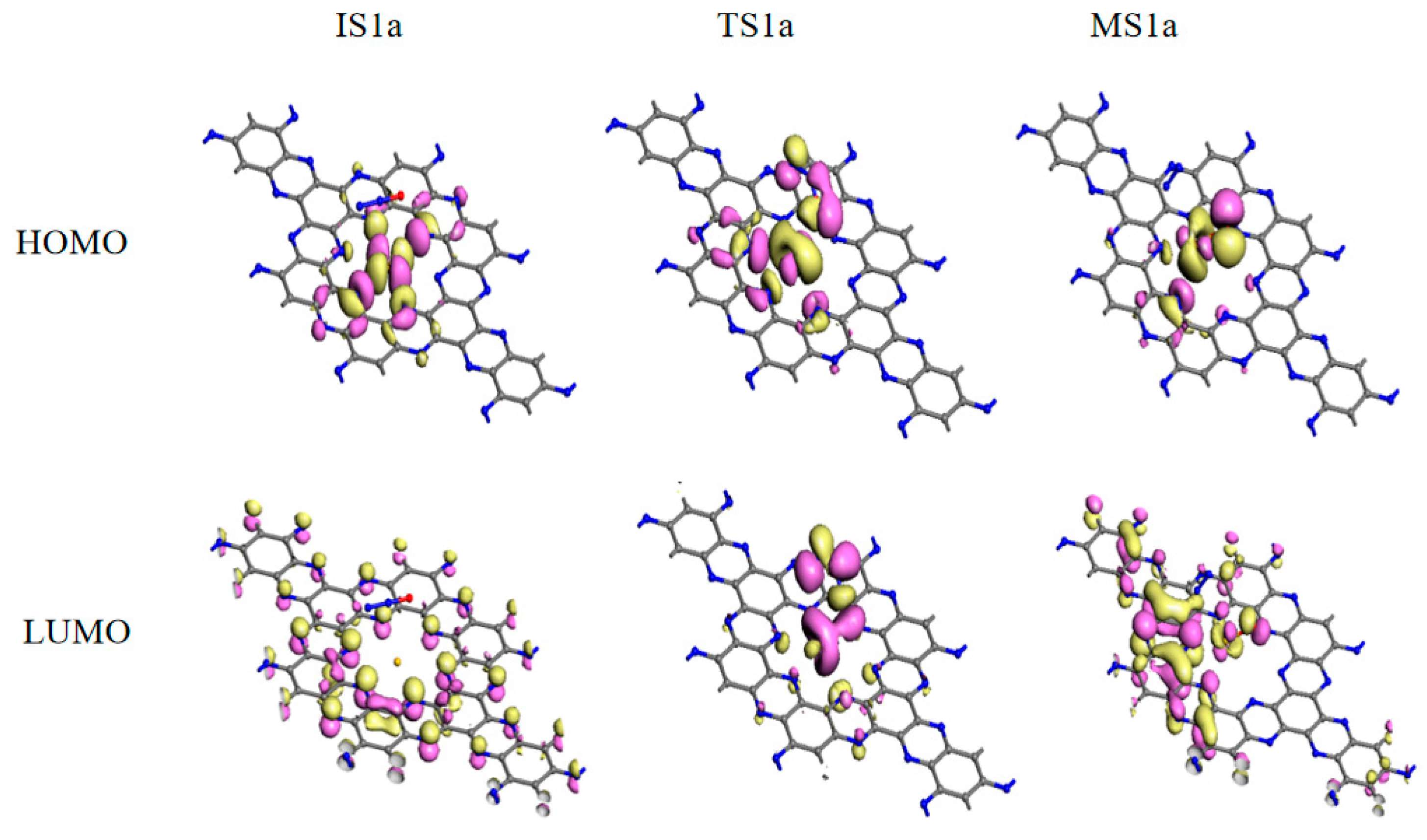
2.2.1. Mechanism I: N2O Adsorption Mechanism
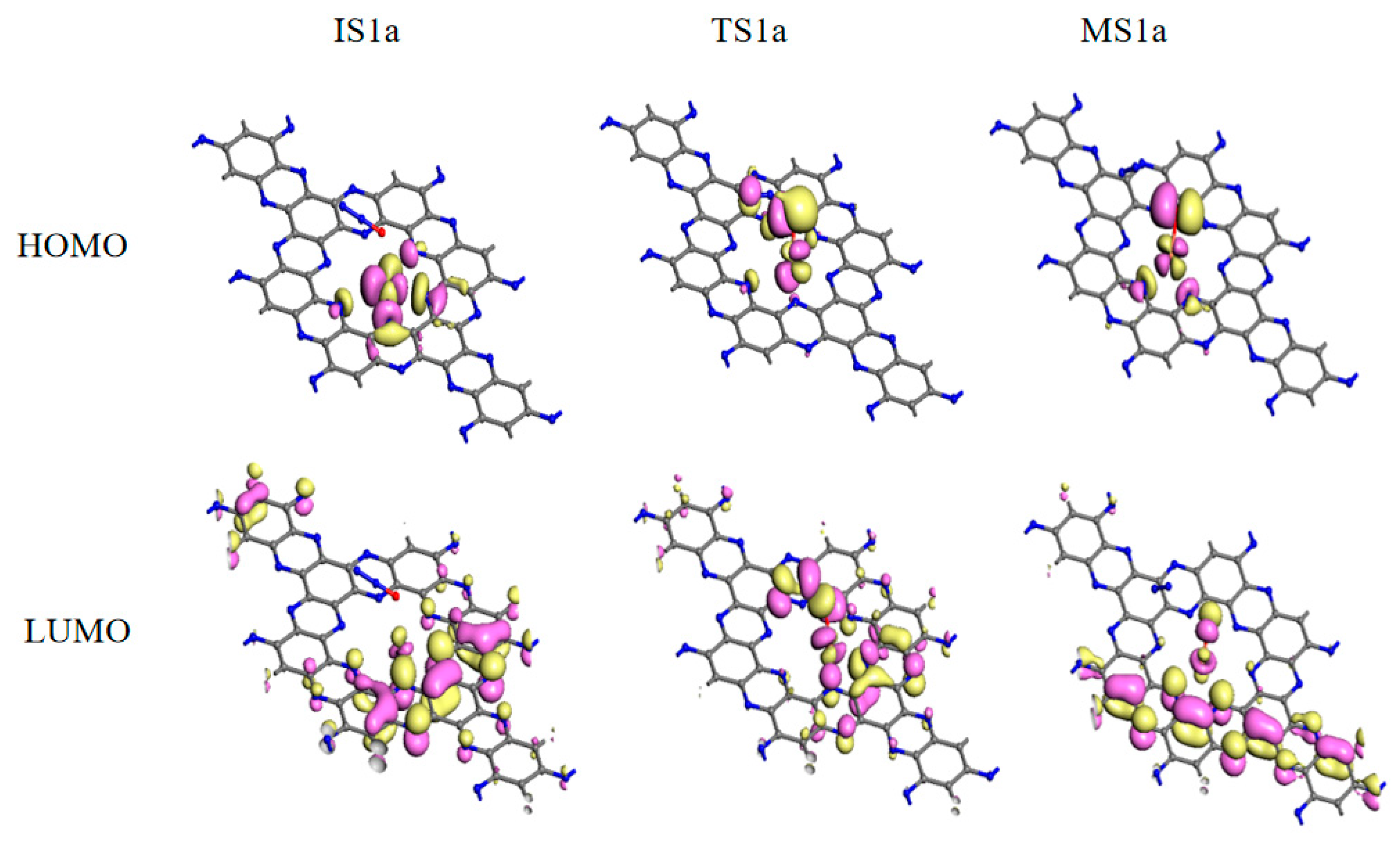
Au/C2N-Catalyzed N2O Reduction
Cu/C2N-Catalyzed N2O Reduction
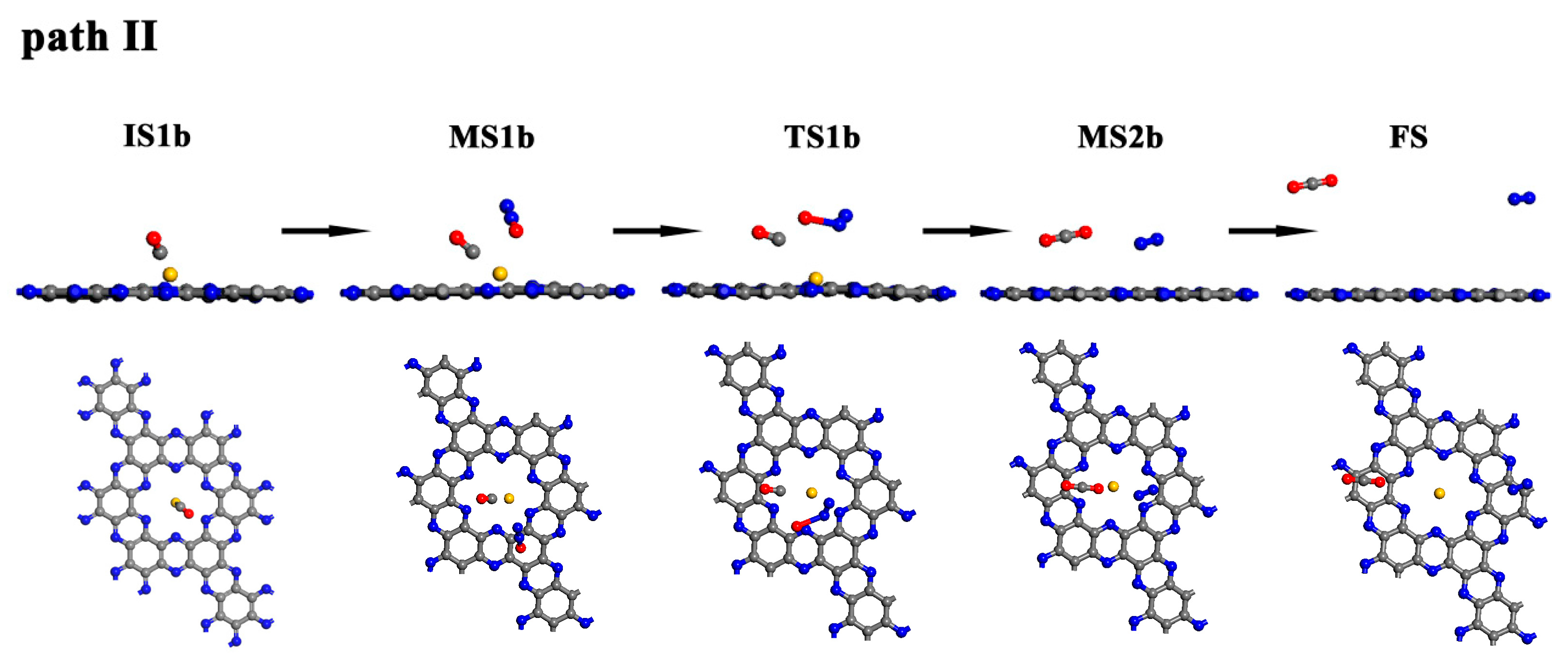
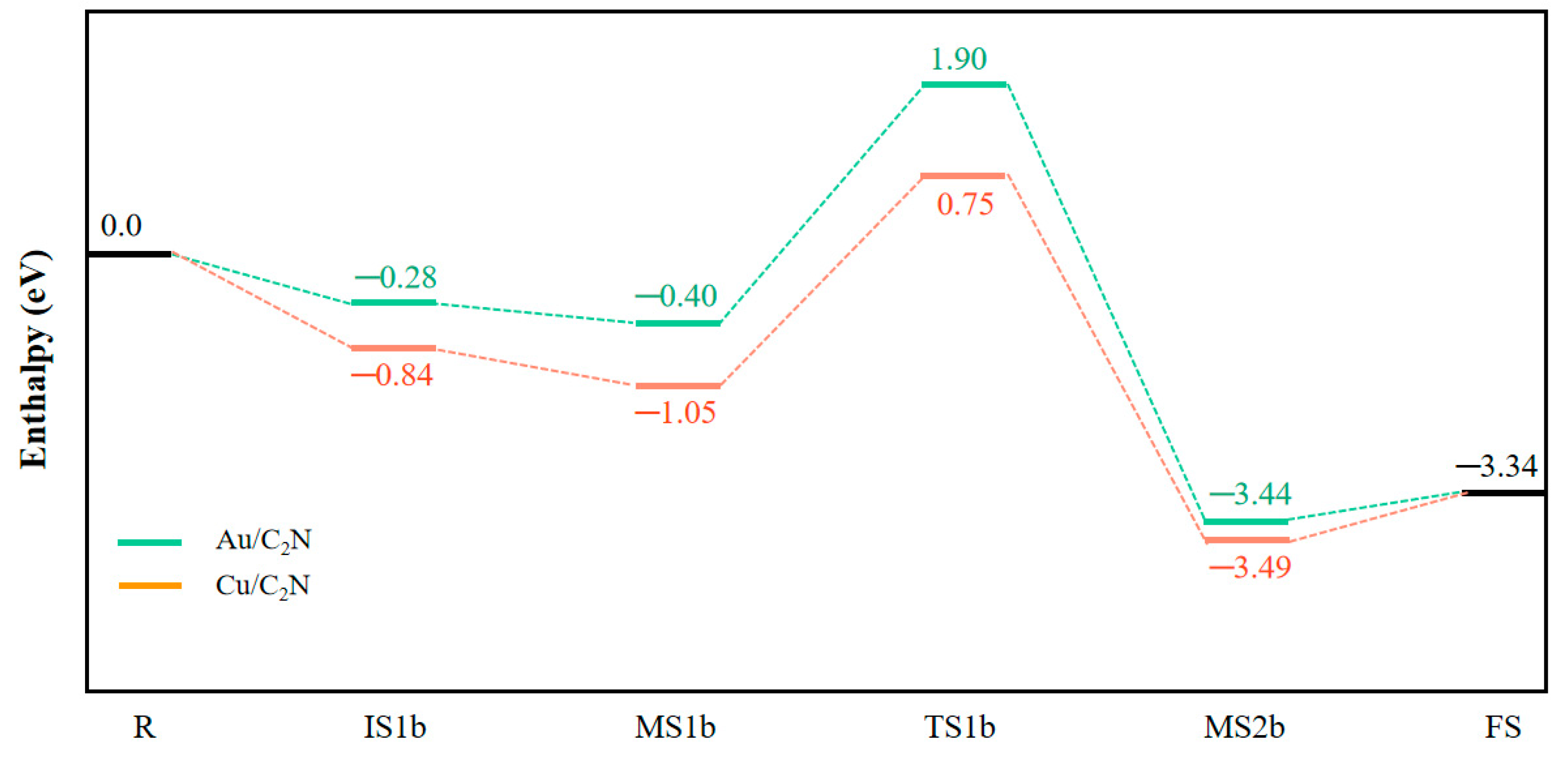
2.2.2. Mechanism II: CO Adsorption Mechanism
Au/C2N-Catalyzed N2O Reduction
Cu/C2N-Catalyzed N2O Reduction
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rockmann, T.; Kaiser, J.; Brenninkmeijer, C.A.M. The isotopic fifingerprint of the pre-industrial and the anthropogenic N2O source. Atmos Chem. Phys. Discuss. 2002, 2, 2021–2043. [Google Scholar]
- Zhang, X.X.; Wang, X.P.; Zhao, X.J.; Xu, Y.Z.; Zhang, F.F. An investigation on N2O formation route over Pt/Hy in H2-SCR. Chem. Eng. J. 2014, 252, 288–297. [Google Scholar] [CrossRef]
- Duce, R.A.; Laroche, J.; Altieri, K.; Arrigo, K.R.; Baker, A.R.; Capone, D.G.; Cornell, S.; Dentener, F.; Galloway, J.; Ganeshram, R.S.; et al. Impacts of atmospheric anthropogenic nitrogen on the open ocean. Science 2008, 320, 893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123. [Google Scholar] [CrossRef] [Green Version]
- Dameris, M. Depletion of the ozone layer in the 21st century, Angew. Chem. Int. Edit. 2010, 49, 489–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montzka, S.A.; Dlugokencky, E.J.; Butler, J.H. Non-CO2 greenhouse gases and climate change. Nature 2011, 476, 43. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Liu, Y.Q.; Liu, Y.; Lian, D.X.; Chen, M.; Ji, Y.J.; Xing, L.W.; Wu, K.; Liu, S.M. Recent advances of Cu-based catalysts for NO reduction by CO under O2-containing conditions. Catalysts 2022, 12, 1402. [Google Scholar] [CrossRef]
- Blagojevic, V.; Orlova, G.; Bohme, D.K. O-atom transport catalysis by atomic cations in the gas phase: reduction of N2O by CO. J. Am. Chem. Soc. 2005, 127, 3545–3555. [Google Scholar] [CrossRef]
- Saramok, M.; Szymaszek, A.; Inger, M.; Antoniak-Jurak, K.; Samojeden, B.; Motak, M. Modified zeolite catalyst for a NOx selective catalytic reduction process in nitric acid plants. Catalysts 2021, 11, 450. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Z.; Guo, W.; Zhang, L.; Zhang, F.; Zhu, H.; Shan, H. Theoretical investigation of the gas-phase Mn+- and Co+-catalyzed oxidation of benzene by N2O. Phys. Chem. Chem. Phys. 2009, 11, 4219–4229. [Google Scholar] [CrossRef]
- Kartha, K.K.; Pai, M.R.; Banerjee, A.M.; Pai, R.V.; Meena, S.S.; Bharadwaj, S.R. Modifified surface and bulk properties of Fe-substituted lanthanum titanates enhances catalytic activity for CO + N2O reaction. J. Mol. Catal. A Chem. 2011, 335, 158–168. [Google Scholar] [CrossRef]
- Loirat, H.; Caralp, F.; Destriau, M.; Lesclaux, R. Oxidation of CO by N2O between 1076 and 1228 K: Determination of the rate constant of the exchange reaction. J. Phys. Chem. 1987, 91, 6538–6542. [Google Scholar] [CrossRef]
- Balaj, O.P.; Balteanu, I.; Roßteuscher, T.T.J.; Beyer, M.K.; Bondybey, V.E. Catalytic oxidation of CO with N2O on gas-phase platinum clusters. Angew. Chem. Int. Edit. 2004, 43, 6519–6522. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Wang, Y.; Wang, Q. Theoretical investigation for the reaction of N2O with CO catalyzed by Pt-graphene. Struct. Chem. 2017, 28, 1679–1685. [Google Scholar] [CrossRef]
- Elizundia, U.; Duraiswami, D.; Pereda-Ayo, B.; López-Fonseca, R.; González-Velasco, J.R. Controlling the selectivity to N2O over Pt/Ba/Al2O3 NOX storage/reduction catalysts. Catal. Today 2011, 176, 324–327. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Yu, Z.; Liu, C. Mechanism of N2O formation during NO reduction on the Au(111) Surface. J. Phys. Chem. C 2010, 114, 2711–2716. [Google Scholar] [CrossRef]
- Wei, X.; Yang, X.-F.; Wang, A.-Q.; Li, L.; Liu, X.-Y.; Zhang, T.; Mou, C.-Y.; Li, J. Bimetallic Au-Pd alloy catalysts for N2O decomposition: Effects of surface structures on catalytic activity. J. Phys. Chem. C 2012, 116, 6222–6232. [Google Scholar] [CrossRef]
- Gholizadeh, R.; Yu, Y.-X. N2O+CO reaction over Si- and Se-doped graphenes: An ab initio DFT study. Appl. Surf. Sci. 2015, 357, 1187–1195. [Google Scholar] [CrossRef]
- Lambeets, S.V.; Barroo, C.; Owczarek, S.; Jacobs, L.; Genty, E.; Gilis, N.; de Bocarmé, T.V. Adsorption and hydrogenation of CO2 on Rh nanosized crystals: Demonstration of the role of interfacet oxygen spillover and comparative studies with O2, N2O, and CO. J. Phys. Chem. C 2017, 121, 16238–16249. [Google Scholar] [CrossRef]
- Kokalj, A.; Matsushima, T. A density-functional theory study of the interaction of N2O with Rh(110). J. Chem. Phys. 2005, 122, 034708. [Google Scholar] [CrossRef] [Green Version]
- Paul, J.-F.; Pérez-Ramírez, J.; Ample, F.; Ricart, J.M. Theoretical studies of N2O adsorption and reactivity to N2 and NO on Rh(111). J. Phys. Chem. B 2004, 108, 17921–17927. [Google Scholar] [CrossRef]
- Song, E.H.; Yan, J.M.; Lian, J.S.; Jiang, Q. External electric field catalyzed N2O decomposition on Mn-embedded graphene. J. Phys. Chem. C 2012, 116, 20342–20348. [Google Scholar] [CrossRef]
- Wannakao, S.; Nongnual, T.; Khongpracha, P.; Maihom, T.; Limtrakul, J. Reaction mechanisms for co catalytic oxidation by N2O on Fe-embedded graphene. J. Phys. Chem. C 2012, 116, 16992–16998. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Sharifi, F.; Nematollahi, P. Al- or Si-decorated graphene oxide: A favorable metal-free catalyst for the N2O reduction. Appl. Surf. Sci. 2016, 387, 454–460. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mohammadian-Sabet, F.; Nematollahi, P. Oxidation of CO by N2O over Al- and Ti-doped graphene: A comparative study. RSC Adv. 2016, 6, 64832–64840. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of co oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Tosoni, S.; Pacchioni, G. Role of support in tuning the properties of single atom catalysts: Cu, Ag, Au, Ni, Pd, and Pt adsorption on SiO2/Ru, SiO2/Pt, and SiO2/Si ultrathin films. J. Chem. Phys. 2021, 154, 134706. [Google Scholar] [CrossRef]
- Tosoni, S.; Liberto, G.D.; Matanovic, I.; Pacchioni, G. Modelling single atom catalysts for water splitting and fuel cells: A tutorial review. J. Power Sour. 2023, 556, 232492. [Google Scholar] [CrossRef]
- Liberto, G.D.; Tosoni, S.; Cipriano, L.A.; Pacchioni, G. A few questions about single-atom catalysts: When modeling helps. Acc. Mater. Res. 2023, 3, 986–995. [Google Scholar] [CrossRef]
- Cipriano, L.A.; Liberto, G.D.; Pacchioni, G. Superoxo and peroxo complexes on single-atom catalysts: Impact on the oxygen evolution reaction. ACS Catal. 2022, 12, 11682–11691. [Google Scholar] [CrossRef]
- Zhang, L.L.; Chen, X.M.; Liu, C.G. Reduction of N2O by CO via Mars-van Krevelen mechanism over phosphotungstic acid supported single-atom catalysts: A density functional theory study. Inorg. Chem. 2019, 58, 5221–5229. [Google Scholar] [CrossRef] [PubMed]
- Anchalee, J.; Supawadee, N.; Phornphimon, M.; Masahiro, E. Silicon-coordinated nitrogen-doped graphene as a promising metal-free catalyst for N2O reduction by CO: A theoretical study. RSC Adv. 2018, 8, 22322–22330. [Google Scholar]
- Liu, C.G.; Sun, C.; Jiang, M.X.; Zhang, L.L.; Sun, M.J. Calculations of NO reduction with CO over a Cu1/PMA single-atom catalyst: A study of surface oxygen species, active sites, and the reaction mechanism. Phys. Chem. Chem. Phys. 2019, 21, 9975–9986. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, M.D.; Heidari, S. Catalytic reduction of nitrous oxide over boron-doped C3N monolayers: A DFT study. Chem. Phys. Lett. 2019, 725, 52–58. [Google Scholar] [CrossRef]
- Fan, G.H.; Wang, Q.; Xu, H.; Wang, X.H.; Tu, X.X.; Chu, X.F. Single Cr atom supported on boron nitride nanotubes for the reaction of N2O reduction by CO: A density functional theory study. Appl. Surf. Sci. 2021, 544, 148776–148782. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Vessally, E. N2O + CO reaction over single Ga or Ge atom embedded graphene: A DFT study. Surf. Sci. 2017, 667, 105–111. [Google Scholar] [CrossRef]
- Mahmood, J.; Lee, E.K.; Jung, M.; Shin, D.; Jeon, I.-Y.; Jung, S.-M.; Choi, H.-J.; Seo, J.-M.; Bae, S.-Y.; Sohn, S.-D.; et al. Nitrogenated holey two-dimensional structures. Nat. Commun. 2015, 6, 6486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarty, S.; Das, T.; Banerjee, P.; Thapa, R.; Das, G.P. Electron doped C2N monolayer as effiffifficient noble metal-free catalysts for CO oxidation. Appl. Surf. Sci. 2017, 418, 92–98. [Google Scholar] [CrossRef]
- Mahmood, J.; Li, F.; Kim, C.; Choi, H.-J.; Gwon, O.; Jung, S.-M.; Seo, J.-M.; Cho, S.-J.; Ju, Y.-W.; Jeong, H.Y.; et al. Fe@C2N: A highly-effiffifficient indirect-contact oxygen reduction catalyst. Nano Energy 2018, 44, 304–310. [Google Scholar] [CrossRef]
- Mahmood, J.; Li, F.; Jung, S.-M.; Okyay, M.S.; Ahmad, I.; Kim, S.-J.; Park, N.; Jeong, H.Y.; Baek, J.-B. An effiffifficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction. Nat. Nanotechnol. 2017, 12, 441–446. [Google Scholar] [CrossRef]
- Ma, J.; Gong, H.; Zhang, T.; Yu, H.; Zhang, R.; Liu, Z.; Yang, G.; Sun, H.; Tang, S.; Qiu, Y. Hydrogenation of CO2 to formic acid on the single atom catalysis Cu/C2N: A first principles study. Appl. Surf. Sci. 2019, 488, 1–9. [Google Scholar] [CrossRef]
- Ma, D.W.; Wang, Q.; Yan, X.; Zhang, X.; He, C.; Zhou, D.; Tang, Y.; Lu, Z.; Yang, Z. 3d transition metal embedded C2N monolayers as promising single-atom catalysts: A first-principles study. Carbon 2016, 105, 463–473. [Google Scholar] [CrossRef]
- He, B.L.; Shen, J.S.; Tian, Z.X. Iron-embedded C2N monolayer: A promising low-cost and high-activity single-atom catalyst for CO oxidation. Phys. Chem. Chem. Phys. 2016, 18, 24261–24269. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756. [Google Scholar] [CrossRef]
- Perdew, J.P.; Yue, W. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8800–8802. [Google Scholar] [CrossRef] [PubMed]
- Basiuk, V.A.; Henao-Holguín, L.V. Dispersion-Corrected Density Functional Theory Calculations of meso-Tetraphenylporphine-C60 Complex by Using DMol3 Module. J. Comput. Theor. Nanosci. 2014, 11, 1609–1615. [Google Scholar] [CrossRef]
- Tkatchenko, A.; Scheffler, M. Accurate Molecular Van Der Waals Interactions from ground-state electron density and free-atom reference data. Phys. Rev. Lett. 2009, 102, 073005. [Google Scholar] [CrossRef] [Green Version]
- Delley, B. Hardness conserving semilocal pseudopotentials. Phys. Rev. B 2002, 66, 155125. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Halgren, T.A.; Lipscomb, W.N. The synchronous-transit method for determining reaction pathways and locating molecular transition states. Chem. Phys. Lett. 1977, 49, 225–232. [Google Scholar] [CrossRef]
- Govind, N.; Petersen, M.; Fitzgerald, G.; King-Smith, D.; Andzelm, J. A generalized synchronous transit method for transition state location. Comput. Mater. Sci. 2003, 28, 250–258. [Google Scholar] [CrossRef]


| Au/C2N | Cu/C2N | |||||
|---|---|---|---|---|---|---|
| ΔEcorr | ΔHcorr | ΔGcorr | ΔEcorr | ΔHcorr | ΔGcorr | |
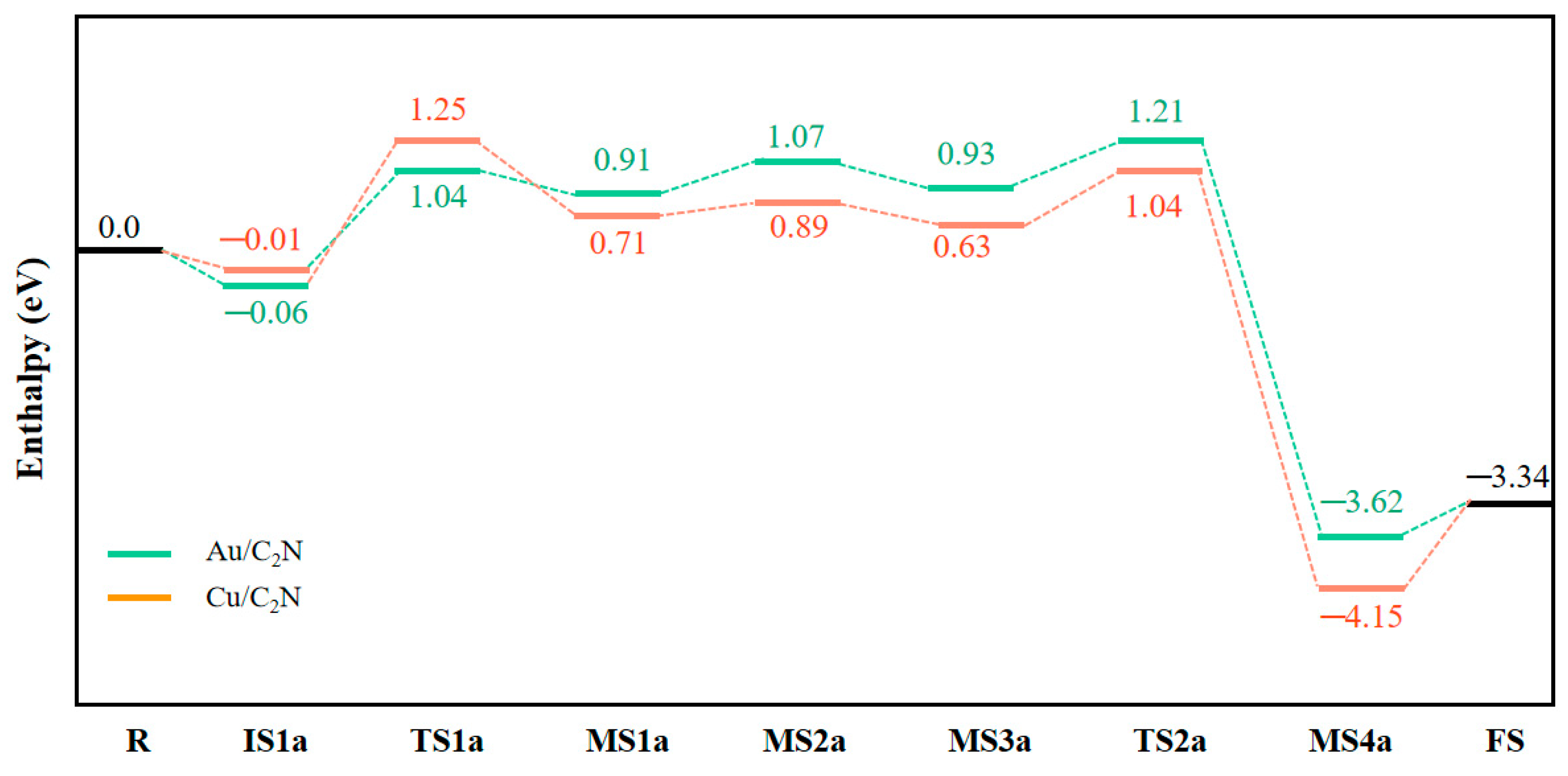
| R | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| IS1a | −0.05 | −0.06 | −0.09 | −0.02 | −0.01 | −0.11 |
| TS1a | 0.90 | 1.04 | 0.74 | 1.07 | 1.25 | 0.80 |
| MS1a | 0.84 | 0.91 | 0.40 | 0.63 | 0.71 | 0.20 |
| MS2a | 0.98 | 1.07 | 0.47 | 0.81 | 0.89 | 0.34 |
| MS3a | 0.83 | 0.93 | 0.60 | 0.56 | 0.63 | 0.29 |
| TS2a | 1.13 | 1.21 | 0.95 | 0.99 | 1.04 | 0.83 |
| MS4a | −3.69 | −3.62 | −3.97 | −4.13 | −4.15 | −3.80 |
| IS1b | −0.32 | −0.28 | −0.28 | −0.90 | −0.84 | −0.92 |
| MS1b | −0.43 | −0.40 | −0.14 | −1.10 | −1.05 | −0.96 |
| TS1b | 1.87 | 1.90 | 2.08 | 0.72 | 0.75 | 0.86 |
| MS2b | −3.48 | −3.44 | −3.30 | −3.58 | −3.49 | −3.54 |
| FS | −3.40 | −3.34 | −3.25 | −3.40 | −3.34 | −3.25 |
| Atom/ Molecule | Charges/e | |||||||
|---|---|---|---|---|---|---|---|---|
| Isolated Systems | IS1a | TS1a | MS1a | MS2a | MS3a | TS2a | MS4a | |
| Au | 0.548 | 0.551 | 0.705 | 0.734 | 0.739 | 0.736 | 0.708 | 0.561 |
| O(N2O) | −0.278 | −0.291 | −0.466 | −0.478 | −0.474 | −0.474 | −0.475 | −0.275 |
| N(a) | 0.420 | 0.435 | 0.141 | 0.013 | ||||
| N(b) | −0.143 | −0.140 | −0.112 | −0.016 | ||||
| N2O | 0 | 0.004 | −0.437 | |||||
| C | 0.08 | 0.080 | 0.102 | 0.543 | ||||
| O(CO) | −0.08 | −0.088 | −0.164 | −0.261 | ||||
| CO | 0 | −0.008 | ||||||
| Atom/ Molecule | Charges of the Systems/e | |||||||
|---|---|---|---|---|---|---|---|---|
| Isolated Systems | IS1a | TS1a | MS1a | MS2a | MS3a | TS2a | MS4a | |
| Cu | 0.622 | 0.639 | 0.785 | 0.809 | 0.807 | 0.806 | 0.725 | 0.685 |
| O(N2O) | −0.278 | −0.306 | −0.515 | −0.530 | −0.526 | −0.527 | −0.504 | −0.265 |
| N(a) | 0.420 | 0.449 | 0.148 | 0.006 | ||||
| N(b) | −0.143 | −0.120 | −0.095 | −0.006 | ||||
| N2O | 0 | 0.023 | −0.462 | |||||
| C | 0.08 | 0.080 | 0.156 | 0.538 | ||||
| O(CO) | −0.08 | −0.086 | −0.123 | −0.237 | ||||
| CO | 0 | 0.006 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, S.; Ma, J.; Liu, Z.; Holiharimanana, D.; Sun, H. Catalytic Reduction of N2O by CO on Single-Atom Catalysts Au/C2N and Cu/C2N: A First-Principles Study. Catalysts 2023, 13, 578. https://doi.org/10.3390/catal13030578
Su S, Ma J, Liu Z, Holiharimanana D, Sun H. Catalytic Reduction of N2O by CO on Single-Atom Catalysts Au/C2N and Cu/C2N: A First-Principles Study. Catalysts. 2023; 13(3):578. https://doi.org/10.3390/catal13030578
Chicago/Turabian StyleSu, Shengyang, Junmei Ma, Zhenhua Liu, Domoina Holiharimanana, and Hao Sun. 2023. "Catalytic Reduction of N2O by CO on Single-Atom Catalysts Au/C2N and Cu/C2N: A First-Principles Study" Catalysts 13, no. 3: 578. https://doi.org/10.3390/catal13030578