Polyoxometalates Encapsulated into Hollow Periodic Mesoporous Organosilica as Nanoreactors for Extraction Oxidation Desulfurization
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Catalyst Preparation
2.2.1. Preparation of PW2Mo2
2.2.2. Preparation of Hollow Periodic Mesoporous Organosilica (HPMOS)
2.2.3. Preparation of Composite PW2Mo2@HPMOS
2.3. Characterizations
2.4. Catalytic Testing
3. Results and Discussion
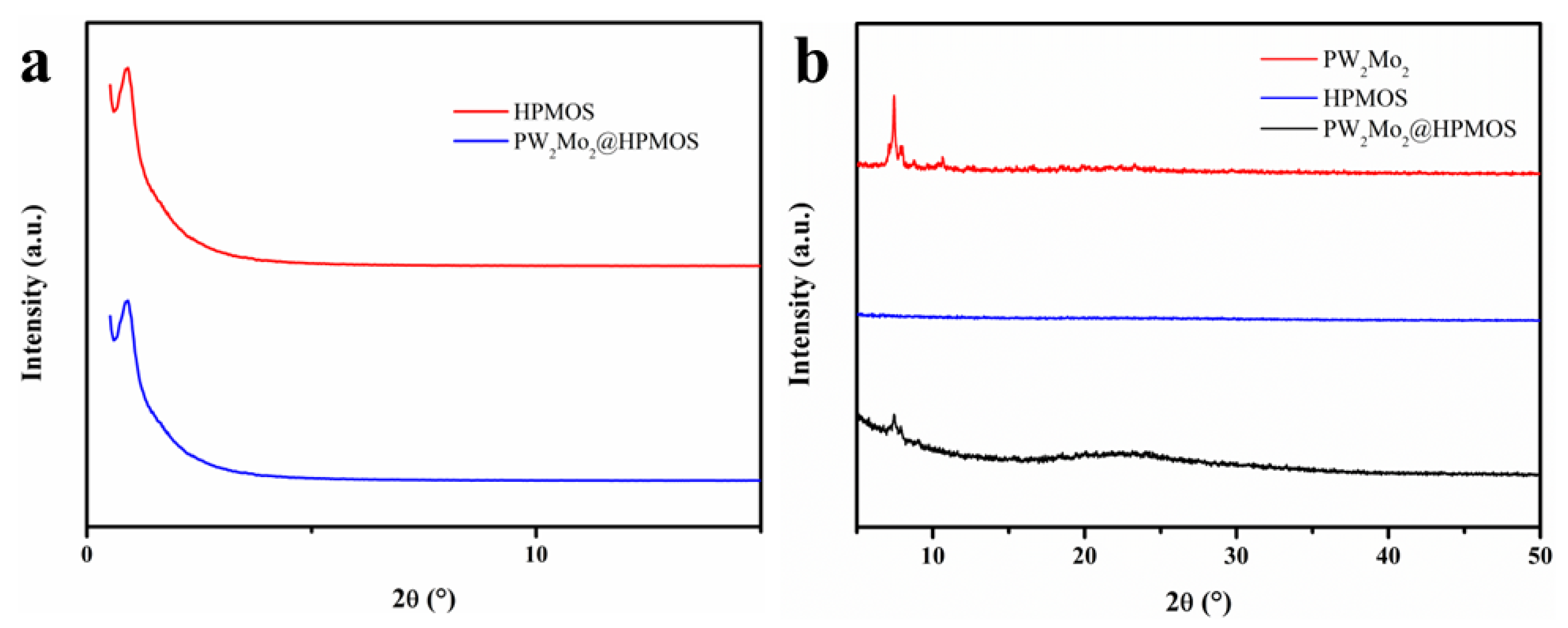
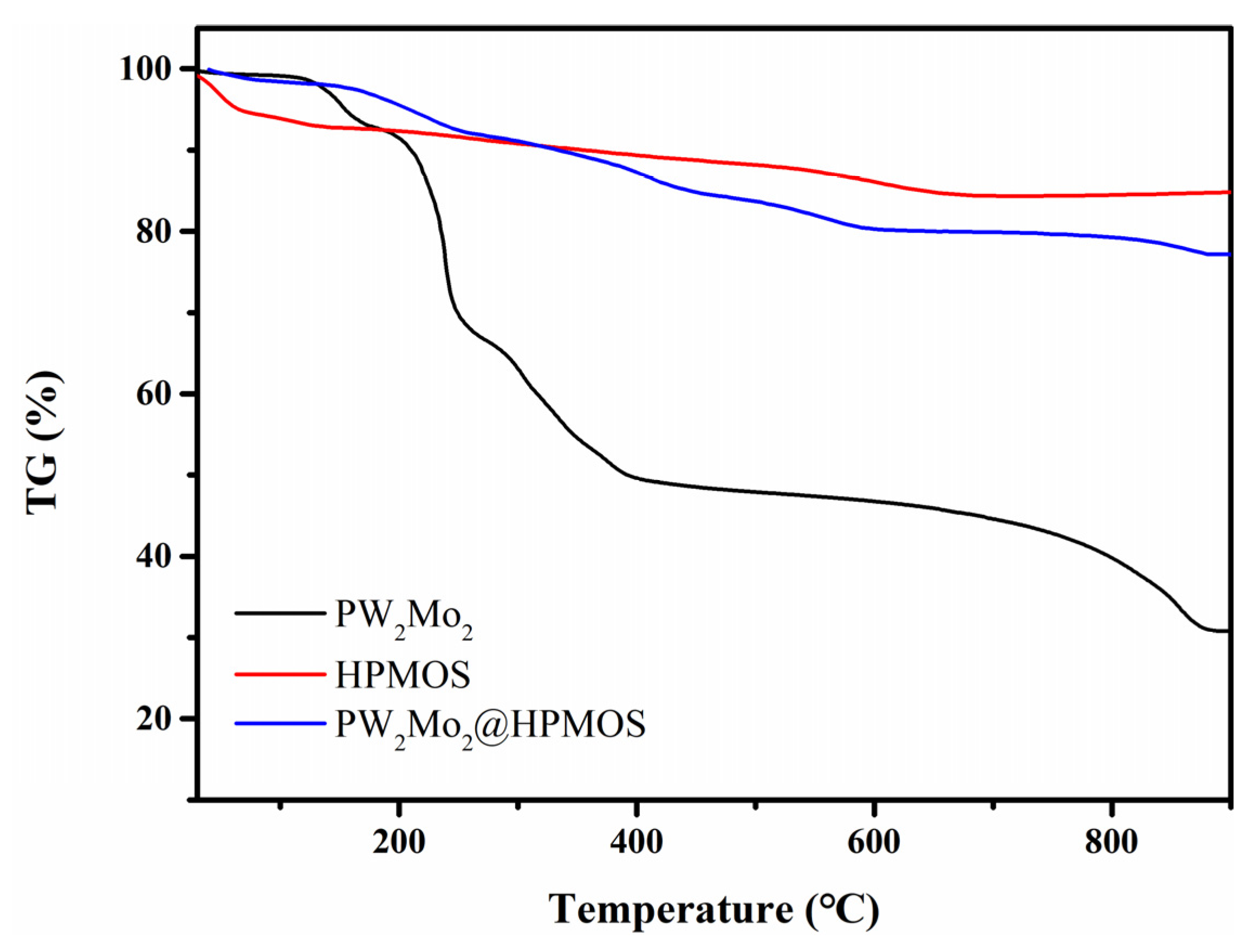
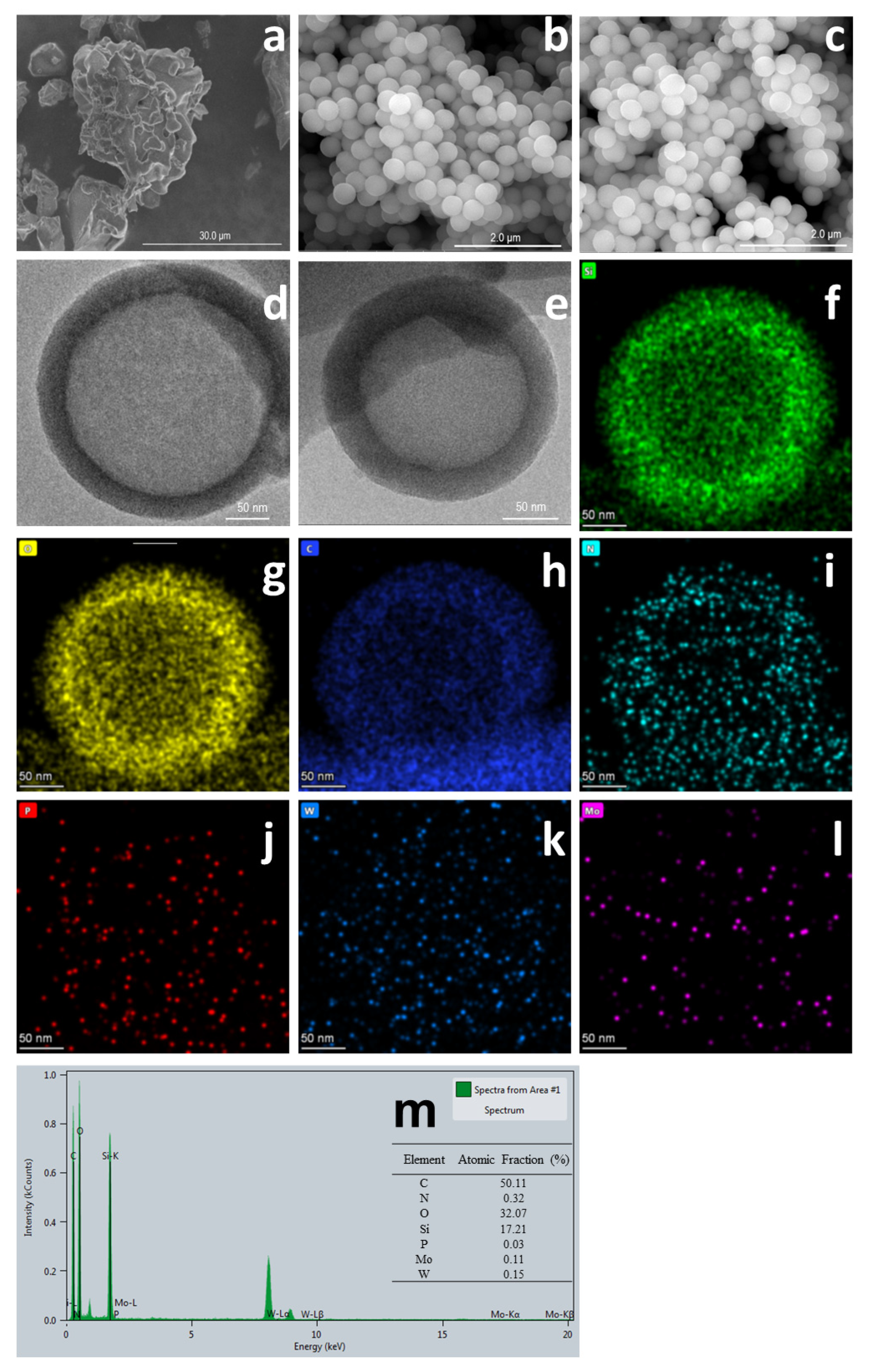
3.1. Catalyst Characterization
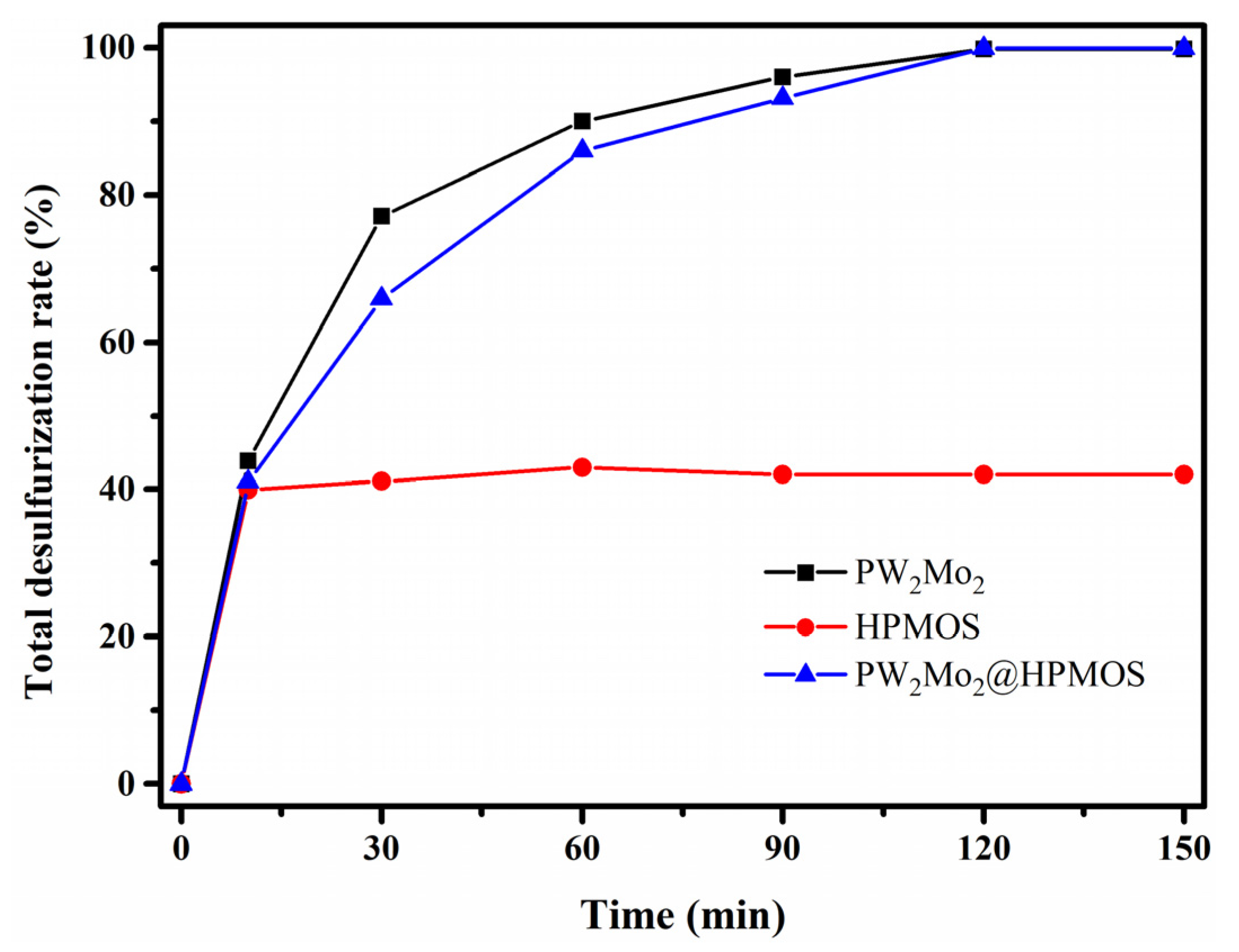
3.2. Desulfurization Efficiency Catalyzed by PW2Mo2@HPMOS Nanoreactor
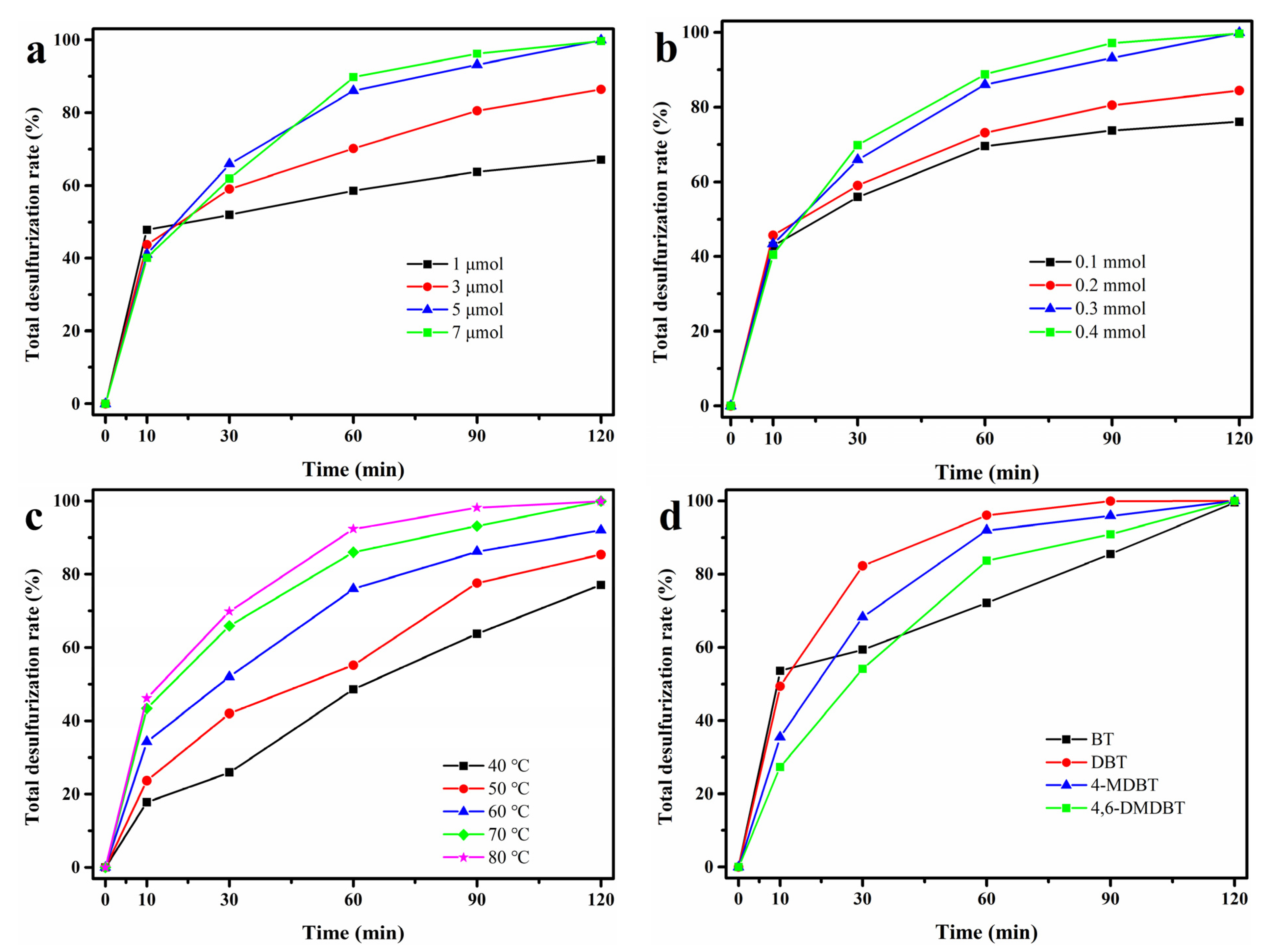
3.3. Condition Optimization
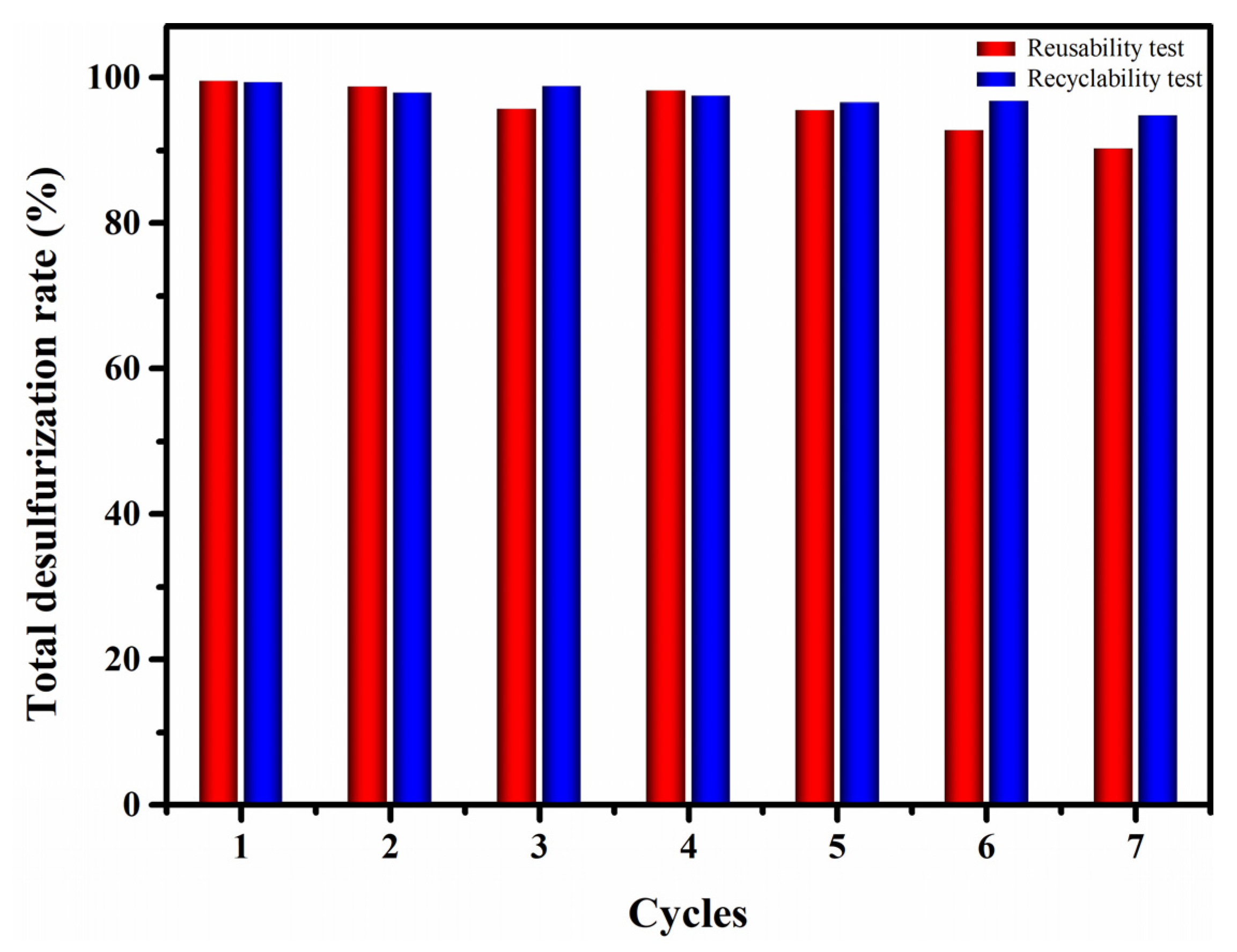
3.4. Reusability of Catalyst and Recyclability of the EODS System
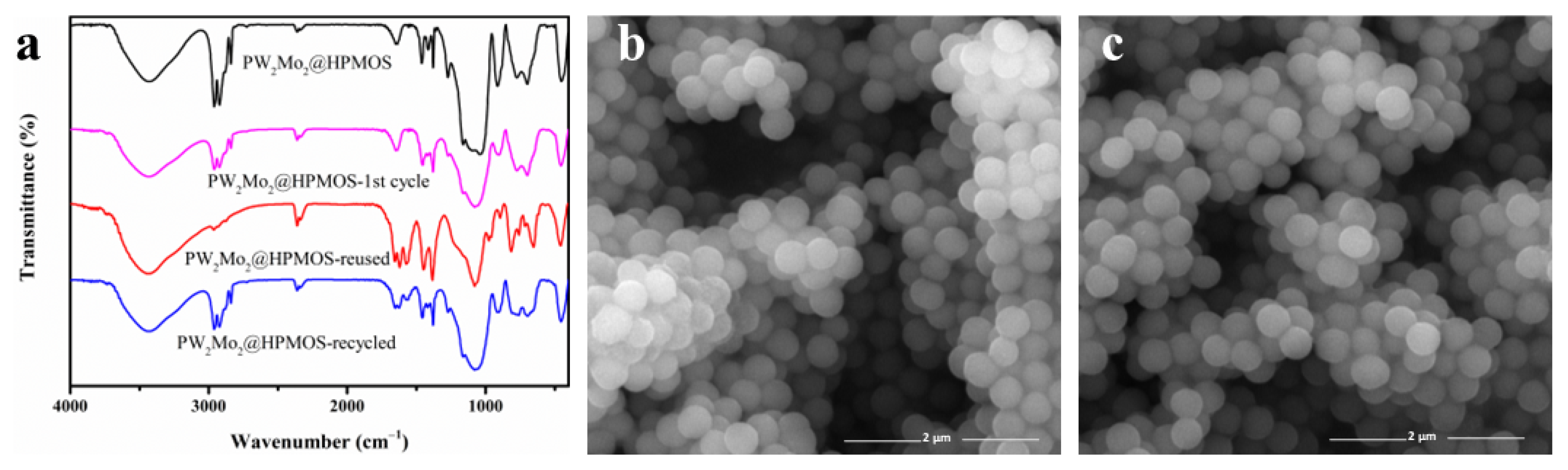
3.5. Stability of PW2Mo2@HPMOS Catalyst
3.6. Comparison of Desulfurization of Fuel in a Nanoreactor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haruna, A.; Merican, Z.M.A.; Musa, S.G. Abubakar, Sulfur removal technologies from fuel oil for safe and sustainable environment. Fuel 2022, 329, 125370. [Google Scholar] [CrossRef]
- Mishra, H.; Siddiqi, U.; Kumari, I.D.; Behera, S.; Mukherjee, B.C. Meikap, Pyrolysis of waste lubricating oil/waste motor oil to generate high-grade fuel oil: A comprehensive review. Renew. Sustain. Energy Rev. 2021, 150, 111446. [Google Scholar] [CrossRef]
- Nikokavoura, A.; Trapalis, C. Graphene and g-C3N4 based photocatalysts for NOx removal: A review. Appl. Surf. Sci. 2018, 430, 18–52. [Google Scholar] [CrossRef]
- Chatrattanawet, N.; Saebea, D.; Authayanun, S.; Arpornwichanop, A.; Patcharavorachot, Y. Performance and environmental study of a biogas-fuelled solid oxide fuel cell with different reforming approaches. Energy 2018, 146, 131–140. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Sun, Y.; Jiang, B.; Yang, H. Deep oxidative desulfurization of fuels by superbase-derived Lewis acidic ionic liquids. Chem. Eng. J. 2017, 328, 445–453. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.; Jiang, M.; Huo, Y.; Wang, X.; Wang, X. Achieving deep desulfurization with inverse-micellar polyoxometalates and oxygen. RSC Adv. 2021, 11, 9043–9047. [Google Scholar] [CrossRef]
- Lǖ, J.; Fu, Y.; Wang, J.; Chen, H. Study on the desulfurization performance of calcium-based desulfurizer and NaHCO3 desulfurizer. Environ. Sci. Pollut. Res. 2023, 30, 20357–20368. [Google Scholar] [CrossRef]
- Ghahremani, H.; Nasri, Z.; Eikani, M.H. Ultrasound-assisted oxidative desulfurization (UAOD) of Iranian heavy crude oil: Investigation of process variables. J. Pet. Sci. Eng. 2021, 204, 108709. [Google Scholar] [CrossRef]
- Petrova, D.; Lyubimenko, V.; Ivanov, E.; Gushchin, P.; Kolesnikov, I. Energy basics of catalytic hydrodesulfurization of diesel fuels. Catalysts 2022, 12, 1301. [Google Scholar] [CrossRef]
- Fedushchak, T.A.; Uimin, M.A.; Maikov, V.V.; Akimov, A.S.; Zhuravkov, S.P.; Vosmerikov, A.V.; Prosvirin, I.P.; Velichkina, L.M.; Stepanov, A.A.; Kogan, V.M. Novel molybdenite-based nanopowder catalysts for hydrodesulfurization. Pet. Chem. 2021, 61, 794–805. [Google Scholar] [CrossRef]
- Huo, Q.; Liu, G.; Sun, H.; Fu, Y.; Ning, Y.; Zhang, B.; Zhang, X.; Gao, J.; Miao, J.; Zhang, X.; et al. CeO2-modified MIL-101(Fe) for photocatalysis extraction oxidation desulfurization of model oil under visible light irradiation. Chem. Eng. J. 2021, 422, 130036. [Google Scholar] [CrossRef]
- Harandi, M.S.; Shams, E.; Sharifi, E.; Momenbeik, F. A new approach in deep desulfurization of model fuel through integration of electrochemical oxidation and liquid-liquid extraction in a biphasic system. Sep. Purif. Technol. 2021, 275, 119087. [Google Scholar] [CrossRef]
- Nojima, S.; Kamata, K.; Suzuki, K.; Yamaguchi, K.; Mizuno, N. Selective oxidation with aqueous hydrogen peroxide by [PO4{WO(O2)2}4]3- supported on zinc-modified tin dioxide. ChemCatChem 2015, 7, 1097–1104. [Google Scholar] [CrossRef]
- Kamata, K.; Sugahara, K.; Ishimoto, R.; Nojima, S.; Mizuno, N. Highly selective epoxidation of cycloaliphatic alkenes with aqueous hydrogen peroxide catalyzed by [PO4{WO(O2)2}4]3−/imidazole. Chemcatchem 2014, 6, 2327–2332. [Google Scholar] [CrossRef]
- Han, W.; Yang, Y.; Fang, D.; Zang, S. Application of 12-heteropolyacids of molybdenum and tungsten ([Smim]3[PMoW3O24]) in epoxidation of olefins. Oxid. Commun. 2014, 37, 112–120. [Google Scholar]
- Zhang, L.; Ji, X.; Ren, X.; Ma, Y.; Shi, X.; Tian, Z.; Asiri, A.M.; Chen, L.; Tang, B.; Sun, X. Electrochemical Ammonia Synthesis via Nitrogen Reduction Reaction on a MoS(2) Catalyst: Theoretical and Experimental Studies. Adv. Mater. 2018, 30, e1800191. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, J.; Wang, Y.; Mou, T.; Lin, Y.; Yue, L.; Li, T.; Liu, Q.; Luo, Y.; Li, N.; et al. High-Performance Electrochemical NO Reduction into NH(3) by MoS(2) Nanosheet. Angew. Chem. Int. Ed. Engl. 2021, 60, 25263–25268. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, E.; Eavani, S. Organic–inorganic polyoxometalate based salts as thermoregulated phase-separable catalysts for selective oxidation of thioethers and thiophenes and deep desulfurization of model fuels. J. Mol. Catal. A Chem. 2013, 380, 18–27. [Google Scholar] [CrossRef]
- Julião, D.; Mirante, F.; Ribeiro, S.O.; Gomes, A.C.; Valença, R.; Ribeiro, J.C.; Pillinger, M.; De Castro, B.; Gonçalves, I.S.; Balula, S.S. Deep oxidative desulfurization of diesel fuels using homogeneous and SBA-15-supported peroxophosphotungstate catalysts. Fuel 2019, 241, 616–624. [Google Scholar] [CrossRef]
- Lin, C.X.; Li, Z.; Brumbley, S.; Petrasovits, L.; Mcqualter, R.; Yu, C.; Lu, G.Q. Synthesis of magnetic hollow periodic mesoporous organosilica with enhanced cellulose tissue penetration behaviour. J. Mater. Chem. 2011, 21, 7565–7571. [Google Scholar] [CrossRef]
- Yang, L.; Guo, H.; Wang, L.; Zhang, J. A facile "polystyrene-dissolving" strategy to hollow periodic mesoporous organosilica with flexible structure-tailorability. Microporous Mesoporous Mater. 2017, 239, 173–179. [Google Scholar] [CrossRef]
- Qian, C.; Al-Hamyari, B.; Tang, X.; Hou, B.; Yang, S.; Zhang, G.; Lv, H.; Yang, Z.; Wang, Z.; Shi, Y. Interface-engineered paclitaxel-based hollow mesoporous organosilica nanoplatforms for photothermal-enhanced chemotherapy of tumor. Mol. Pharm. 2021, 18, 4531–4542. [Google Scholar] [CrossRef]
- Ying, Y.A.; Feng, Y.A.; Hai, W.B.; Bz, A.; Sh, A. Amine-promoted Ru1/Fe3O4 encapsulated in hollow periodic mesoporousorganosilica sphere as a highly selective and stable catalyst for aqueous levulinic acid hydrogenation. J. Colloid Interface Sci. 2021, 581, 167–176. [Google Scholar] [CrossRef]
- Li, S.W.; Gao, R.M.; Zhang, R.L.; Zhao, J.S. Template method for a hybrid catalyst material POM@MOF-199 anchored on MCM-41: Highly oxidative desulfurization of DBT under molecular oxygen. Fuel 2016, 184, 18–27. [Google Scholar] [CrossRef]
- Furukawa, H.; Nakamura, T.; Inagaki, H.; Nishikawa, E.; Imai, C.; Misono, M. Oxidation of cyclopentene with hydrogen peroxide catalyzed by 12-heteropoly acids. Chem. Lett. 1988, 24, 877–880. [Google Scholar] [CrossRef]
- Yan, G.; Dj, A.; Dfs, A.; Bdc, A.; Jz, B.; Ssb, A. A simple desulfurization process to achieve high efficiency, sustainability and cost-effectivity via peroxotungstate catalyst. Mol. Catal. 2021, 505, 1–7. [Google Scholar] [CrossRef]
- Salles, L.; Thouvenot, R.; Brégeault, J. Redistribution and fluxionality in heteropolyoxoperoxo complexes: [PO4{M2O2(-O2)2(O2)2}2]3− with M = Mo and/or W. Dalton Trans. 2004, 6, 904. [Google Scholar] [CrossRef]
- Zhu, W.; Zhu, G.; Li, H.; Chao, Y.; Zhang, M.; Du, D.; Wang, Q.; Zhao, Z. Catalytic kinetics of oxidative desulfurization with surfactant-type polyoxometalate-based ionic liquids. Fuel Process. Technol. 2013, 106, 70–76. [Google Scholar] [CrossRef]
- Zhu, W.; Zhu, G.; Li, H.; Chao, Y.; Chang, Y.; Chen, G.; Han, C. Oxidative desulfurization of fuel catalyzed by metal-based surfactant-type ionic liquids. J. Mol. Catal. A Chem. 2011, 347, 8–14. [Google Scholar] [CrossRef]
- Zhou, W.; Ma, H.; Dai, Y.; Du, Y.; Guo, C.; Wang, J. Architecture of nanoantioxidant based on mesoporous organosilica trp-met-PMO with dipeptide skeleton. Materials 2023, 16, 638. [Google Scholar] [CrossRef]
- Teng, S.; Han, Y.; Hu, Y.; Li, J.; Wang, M.; Guo, Z.; Yang, W. Swellable hollow periodic mesoporous organosilica capsules with ultrahigh loading capacity for hydrophobic drugs. J. Colloid Interface Sci. 2023, 630, 266–273. [Google Scholar] [CrossRef]
- Huo, H.; Jiang, Y.; Zhao, T.; Wang, J.; Li, D.; Xu, X.; Lin, K. Periodic Mesoporous Organosilicas as Efficient Nanoreactors in Cascade Reactions Preparing Cyclopropanic Derivatives. Chem. Asian J. 2019, 14, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, M.; Shirini, F.; Golshekan, M. Efficiency of NaHSO4 modified periodic mesoporous organosilica magnetic nanoparticles as a new magnetically separable nanocatalyst in the synthesis of [1,2,4]triazolo quinazolinone/pyrimidine derivatives. J. Mol. Struct. 2018, 1171, 168–178. [Google Scholar] [CrossRef]
- Ribeiro, S.O.; Nogueira, L.S.; Gago, S.; Almeida, P.L.; Corvo, M.C.; Castro, B.d.; Granadeiro, C.M.; Balula, S.S. Desulfurization process conciliating heterogeneous oxidation and liquid extraction: Organic solvent or centrifugation/water? Appl. Catal. A Gen. 2017, 542, 359–367. [Google Scholar] [CrossRef]
- Mirante, F.; Dias, L.; Silva, M.; Ribeiro, S.O.; Corvo, M.C.; de Castro, B.; Granadeiro, C.M.; Balula, S.S. Efficient heterogeneous polyoxometalate-hybrid catalysts for the oxidative desulfurization of fuels. Catal. Commun. 2018, 104, 1–8. [Google Scholar] [CrossRef]
- Jiang, W.; Xiao, J.; Gao, X.; An, X.; Li, H. In situ fabrication of hollow silica confined defective molybdenum oxide for enhanced catalytic oxidative desulfurization of diesel fuels. Fuel 2021, 305, 121470. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Dong, J.; Liu, D.; Liu, C.; Chi, Y.; Hu, C. Polyoxometalates encapsulated into hollow double-shelled nanospheres as amphiphilic nanoreactors for an effective oxidative desulfurization. Nanoscale 2020, 12, 16586–16595. [Google Scholar] [CrossRef] [PubMed]
- Astle, M.A.; Rance, G.A.; Loughlin, H.; Peters, T.D.; Khlobystov, A.N. Molybdenum dioxide in carbon nanoreactors as a catalytic nanosponge for the efficient desulfurization of liquid fuels. Adv. Funct. Mater. 2019, 29, 1808092. [Google Scholar] [CrossRef]
- Yang, G.; Feng, F.; Luo, Y.; Qin, J.; Yuan, F.; Wang, S.; Luo, S.; Ma, J. Facile synthesis of MoO3 nanodots self-assembled into hollow mesoporous silica: Enhancing efficient oxidative desulfurization and investigating reaction mechanism. J. Environ. Chem. Eng. 2021, 9, 106309. [Google Scholar] [CrossRef]


| Sample | SBET (m²/g) | V (cm3/g) | D (nm) |
|---|---|---|---|
| HPMOS | 942.05 | 1.45 | 5.62 |
| PW2Mo2@HPMOS | 326.46 | 0.48 | 5.88 |
| Catalyst | Substrate | Test Conditions | Desulfurization Rate, % | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Species | Concentration, ppm | Oxidant | [O]/S | T, °C | Reaction Time, min | |||
| MoOx/HS | DBT | 200 | O2 | 3 | 50 | 60 | 99.7 | [36] |
| 4-MDBT | 200 | 60 | 99.6 | |||||
| 4,6-DMDBT | 200 | 90 | 39.3 | |||||
| PMo12/AmHMSiO2@C | DBT | 800 | H2O2 | 6 | 40 | 180 | >99 | [37] |
| MoO2@GNF | BT | 125 | TBHP * | 20 | 60 | 120 | 95.3 | [38] |
| DBT | 500 | 98.8 | ||||||
| 4,6-DMDBT | 125 | 94.8 | ||||||
| MoO3/SiO2-1 HN | DBT | 500 | H2O2 | 6 | 60 | 30 | 99.9 | [39] |
| PW2Mo2@ HPMOS | BT | 500 | H2O2 | 10 | 70 | 90 | 100.0 | This work |
| DBT | 500 | 120 | 99.5 | |||||
| 4-MDBT | 500 | 120 | 100.0 | |||||
| 4,6-DMDBT | 500 | 120 | 99.9 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Chen, Y.; Wang, C.; Yin, A.; Li, H.; Zhao, J. Polyoxometalates Encapsulated into Hollow Periodic Mesoporous Organosilica as Nanoreactors for Extraction Oxidation Desulfurization. Catalysts 2023, 13, 747. https://doi.org/10.3390/catal13040747
Gao Y, Chen Y, Wang C, Yin A, Li H, Zhao J. Polyoxometalates Encapsulated into Hollow Periodic Mesoporous Organosilica as Nanoreactors for Extraction Oxidation Desulfurization. Catalysts. 2023; 13(4):747. https://doi.org/10.3390/catal13040747
Chicago/Turabian StyleGao, Yan, Yu Chen, Cuiying Wang, Aiping Yin, Hailong Li, and Jianshe Zhao. 2023. "Polyoxometalates Encapsulated into Hollow Periodic Mesoporous Organosilica as Nanoreactors for Extraction Oxidation Desulfurization" Catalysts 13, no. 4: 747. https://doi.org/10.3390/catal13040747







