SOEing PCR/Docking Optimization of Protein A-G/scFv-Fc-Bioconjugated Au Nanoparticles for Interaction with Meningitidis Bacterial Antigen
Abstract
:1. Introduction
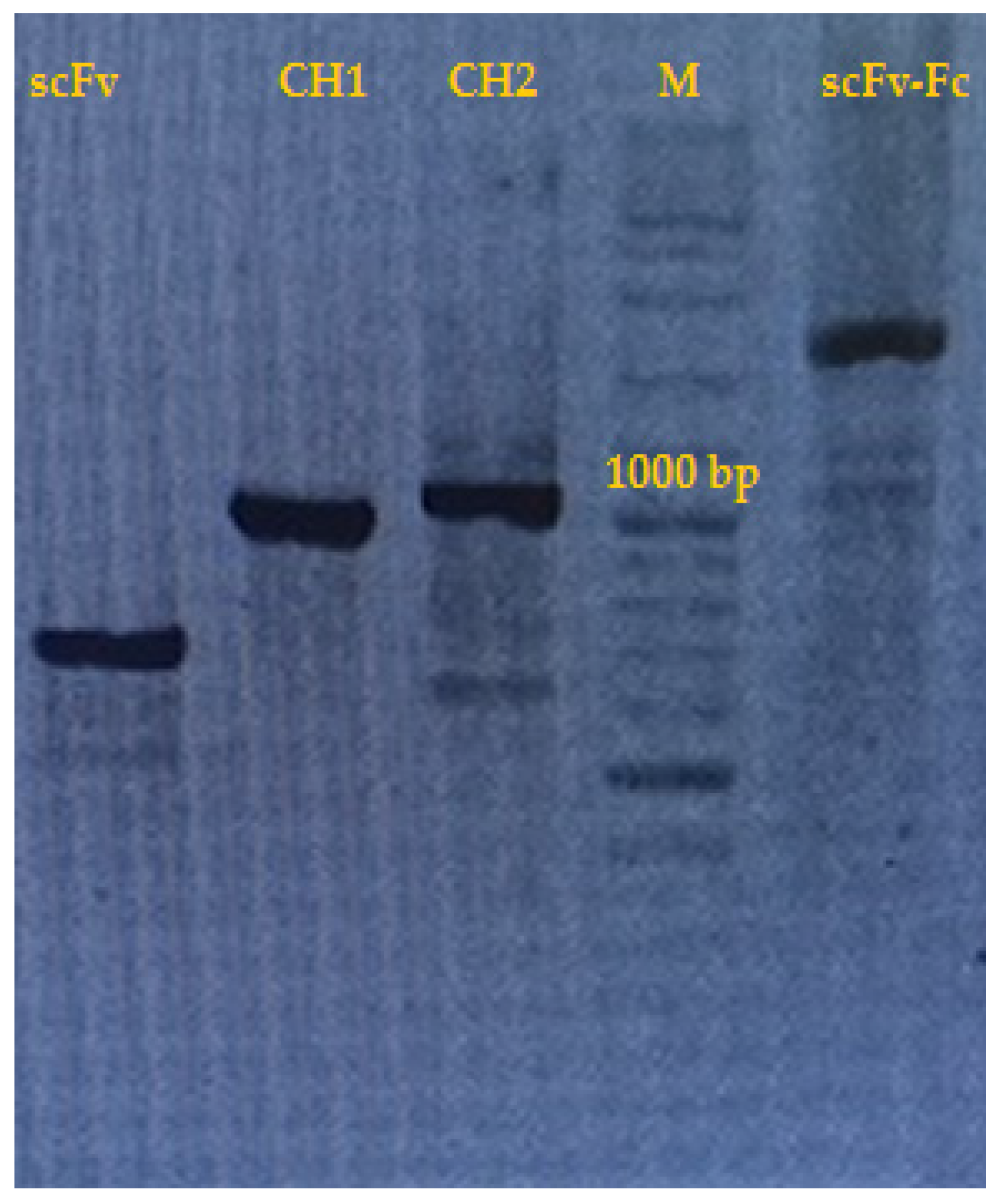
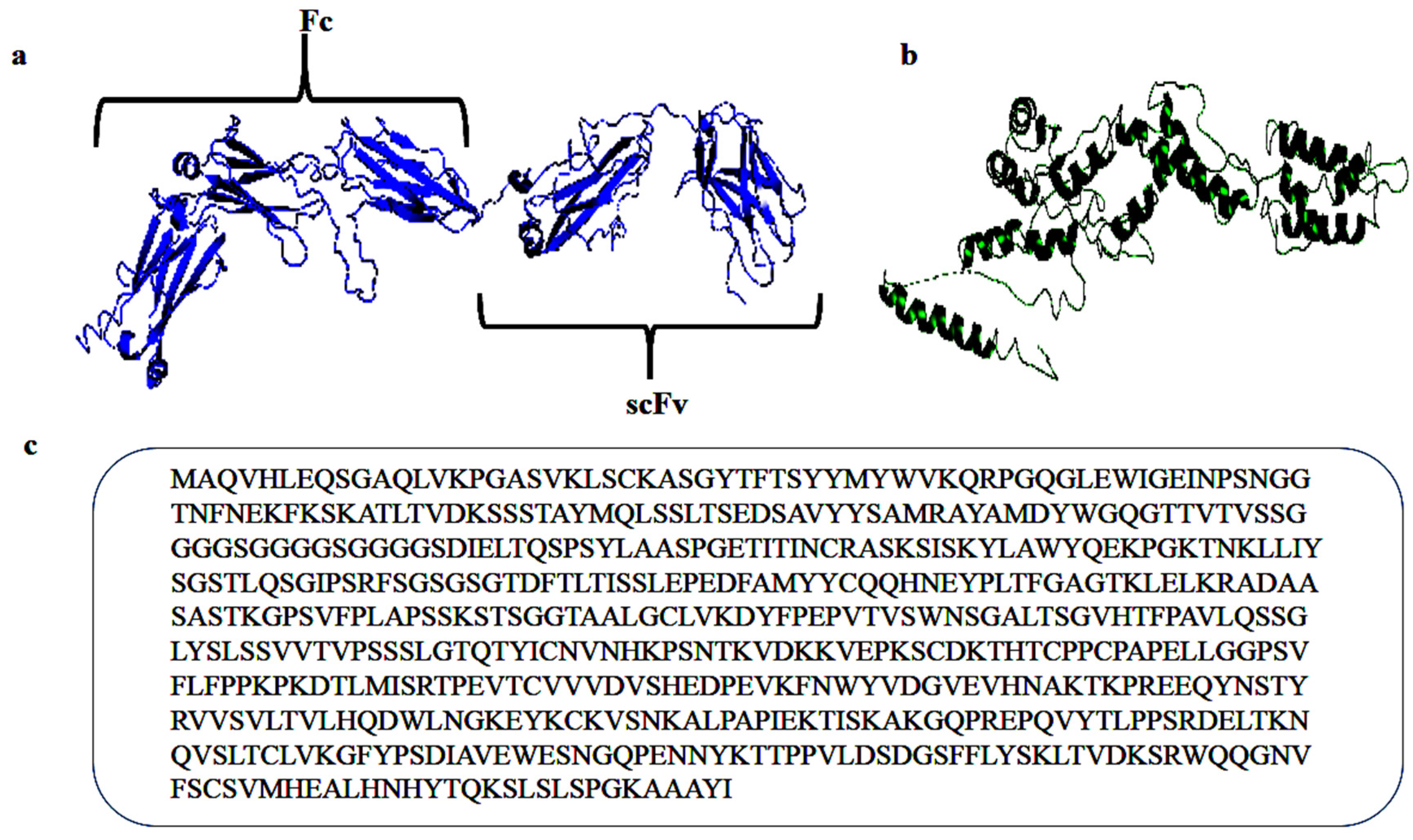
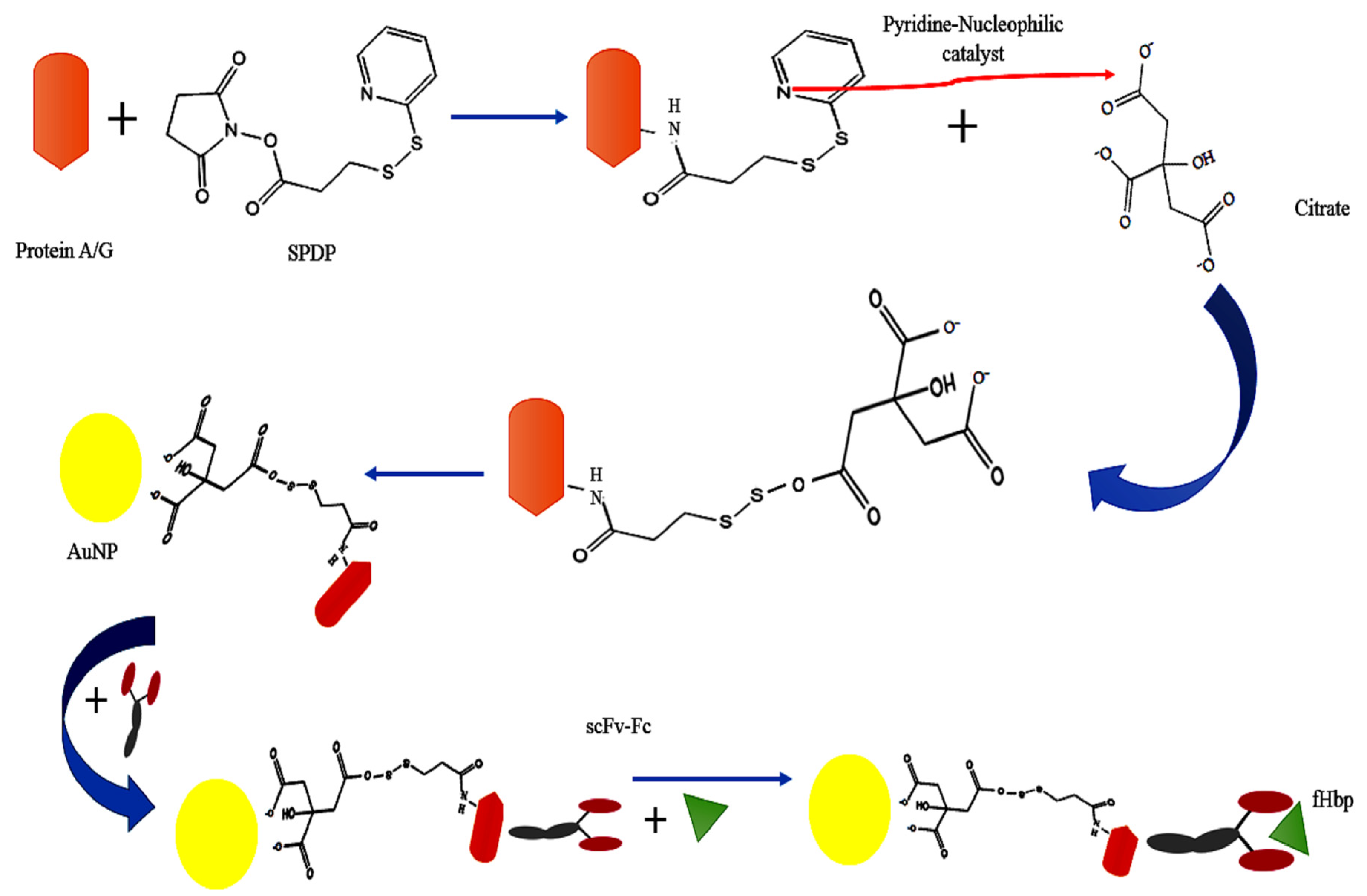
2. Results and Discussion
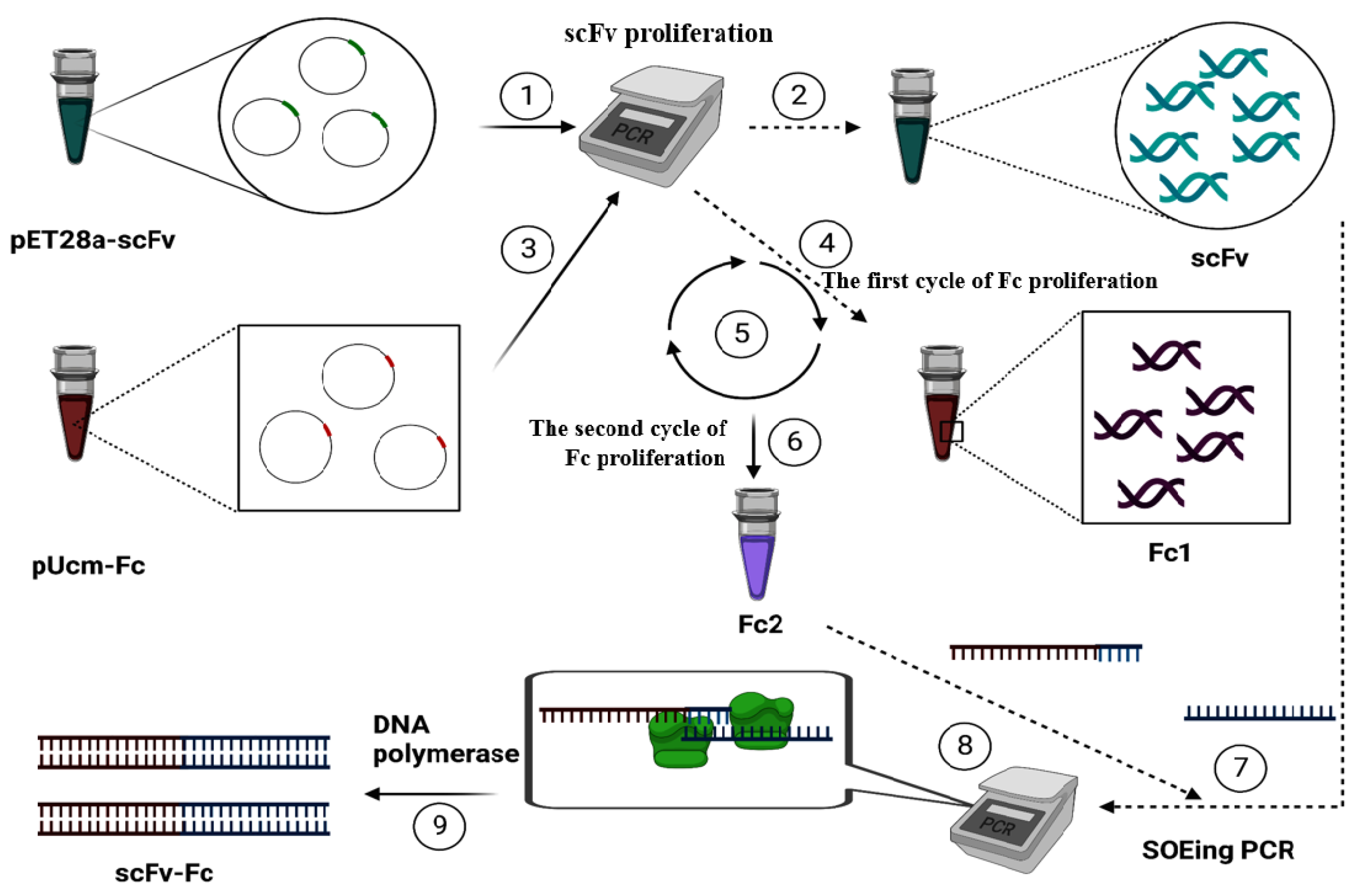
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jazayeri, M.H.; Amani, H.; Pourfatollah, A.A.; Pazoki-Toroudi, H.; Sedighimoghaddam, B. Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens. Bio-Sens. Res. 2016, 9, 17–22. [Google Scholar] [CrossRef]
- Yao, J.; Wang, Y.; Dai, Y.; Liu, C.C. Bioconjugated, single-use biosensor for the detection of biomarkers of prostate cancer. ACS Omega 2018, 3, 6411–6418. [Google Scholar] [CrossRef]
- Okyem, S.; Awotunde, O.; Ogunlusi, T.; Riley, M.B.; Driskell, J.D. High-Affinity Points Of Interaction on Antibody Allow Synthesis of Stable and Highly Functional Antibody–Gold Nanoparticle Conjugates. Bioconjug. Chem. 2021, 32, 1753–1762. [Google Scholar] [CrossRef]
- Conroy, P.J.; Hearty, S.; Leonard, P.; O’Kennedy, R.J. (Eds.) Antibody production, design and use for biosensor-based applications. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Assali, A.; Razzazan, S.; Akhavan, O.; Mottaghitalab, F.; Adeli, M.; Atyabi, F. The bio-interface between functionalized Au NR@ GO nanoplatforms with protein corona and their impact on delivery and release system. Coll. Surf. B Biointerfaces 2019, 173, 891–898. [Google Scholar] [CrossRef]
- Raval, K.; Ganatra, T. Basics, types and applications of molecular docking: A review. IP Int. J. Compr. Adv. Pharmacol. 2022, 7, 12–16. [Google Scholar] [CrossRef]
- Bartuzi, D.; Kaczor, A.A.; Targowska-Duda, K.M.; Matosiuk, D. Recent advances and applications of molecular docking to G protein-coupled receptors. Molecules 2017, 22, 340. [Google Scholar] [CrossRef] [PubMed]
- Assali, A.; Akhavan, O.; Adeli, M.; Razzazan, S.; Dinarvand, R.; Zanganeh, S.; Soleimani, M.; Dinarvand, M.; Atyabi, F. Multifunctional core-shell nanoplatforms (gold@ graphene oxide) with mediated NIR thermal therapy to promote miRNA delivery. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1891–1903. [Google Scholar] [CrossRef] [PubMed]
- Rad, M.; Ebrahimipour, G.; Bandehpour, M.; Akhavan, O.; Yarian, F. Enzymatic Formation of Recombinant Antibody-Conjugated Gold Nanoparticles in the Presence of Citrate Groups and Bacteria. Catalysts 2022, 12, 1048. [Google Scholar] [CrossRef]
- Mirica, A.-C.; Stan, D.; Chelcea, I.-C.; Mihailescu, C.M.; Ofiteru, A.; Bocancia-Mateescu, L.-A. Latest Trends in Lateral Flow Immunoassay (LFIA) Detection Labels and Conjugation Process. Front. Bioeng. Biotechnol. 2022, 10, 922772. [Google Scholar] [CrossRef]
- Tripathi, K.; Driskell, J.D. Quantifying bound and active antibodies conjugated to gold nanoparticles: A comprehensive and robust approach to evaluate immobilization chemistry. ACS Omega 2018, 3, 8253–8259. [Google Scholar] [CrossRef]
- Gao, S.; Rojas-Vega, F.; Rocha-Martin, J.; Guisán, J.M. Oriented immobilization of antibodies through different surface regions containing amino groups: Selective immobilization through the bottom of the Fc region. Int. J. Biol. Macromol. 2021, 177, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Rispens, T.; Vidarsson, G. Human IgG Subclasses. In Antibody Fc; Elsevier: Amsterdam, The Netherlands, 2014; pp. 159–177. [Google Scholar]
- Zeng, X.; Shen, Z.; Mernaugh, R. Recombinant antibodies and their use in biosensors. Anal. Bioanal. Chem. 2012, 402, 3027–3038. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, R.; Chen, X. Application of a single-chain fragment variable (scFv) antibody for the confirmatory diagnosis of hydatid disease in non-endemic areas. Electron. J. Biotechnol. 2017, 29, 57–62. [Google Scholar] [CrossRef]
- Ahangarzadeh, S.; Bandehpour, M.; Kazemi, B. Selection of single-chain variable fragments specific for Mycobacterium tuberculosis ESAT-6 antigen using ribosome display. Iran. J. Basic Med. Sci. 2017, 20, 327. [Google Scholar] [PubMed]
- Eyvazi, S.; Bandehpour, M.; Kazemi, B. Study of the production and the application of monoclonal antibodies: ScFv. Res. Med. 2017, 41, 138–151. [Google Scholar]
- Yang, H.; Zhong, Y.; Wang, J.; Zhang, Q.; Li, X.; Ling, S.; Wang, S.; Wang, R. Screening of a ScFv antibody with high affinity for application in human IFN-γ immunoassay. Front. Microbiol. 2018, 9, 261. [Google Scholar] [CrossRef]
- Gaba, M.; Gaba, P.; Singh, S.; Gupta, G. An overview on molecular docking. Int. J. Drug Dev. Res. 2010, 2, 219–231. [Google Scholar]
- Alabduladhem, T.O.; Bordoni, B. Physiology, Krebs Cycle. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Wiley, D.T.; Webster, P.; Gale, A.; Davis, M.E. Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor. Proc. Natl. Acad. Sci. USA 2013, 110, 8662–8667. [Google Scholar] [CrossRef]
- Yamazaki, S.; Shikida, N.; Takahashi, K.; Matsuda, Y.; Inoue, K.; Shimbo, K.; Mihara, Y. Lipoate-acid ligase a modification of native antibody: Synthesis and conjugation site analysis. Bioorgan. Med. Chem. Lett. 2021, 51, 128360. [Google Scholar] [CrossRef]
- Yarian, F.; Kazemi, B.; Bandehpour, M. Identification and characterization of a novel single-chain variable fragment (scFv) antibody against Neisseria meningitidis factor H-binding protein (fHbp). J. Med. Microbiol. 2018, 67, 820–827. [Google Scholar] [CrossRef]
- Zarghampoor, F.; Behzad-Behbahani, A.; Azarpira, N.; Khatami, S.R.; Fanian, M.; Aghdaie, M.H.; Dehbidi, G.R. A single tube overlap extension PCR method for splicing of multiple DNA fragments. Avicenna J. Med. Biotechnol. 2020, 12, 37. [Google Scholar] [PubMed]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, C.; Li, Y.; Pearce, R.; Bell, E.W.; Zhang, Y. Folding non-homologous proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Rep. Methods 2021, 1, 100014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Freddolino, P.L.; Zhang, Y. COFACTOR: Improved protein function prediction by combining structure, sequence and protein–protein interaction information. Nucleic Acids Res. 2017, 45, W291–W299. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y. I-TASSER server: New development for protein structure and function predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y. Improving the physical realism and structural accuracy of protein models by a two-step atomic-level energy minimization. Biophys. J. 2011, 101, 2525–2534. [Google Scholar] [CrossRef]
- Krieger, E.; Joo, K.; Lee, J.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins Struct. Funct. Bioinformat. 2009, 77 (Suppl. S9), 114–122. [Google Scholar] [CrossRef]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35 (Suppl. S2), W407–W410. [Google Scholar] [CrossRef]
- Sippl, M.J. Recognition of errors in three-dimensional structures of proteins. Proteins Struct. Funct. Bioinform. 1993, 17, 355–362. [Google Scholar] [CrossRef]
- Colovos, C.; Yeates, T.O. Verification of protein structures: Patterns of nonbonded atomic interactions. Protein Sci. 1993, 2, 1511–1519. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, L.; DeLano, W. PyMOL [Internet]. 2020. Available online: http://www.pymol.org/pymol (accessed on 1 September 2022).
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef] [PubMed]

| Complex | Binding Affinity (Kcal/mol) |
|---|---|
| (scFv-Fc)/citrate | −5.5 |
| (scFv-Fc)/PHA | −2.6 |
| (scFv-Fc)/PVA | −2.2 |
| Protein A-G/citrate | −5.2 |
| Protein A-G/SPDP | −6.4 |
| (scFv’s antigen binding site)/citrate | −4.5 |
| Hydrogen Bonds | |||||||||
| Index | Residue | AA | Distance H-A | Distance D-A | Donor Angle | Protein Donor? | Side Chain? | Donor Atom | Acceptor Atom |
| 1 | 200A | LYS | 2.32 | 3.27 | 156.68 | √ | √ | 3297[N3+] | 6745[O3] |
| 2 | 281A | HIS | 2.44 | 3.44 | 169.68 | √ | √ | 4627[Nar] | 6730[N3] |
| Hydrophobic Interactions | |||||||||
| Index | Residue | AA | Distance | Ligand Atom | Protein Atom | ||||
| 1 | 183A | TYR | 3.55 | 6735 | 3006 | ||||
| 2 | 184A | PHE | 3.83 | 6736 | 3030 | ||||
| 3 | 187A | LEU | 3.95 | 6736 | 3081 | ||||
| 4 | 199A | LYS | 3.98 | 6740 | 3272 | ||||
| Fc Cycle 1 | Fc Cycle 2 | scFv | SOEing | |
|---|---|---|---|---|
| Forward | AGCGCCAGCACCAAGGG | TGAAACGGGCTGATGCTGCAAGCGCCAGCACCAAGG | ATATATATCCATGGGACAGGTCCACC | ATATATATCCATGGGACAGGTCCACC |
| Reverse | ATATATATGCGGCCGCCTTGCCGGGGCTCAGGC | ATATATATGCGGCCGCCTTGCCGGGGCTCAGGC | TGCAGCATCAGCCCGTTTC | ATATATATGCGGCCGCCTTGCCGGGGCTCAGGC |
| Chemical Name | PubChem CID | 2D Structure | 3D Structure | Charge |
|---|---|---|---|---|
| Citrate | 31,348 | 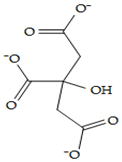 | 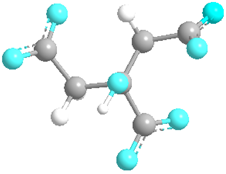 | Anionic |
| Allylamine hydrochloride (PAH) | 82,291 | 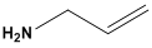 | 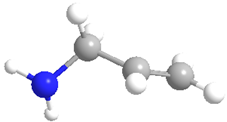 | Cationic |
| Polyvinyl alcohol (PVA) | 11,199 |  |  | Neutral |
| N-Succinimidyl 3-(2-pyridyldithio)propionate (SPDP) | 100,682 |  |  | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rad, M.; Ebrahimipour, G.; Bandehpour, M.; Akhavan, O.; Yarian, F. SOEing PCR/Docking Optimization of Protein A-G/scFv-Fc-Bioconjugated Au Nanoparticles for Interaction with Meningitidis Bacterial Antigen. Catalysts 2023, 13, 790. https://doi.org/10.3390/catal13050790
Rad M, Ebrahimipour G, Bandehpour M, Akhavan O, Yarian F. SOEing PCR/Docking Optimization of Protein A-G/scFv-Fc-Bioconjugated Au Nanoparticles for Interaction with Meningitidis Bacterial Antigen. Catalysts. 2023; 13(5):790. https://doi.org/10.3390/catal13050790
Chicago/Turabian StyleRad, Maryam, Gholamhossein Ebrahimipour, Mojgan Bandehpour, Omid Akhavan, and Fatemeh Yarian. 2023. "SOEing PCR/Docking Optimization of Protein A-G/scFv-Fc-Bioconjugated Au Nanoparticles for Interaction with Meningitidis Bacterial Antigen" Catalysts 13, no. 5: 790. https://doi.org/10.3390/catal13050790







