The Promotional Effect of Rare Earth on Pt for Ethanol Electro-Oxidation and Its Application on DEFC
Abstract
:1. Introduction
2. Results and Discussions
2.1. Physicochemical Characterization
2.2. Electrochemical Measurements
2.2.1. Three-Electrode Glass Cell Results
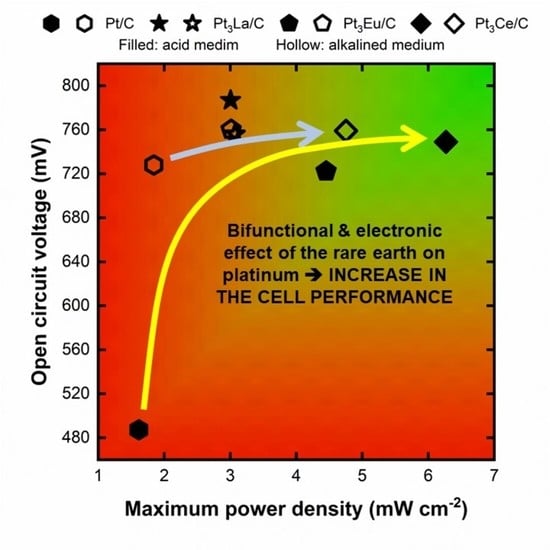
2.2.2. Single-Cell DEFC Tests
2.3. Identification and Quantification of the Oxidation Products
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of the Catalysts
3.3. Structural and Morphological Characterization
3.4. Electrochemical Measurements
3.5. DEFC Experiments and EEO Products Quantification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Cao, X.; Liu, Y.; Shao, Y.; Nan, Z.; Teng, L.; Peng, W.; Bian, J. Safety of Hydrogen Storage and Transportation: An Overview on Mechanisms, Techniques, and Challenges. Energy Rep. 2022, 8, 6258–6269. [Google Scholar] [CrossRef]
- Blanco, H.; Nijs, W.; Ruf, J.; Faaij, A. Potential for Hydrogen and Power-to-Liquid in a Low-Carbon EU Energy System Using Cost Optimization. Appl. Energy 2018, 232, 617–639. [Google Scholar] [CrossRef]
- Kunwer, R.; Pasupuleti, S.R.; Bhurat, S.S.; Gugulothu, S.K.; Kumar Joshi, S. Economic Assessment and Production of Ethanol-A Review. Mater. Today Proc. 2022, 69, 543–548. [Google Scholar] [CrossRef]
- Abdullah, S.; Hashim, N.; Shobery, N.A.M.; Abdullah, N. Brief Review of Solid Polymer Electrolyte for Direct Ethanol Fuel Cells Applications. In Proceedings of the 3rd International Conference on Chemistry, Chemical Process and Engineering, Yogyakarta, Indonesia, 30 September 2020; Fatimah, I., Oh, W.-C., Sahroni, I., Eds.; AIP Publishing: Yogyakarta, Indonesia, 2021; p. 020032. [Google Scholar]
- Badwal, S.P.S.; Giddey, S.; Kulkarni, A.; Goel, J.; Basu, S. Direct Ethanol Fuel Cells for Transport and Stationary Applications—A Comprehensive Review. Appl. Energy 2015, 145, 80–103. [Google Scholar] [CrossRef]
- de Oliveira, D.S.B.L.; Colmati, F.; de Sousa, R. Reaction Kinetics-Based Modeling and Parameter Sensitivity Analysis of Direct Ethanol Fuel Cells. Energies 2022, 15, 9143. [Google Scholar] [CrossRef]
- Marinkovic, N.S.; Li, M.; Adzic, R.R. Pt-Based Catalysts for Electrochemical Oxidation of Ethanol. Electrocatalysis 2020, 377, 1–39. [Google Scholar] [CrossRef]
- Artyushkova, K.; Halevi, B.; Padilla, M.; Atanassov, P.; Baranova, E.A. Mechanistic Study of Electrooxidation of Ethanol on PtSn Nanoparticles in Alkaline and Acid Media. J. Electrochem. Soc. 2015, 162, H345–H351. [Google Scholar] [CrossRef] [Green Version]
- Neto, A.O.; Watanabe, A.Y.; Rodrigues, R.M.D.S.; Linardi, M.; Forbicini, C.A.; Spinacé, E. Electrooxidation of Ethanol Using Pt Rare Earth–C Electrocatalysts Prepared by an Alcohol Reduction Process. Ionics 2008, 14, 577–581. [Google Scholar] [CrossRef]
- Corradini, P.G.; Antolini, E.; Perez, J. Structural and Electrochemical Characterization of Carbon Supported Pt–Pr Catalysts for Direct Ethanol Fuel Cells Prepared Using a Modified Formic Acid Method in a CO Atmosphere. Phys. Chem. Chem. Phys. 2013, 15, 11730. [Google Scholar] [CrossRef]
- Corradini, P.G.; Antolini, E.; Perez, J. Activity, Short-Term Stability (Poisoning Tolerance) and Durability of Carbon Supported Pt–Pr Catalysts for Ethanol Oxidation. J. Power Sources 2014, 251, 402–410. [Google Scholar] [CrossRef]
- Corradini, P.G.; Santos, N.A.; Silva, G.C.; Perez, J. Pt–Rare Earth Catalysts for Ethanol Electrooxidation: Modification of Polyol Synthesis. J. Solid State Electrochem. 2016, 20, 2581–2587. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Lourenço, J.R.B.; Macciò, D.; Saccone, A.; Sequeira, C.A.C.; Figueiredo, J.L. Ethanol Electrooxidation at Platinum-Rare Earth (RE = Ce, Sm, Ho, Dy) Binary Alloys. Energies 2020, 13, 1658. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Shu, J.; Ma, S.; Yang, H.; Jin, J.; Zhang, X.; Jin, R. Engineering Three-Dimensional Nitrogen-Doped Carbon Black Embedding Nitrogen-Doped Graphene Anchoring Ultrafine Surface-Clean Pd Nanoparticles as Efficient Ethanol Oxidation Electrocatalyst. Appl. Catal. B Environ. 2021, 280, 119464. [Google Scholar] [CrossRef]
- Zhao, J.; Shu, J.; Wang, J.; Yang, H.; Dong, Z.; Li, S. Combining Surface Chemical Functionalization with Introducing Reactive Oxygen Species Boosts Ethanol Electrooxidation. Nanoscale 2022, 14, 17392–17400. [Google Scholar] [CrossRef]
- Kazan-Kaya, E.S.; Bayramoğlu, M. Investigation of Ethanol Fuel Electrooxidation Reaction on Ni-CeO2NRs Anode Electrocatalyst in Alkaline Media. J. Electroanal. Chem. 2022, 927, 116982. [Google Scholar] [CrossRef]
- Xu, C.; Shen, P.K. Novel Pt/CeO2/C Catalysts for Electrooxidation of Alcohols in Alkaline Media. Chem. Commun. 2004, 19, 2238–2239. [Google Scholar] [CrossRef]
- Xie, A.; Zhang, Q.; He, H.; Peng, C. Facile Synthesis of PdAg Nanocatalysts on CeO2/C Composite Supports as High-Performance Catalysts toward Alkaline Ethanol Electro-Oxidation. N. J. Chem. 2020, 44, 17761–17768. [Google Scholar] [CrossRef]
- Alfi, N.; Rezvani, A.R.; Khorasani-Motlagh, M.; Noroozifar, M. Synthesis of Europium Oxide-Promoted Pd Catalyst by an Improved Impregnation Method as a High Performance Catalyst for the Ethanol Oxidation Reaction. N. J. Chem. 2017, 41, 10652–10658. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Lu, N.; Tian, C.; Han, Z.; Zhang, L.; Fang, Y.; Qian, B.; Jiang, X.; Cui, R. Nanoporous PdCe Bimetallic Nanocubes with High Catalytic Activity towards Ethanol Electro-Oxidation and the Oxygen Reduction Reaction in Alkaline Media. J. Mater. Chem. A 2018, 6, 23560–23568. [Google Scholar] [CrossRef]
- Sarma, S.C.; Subbarao, U.; Khulbe, Y.; Jana, R.; Peter, S.C. Are We Underrating Rare Earths as an Electrocatalyst? The Effect of Their Substitution in Palladium Nanoparticles Enhances the Activity towards Ethanol Oxidation Reaction. J. Mater. Chem. A 2017, 5, 23369–23381. [Google Scholar] [CrossRef]
- Leal Filho, W.L.; Kotter, R.; Özuyar, P.G.; Abubakar, I.R.; Eustachio, J.H.P.P.; Matandirotya, N.R. Understanding Rare Earth Elements as Critical Raw Materials. Sustainability 2023, 15, 1919. [Google Scholar] [CrossRef]
- Qin, M.; Chang, Q.; Yu, Y.; Wu, H. Exterior and Internal Uniform Loading of Pt Nanoparticles on Yolk-Shell La2O3 by Acoustic Levitation Synthesis with Enhanced Photocatalytic Performance. Materials 2019, 13, 107. [Google Scholar] [CrossRef] [Green Version]
- Kozlov, S.M.; Neyman, K.M. Effects of Electron Transfer in Model Catalysts Composed of Pt Nanoparticles on CeO2(1 1 1) Surface. J. Catal. 2016, 344, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Lee, C.H.; Nam, T.; Lee, S.; Oh, I.-K.; Yang, J.Y.; Choi, D.W.; Yoo, C.; Kim, H.; Kim, W.-H.; et al. Hydrogen Barrier Performance of Sputtered La2O3 Films for InGaZnO Thin-Film Transistor. J. Mater. Sci. 2019, 54, 11145–11156. [Google Scholar] [CrossRef]
- Kumar, S.; Prakash, R.; Choudhary, R.J.; Phase, D.M. Structural, XPS and Magnetic Studies of Pulsed Laser Deposited Fe Doped Eu2O3 Thin Film. Mater. Res. Bull. 2015, 70, 392–396. [Google Scholar] [CrossRef]
- Soni, S.; Vats, V.S.; Kumar, S.; Dalela, B.; Mishra, M.; Meena, R.S.; Gupta, G.; Alvi, P.A.; Dalela, S. Structural, Optical and Magnetic Properties of Fe-Doped CeO2 Samples Probed Using X-Ray Photoelectron Spectroscopy. J. Mater. Sci. Mater. Electron. 2018, 29, 10141–10153. [Google Scholar] [CrossRef]
- Borges, L.R.; Silva, A.G.M.; Braga, A.H.; Rossi, L.M.; Suller Garcia, M.A.; Vidinha, P. Towards the Effect of Pt0/PtΔ+ and Ce3+ Species at the Surface of CeO2 Crystals: Understanding the Nature of the Interactions under CO Oxidation Conditions. ChemCatChem 2021, 13, 1340–1354. [Google Scholar] [CrossRef]
- Jiang, Z.; Jing, M.; Feng, X.; Xiong, J.; He, C.; Douthwaite, M.; Zheng, L.; Song, W.; Liu, J.; Qu, Z. Stabilizing Platinum Atoms on CeO2 Oxygen Vacancies by Metal-Support Interaction Induced Interface Distortion: Mechanism and Application. Appl. Catal. B Environ. 2020, 278, 119304. [Google Scholar] [CrossRef]
- Colmati, F.; Antolini, E.; Gonzalez, E.R. Ethanol Oxidation on a Carbon-Supported Pt75Sn25 Electrocatalyst Prepared by Reduction with Formic Acid: Effect of Thermal Treatment. Appl. Catal. B Environ. 2007, 73, 106–115. [Google Scholar] [CrossRef]
- Liu, H.-H.; Wang, Y.; Jia, A.-P.; Wang, S.-Y.; Luo, M.-F.; Lu, J.-Q. Oxygen Vacancy Promoted CO Oxidation over Pt/CeO2 Catalysts: A Reaction at Pt–CeO2 Interface. Appl. Surf. Sci. 2014, 314, 725–734. [Google Scholar] [CrossRef]
- Rheinländer, P.J.; Herranz, J.; Durst, J.; Gasteiger, H.A. Kinetics of the Hydrogen Oxidation/Evolution Reaction on Polycrystalline Platinum in Alkaline Electrolyte Reaction Order with Respect to Hydrogen Pressure. J. Electrochem. Soc. 2014, 161, F1448–F1457. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Q.; Yu, H.; Peng, F. Platinum-Based Ternary Catalysts for the Electrooxidation of Ethanol. Particuology 2021, 58, 169–186. [Google Scholar] [CrossRef]
- Rousseau, S.; Coutanceau, C.; Lamy, C.; Léger, J.-M. Direct Ethanol Fuel Cell (DEFC): Electrical Performances and Reaction Products Distribution under Operating Conditions with Different Platinum-Based Anodes. J. Power Sources 2006, 158, 18–24. [Google Scholar] [CrossRef]
- Jadali, S.; Kamyabi, M.A.; Solla-Gullón, J.; Herrero, E. Effect of Pd on the Electrocatalytic Activity of Pt towards Oxidation of Ethanol in Alkaline Solutions. Appl. Sci. 2021, 11, 1315. [Google Scholar] [CrossRef]
- Nascimento, A.P.; Linares, J.J. Performance of a Direct Glycerol Fuel Cell Using KOH Doped Polybenzimidazole as Electrolyte. J. Braz. Chem. Soc. 2014, 25, 509–516. [Google Scholar] [CrossRef]
- Binninger, T.; Fabbri, E.; Kötz, R.; Schmidt, T.J. Determination of the Electrochemically Active Surface Area of Metal-Oxide Supported Platinum Catalyst. J. Electrochem. Soc. 2014, 161, H121–H128. [Google Scholar] [CrossRef]
- Souza, F.M.; Pinheiro, V.S.; Gentil, T.C.; Lucchetti, L.E.; Silva, J.C.M.; Santos, M.L.; De Oliveira, I.; Dourado, W.M.C.; Amaral-Labat, G.; Okamoto, S.; et al. Alkaline Direct Liquid Fuel Cells: Advances, Challenges and Perspectives. J. Electroanal. Chem. 2022, 922, 116712. [Google Scholar] [CrossRef]
- Pinheiro, V.S.; Souza, F.M.; Gentil, T.C.; Nascimento, A.N.; Parreira, L.S.; Hammer, P.; Sairre, M.I.; Batista, B.L.; Santos, M.C. Electrocatalysts Based on Low Amounts of Palladium Combined with Tin Nanoparticles and Cerium Dioxide Nanorods for Application as ADEFC Anodes. Int. J. Hydrogen Energy 2021, 46, 39438–39456. [Google Scholar] [CrossRef]
- Mukherjee, A.; Basu, S. Direct Hydrocarbon Low-Temperature Fuel Cell. In Electrocatalysts for Low Temperature Fuel Cells; Maiyalagan, T., Saji, V.S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 113–143. [Google Scholar]








| Catalyst | Label | Metal wt.% | Pt:RE Atomic Ratio (Nominal Value of 75:25) | Average Crystallite Size (nm) |
|---|---|---|---|---|
| Pt/C | Pt | 22.5 | - | 4.6 |
| Pt3La/C | Pt3LaC | 21.9 | 78:22 | 4.3 |
| Pt3Eu/C | Pt3EuC | 21.9 | 77:23 | 5.2 |
| Pt3Ce/C | Pt3CeC | 21.6 | 78:22 | 3.8 |
| Catalyst | At. % on the Nanoparticle Surface | Pt 4f7/2 XPS BE (% of Each Species in Parentheses) | ||
|---|---|---|---|---|
| Pt | RE | Pt0 | Oxidized (II or IV) Pt | |
| PtC | 100 | 71.10 (61) | 71.90 (39) | |
| Pt3LaC | 56.65 | 43.35 | 71.58 (46) | 72.71 (54) |
| Pt3EuC | 59.04 | 40.96 | 71.53 (51) | 72.46 (49) |
| Pt3CeC | 53.53 | 46.47 | 71.52 (50) | 72.46 (50) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, A.R.; Linares, J.J.; Crisafulli, R.; Zignani, S.C.; Colmati, F. The Promotional Effect of Rare Earth on Pt for Ethanol Electro-Oxidation and Its Application on DEFC. Catalysts 2023, 13, 1011. https://doi.org/10.3390/catal13061011
Nunes AR, Linares JJ, Crisafulli R, Zignani SC, Colmati F. The Promotional Effect of Rare Earth on Pt for Ethanol Electro-Oxidation and Its Application on DEFC. Catalysts. 2023; 13(6):1011. https://doi.org/10.3390/catal13061011
Chicago/Turabian StyleNunes, Alécio Rodrigues, José J. Linares, Rudy Crisafulli, Sabrina C. Zignani, and Flávio Colmati. 2023. "The Promotional Effect of Rare Earth on Pt for Ethanol Electro-Oxidation and Its Application on DEFC" Catalysts 13, no. 6: 1011. https://doi.org/10.3390/catal13061011